Abstract
Opportunistic pathogenic bacteria can engage in biofilm-based infections that evade immune responses and develop into chronic conditions. Because conventional antimicrobials cannot efficiently eradicate biofilms, there is an urgent need to develop alternative measures to combat biofilm infections. It has recently been established that the secondary messenger cyclic diguanosine monophosphate (c-di-GMP) functions as a positive regulator of biofilm formation in several different bacteria. In the present study we investigated whether manipulation of the c-di-GMP level in bacteria potentially can be used for biofilm control in vivo. We constructed a Pseudomonas aeruginosa strain in which a reduction in the c-di-GMP level can be achieved via induction of the Escherichia coli YhjH c-di-GMP phosphodiesterase. Initial experiments showed that induction of yhjH expression led to dispersal of the majority of the bacteria in in vitro-grown P. aeruginosa biofilms. Subsequently, we demonstrated that P. aeruginosa biofilms growing on silicone implants, located in the peritoneal cavity of mice, dispersed after induction of the YhjH protein. Bacteria accumulated temporarily in the spleen after induction of biofilm dispersal, but the mice tolerated the dispersed bacteria well. The present work provides proof of the concept that modulation of the c-di-GMP level in bacteria is a viable strategy for biofilm control.
INTRODUCTION
Bacteria are known to live either as single cells or in highly structured biofilms, where they are organized in aggregates in a self-produced matrix. These two life forms are very distinct from each other, and if bacteria succeed in forming biofilm inside a host it often results in a chronic infection that the host immune system and antibiotic treatment fail to eradicate (1–4). Accordingly, biofilms formed by opportunistic pathogenic bacteria are involved in devastating medical device-associated infections, and problematic infections in individuals who are immunocompromised or otherwise impaired in the host defense (1, 5–8). The U.S. National Institutes of Health have estimated that >80% of persistent bacterial infections involve biofilms (9). The importance of new treatment strategies to overcome the massive problems that chronic biofilm infections cause is thus obvious, and substantial research is being carried out in the field. Weakening of biofilms by the use of quorum-sensing inhibitors that subsequently reinstate proper action of the immune system and antibiotics is an example of a new chemotherapeutic strategy to combat biofilms (2, 10–18).
The opportunistic pathogen Pseudomonas aeruginosa is often associated with chronic biofilm infections, and it has therefore become a model organism for biofilm research (16). Dispersal of bacteria from P. aeruginosa biofilms is occurring naturally and completes the biofilm developmental cycle. The phenotype of single cells dispersed from biofilms has been shown to be different from that of the bacteria in the biofilms (19, 20). Thus, cells dispersing from biofilms were found to be more similar to planktonic bacteria than to bacteria isolated from mature biofilms (20). Biofilm dispersal has been shown to occur in response to changes in nutrient and oxygen availability for some bacterial species (21–23). Furthermore, intracellular levels of the secondary messenger cyclic diguanosine monophosphate (c-di-GMP) have been found to play a role in the dispersal of biofilms (21, 24–26). The c-di-GMP molecule and proteins with GGDEF and EAL or HD-GYP domains have been linked to regulation of cellular adhesiveness and biofilm formation in several different bacteria. Studies of P. aeruginosa, Burkholderia cenocepacia, Pseudomonas putida, Pseudomonas fluorescens, Salmonella enterica serovar Typhimurium, Escherichia coli, and Vibrio cholerae have shown that GGDEF domain proteins elevate the intracellular c-di-GMP concentration, whereas EAL or HD-GYP domain proteins diminish the c-di-GMP concentration in the cells, by functioning as c-di-GMP cyclases and phosphodiesterases, respectively (24, 27–33). In general, low levels of intracellular c-di-GMP result in reduced matrix production and induce a planktonic lifestyle, whereas high intracellular c-di-GMP levels cause an increase in matrix production and promote biofilm formation. An interesting and obvious new drug target is therefore c-di-GMP metabolism, since drugs that are able to lower the intracellular levels of c-di-GMP presumably will force the bacteria into the much more treatable planktonic lifestyle. Recently, Rybtke et al. (34) created a fluorescence-based reporter that enables gauging of the intracellular level of c-di-GMP in P. aeruginosa and is well suited for high-throughput screens for compounds that can lower the c-di-GMP level in bacteria and thus serve as lead compounds in the development of novel antibiofilm drugs.
In the present report we provide proof of the concept of induced dispersal as a treatment strategy against biofilm-based infections. We have created a P. aeruginosa strain in which overexpression of the E. coli YhjH c-di-GMP phosphodiesterase can be induced. Using an in vitro biofilm assay we show that overexpression of the YhjH protein inhibits P. aeruginosa in forming biofilms. Furthermore, we demonstrate that the induction of elevated c-di-GMP phosphodiesterase levels in established in vitro-grown P. aeruginosa biofilms leads to dispersal of the majority of the bacteria from the biofilms. Using an intraperitoneal foreign-body infection model in mice, we show that induction of the yhjH gene results in dispersal of P. aeruginosa biofilms from silicone implants so that bacteria could not be cultivated from the majority of the implants 15 h after the induction of dispersal. We also undertook the present study to investigate whether massive dispersal of bacteria from sites of infection could result in development of sepsis with subsequent lethal consequences. Although bacteria accumulated transiently in the spleens of the mice after induction of biofilm dispersal, the mice did not show any signs of discomfort. Thus, the current findings suggest that compounds that can lower the c-di-GMP level in bacteria may be effective drugs against biofilm-based infections.
MATERIALS AND METHODS
Bacterial strains.
The flow-chamber biofilm experiments presented here were performed with the sequenced wild-type P. aeruginosa PAO1 strain, obtained from the Pseudomonas Genetic Stock Center, and conjugated with plasmid pPBAD-yhjH or pJN105. The microtiter tray-based biofilm experiments were performed both with the sequenced P. aeruginosa PAO1 strain and with a P. aeruginosa PAO1 strain obtained from Barbara Iglewski's laboratory (University of Rochester Medical Center, Rochester, NY) and conjugated with pPBAD-yhjH or pJN105. The microtiter tray biofilm assay results obtained with the two PAO1 strains are essentially identical, but only the results obtained with the sequenced PAO1 strain are presented here. The animal experiments were performed with the wild-type P. aeruginosa PAO1 strain, obtained from Barbara Iglewski's laboratory, and conjugated with pPBAD-yhjH or pJN105. Colony morphology assays were performed using P. aeruginosa wspF deletion mutants containing pPBAD-yhjH or pJN105. The P. aeruginosa wspF deletion mutants were constructed in the sequenced PAO1 strain and in the PAO1 strain from Barbara Iglewski's laboratory as previously described by Rybtke et al. (34) using the pΔwspF deletion vector (28). The colony morphology assay results obtained with the two PAO1 strains were identical, but only the results obtained with the sequenced PAO1 strain are presented here. The pPBAD-yhjH and pJN105 plasmids were transformed into E. coli S17-1 by electroporation and hereafter conjugated into the P. aeruginosa strains with selection for plasmid-containing bacteria on ABTC plates (AB minimal medium [35] with 1 mg of thiamine/ml and 10 mM sodium citrate) supplemented with 30 μg of gentamicin/ml. The strains were gfp tagged by the use of a plasmid-based mini-Tn7 transposon system (36), so that they constitutively express the green fluorescent protein (GFP).
Construction of plasmid pPBAD-yhjH.
The yhjH gene of E. coli MG1655 was amplified by PCR using primers YhjH rev (5′-AAA TCT AGA GAA AAT GAG GCA GCT TAT AGC GC-3′) and YhjH fwd (5′-AAA CTG CAG TAG TGG AGG AAT TTG ATG ATA AGG CAG GTT ATC CAG C-3′). The PCR product was cloned into the vector pJN105 by digestion with PstI and XbaI. DNA restriction enzyme digestions and modifications were performed according to the manufacturer's instructions (Fermentas/Invitrogen). The resulting plasmid, pPBAD-yhjH, was transferred into E. coli DH5α by electroporation. Correct insertion of the yhjH gene into the vector pJN105 was verified by sequencing. Plasmid pJN105 contains an araC-PBAD promoter, which is induced in the presence of l-arabinose (37). The yhjH gene is placed in plasmid pPBAD-yhjH so that it's expression is controlled by the araC-PBAD promoter.
Colony morphology assay.
Strains were streaked on ABTC plates supplemented with 30 mg of gentamicin/ml and, where indicated, 0.2% l-arabinose. After 20 h of incubation at 37°C, the colony morphologies were visualized by bright-field microscopy, and pictures were taken using AxioVision software.
Assessment of YhjH activity in P. aeruginosa.
The activity of YhjH in P. aeruginosa was assessed using a modified version of the c-di-GMP-level reporter previously described by Rybtke et al. (34). The reporter plasmid pUCP18-CdrA::gfpC was constructed to be compatible with plasmid pPBAD-yhjH. The NotI cassette containing the reporter construct was excised from pCdrA::gfpC, blunt ended with T4 DNA polymerase, and inserted into SmaI-digested pUCP18 (38), creating pUCP18-CdrA::gfpC. The P. aeruginosa wspF pelA pslBCD triple deletion reporter background (34) was cotransformed with pPBAD-yhjH and pUCP18-CdrA::gfpC and selected for by growth on medium containing both 200 μg of carbenicillin/ml and 30 μg of gentamicin/ml. Gauging of c-di-GMP levels were conducted as previously described (34) with modifications. Briefly, overnight cultures of the reporter strains were diluted to an optical density at 600 nm (OD600) of 0.03 and added to the wells of a 96-well microtiter plate with or without 0.05% (wt/vol) l-arabinose. The plate was sealed with a breathable rayon membrane (Nunc) and incubated at 37°C on a microplate shaker (IKA, Germany) set at a shaking speed of 1,000 rpm for 18 h. After the incubation period, the cultures were diluted 10 times with a 0.9% NaCl solution in a black-welled microtiter plate (Nunc). Growth and fluorescent readout was measured as the A450 and arbitrary GFP units on a Victor Vallac X4 plate reader (Perkin-Elmer), respectively. The data are finally presented as GFP units/A450, termed relative fluorescence units.
Microtiter tray biofilm assay.
Biofilm formation in static microtiter dishes (612u96; Sterilin; Thermo Fisher) was quantified by crystal violet (CV) staining as described by O'Toole and Kolter (39) with modifications. Briefly, 100 μl of AB trace minimal medium containing 0.5% glucose (wt/vol), 0.5% (wt/vol) Casamino Acids, and 0.1, 0.05, 0.025, or 0% l-arabinose (A3256; Sigma) was inoculated with an overnight culture of PAO1/pPBAD-yhjH or PAO1/pJN105, adjusted to an OD600 of 0.1, and incubated at 37°C for 24 h. The wells were aspirated and washed with 120 μl of 0.9% NaCl, followed by 15 min staining with 120 μl of 0.1% crystal violet (Sigma) in 0.9% NaCl. After staining, the wells were washed twice with 150 μl of 0.9% NaCl. The crystal violet was solubilized with 96% ethanol for 30 min before measuring the absorbance at 590 nm.
Microtiter tray biofilm dispersal assay.
One hundred microliters of ABTG medium inoculated with an overnight culture of PAO1/pPBAD-yhjH or PAO1/pJN105 adjusted to an OD600 of 0.1 was added to the wells in a microtiter dish. Biofilm was allowed to develop for 24 h at 37°C. The medium was then removed from the wells, and each well was washed twice with 120 μl of 0.9% NaCl. Then, 100 μl of ABTG medium containing 0.05% arabinose (or 0.9% NaCl as a control) was added to the wells. At different time points after the addition of arabinose (0, 15, 30, 60, 120, and 180 min), 10-μl samples from two wells for each treatment were serially diluted and plated onto Luria-Bertani (LB) and blue agar plates to determine the CFU/ml. Blue agar plates are selective for Gram-negative bacilli (40). The experiment was performed three times.
Flow-chamber experiments.
The flow-chamber system was assembled and operated as described previously (41). Biofilms were grown at 37°C in continuous-culture, once-through, three-channel flow chambers with individual channel dimensions of 1 by 4 by 40 mm perfused with sterile AB trace minimal medium (42) containing 0.3 mM glucose and 30 μg of gentamicin/ml. Overnight cultures were diluted to an OD600 of 0.1 in 0.9% NaCl, and 250 μl was used for inoculation per channel. We added 0.05% l-arabinose or 0.9% NaCl to the medium after 24 h of biofilm growth. The biofilms were visualized by confocal laser scanning microscopy (CLSM) 0, 3, 10, and 15 h after the shift to arabinose-containing medium. In addition, flow-chamber experiments were performed where effluent was collected for 5 min from each flow-chamber channel through a 4-cm silicone tube 0, 15, 30, 60, 120, 180, and 240 min after the shift to arabinose-containing medium. The samples were kept on ice until serial dilution and plating on LB and blue agar plates to determine the CFU/ml. The experiment was performed three times; the effluent each time was collected from two channels per strain and treatment.
Microscopy and image processing.
Microscopic observation and image acquisition of biofilms were performed with a Zeiss LSM 710 confocal laser scanning microscope (Carl Zeiss, Germany) equipped with a laser and detector and filter sets for monitoring the GFP (excitation, 488 nm; emission, 517 nm). Images were obtained using a ×63/1.4 objective lens. Simulated fluorescence projections were generated using the Imaris software package (Bitplane AG, Switzerland).
Animals.
Female BALB/c mice were purchased from Taconic M&B A/S (Ry, Denmark) at 8 to 9 weeks of age and were maintained on standard mouse chow and water ad libitum for 2 weeks before the challenge. The animal studies were carried out in accordance with the European convention and Directive for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes and the Danish law on animal experimentation. All experiments were authorized and approved by the National Animal Ethics Committee of Denmark (The Animal Experiments Inspectorate, dyreforsoegstilsynet.dk) and assigned the permit number 2010/561-1817.
Foreign-body infection model.
Silicone implants were prepared as described previously by Christensen et al. (11) with modifications. Silicone tube implants with a length of 4 mm (inner diameter, 4 mm; outer diameter, 6 mm; Ole Dich, Denmark) were cut in half. A bacterial pellet from a centrifuged P. aeruginosa overnight culture was resuspended in 0.9% NaCl to an OD600 of 0.1, and the tube pieces were left in this solution for 20 h at 37°C with shaking at 110 rpm to allow bacterial colonization of them before insertion in the peritoneal cavities of mice. Animals were challenged according to the method of Christensen et al. (11). Mice were anesthetized by subcutaneous injections in the groin area with Hypnorm/Midazolam (Roche) (one part Hypnorm [0.315 mg of fentanyl citrate/ml and 10 mg of fluanisone/ml], one part Midazolam [5 mg/ml], and three parts sterile water), and the tube pieces with bacteria were inserted into the peritoneal cavity of each animal.
In vivo sampling and bacteriology.
Pentobarbital (200 mg/ml at 10 ml/kg of body weight) with lidocaine hydrochloride (20 mg/ml) was injected intraperitoneally to euthanize mice at the termination of the experiment. Silicone implants were removed from the peritoneal cavities of the mice, placed in 15-ml centrifuge tubes containing 2 ml of 0.9% NaCl, and kept on ice until the tubes were placed in an ultrasound bath (Bransonic model 2510; Branson Ultrasonic Corp.) for 10 min (a 5-min degas step followed by a 5-min sonic step). Second, the opening in the peritoneal cavity, from which the silicone implant was removed, was closed with silk to avoid leakage of fluid when the cavity was then flushed with 2 ml of 0.9% NaCl using a 25G needle injected in the groin area. The cavity area was massaged gently from the outside of the mouse, and the cavity was opened again to collect the saline, which was kept on ice until assay. The spleen was then removed from the mouse and placed in a 50-ml centrifuge tube containing 2 ml of 0.9% NaCl and kept on ice until the spleen cells were isolated by forcing the spleen through a 40-μm-pore-size cell strainer (BD Biosciences) using the plunger of a 1-ml syringe. All of the NaCl-bacterium solutions (from the implant, intraperitoneal cavity, and spleen) were then serially diluted and plated on blue agar plates (State Serum Institute, Copenhagen, Denmark) for colony formation. The plates were incubated at 37°C overnight before determination of the CFU/implant.
Statistical analysis.
Statistical significance was evaluated by one-way or two-way analysis of variance (ANOVA) with Bonferroni post tests for experiments with more than one comparison. To compare the bacterial counts (CFU) between two groups of mice to a single time point, the Mann-Whitney U test was used (analysis of nonparametric data). To compare data that followed normal distribution a Student t test was performed. For calculating P values the statistical program GraphPad Prism version 5.0 was used (GraphPad Software, Inc., San Diego, CA).
RESULTS
Construction and characterization of a P. aeruginosa strain harboring an inducible yhjH gene.
We selected Escherichia coli yhjH to serve as our inducible phosphodiesterase gene. The YhjH protein was chosen because it does not contain any recognizable domains other than the EAL domain (27) and because it was previously shown to have phosphodiesterase activity toward c-di-GMP in B. cenocepacia, P. putida, S. Typhimurium, and Shewanella oneidensis (24, 27, 29, 43). In addition, the S. Typhimurium YhjH ortholog was previously shown to have phosphodiesterase activity toward c-di-GMP in P. aeruginosa (27). We PCR amplified the yhjH gene and cloned it into the vector pJN105, so that its expression is controlled by an araC-PBAD promoter, allowing induction of gene expression with l-arabinose (37). P. aeruginosa PAO1 carrying the yhjH gene will be referred to as PAO1/pPBAD-yhjH. As a negative control, we used P. aeruginosa PAO1 transformed with the vector pJN105, which will be referred to as PAO1/pJN105.
In order to ascertain that our pPBAD-yhjH plasmid functions as anticipated, we transformed a P. aeruginosa wspF mutant with the pPBAD-yhjH plasmid and pJN105 vector control and observed the colony morphology of the wspF/pPBAD-yhjH strain on agar plates with or without arabinose. The formation of wrinkled colonies by bacteria on solid medium is indicative of the production of adhesive matrix components such as exopolysaccharides, aggregative fimbriae, and large adhesive proteins (27, 44–48). This behavior has been shown to be positively regulated by c-di-GMP in a number of bacteria (24, 27, 32, 49, 50). A mutation in the wspF gene in P. aeruginosa has been shown to result in elevated c-di-GMP levels that leads to increased matrix production, cell aggregation, and a wrinkly colony morphology (28, 49). As expected, the wspF/pPBAD-yhjH bacteria formed wrinkly colonies on agar plates without arabinose and formed smooth colonies on agar plates with arabinose (Fig. 1A and B). In contrast, the wspF/pJN105 vector control strain formed wrinkly colonies both on plates with and without arabinose (Fig. 1C and D). To further confirm the activity of YhjH in P. aeruginosa, the effect of the induced PBAD-yhjH fusion on the c-di-GMP level in P. aeruginosa was assessed using a pUCP18-based version of the fluorescence-based reporter of c-di-GMP levels previously described by us (34). The relative fluorescence levels emitted by the reporter strain containing both pPBAD-yhjH and pUCP18-CdrA::gfpC were 10.274 (n = 6, standard deviation [SD] = 2,015) with addition of 0.05% arabinose and 36.820 (n = 6, SD = 1,306) without arabinose, further indicating that YhjH from E. coli is an active c-di-GMP degrading phosphodiesterase when expressed in P. aeruginosa. The PAO1/pPBAD-yhjH and PAO1/pJN105 strains grew with the same growth rate in cultures with or without arabinose (data not shown).
Fig 1.
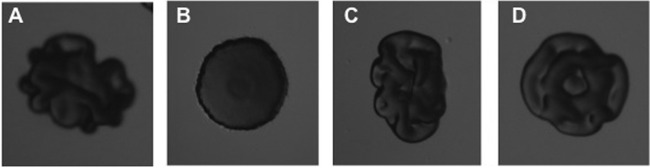
Colony morphology of P. aeruginosa wspF/pPBAD-yhjH on agar plates without (A) or with (B) l-arabinose and of P. aeruginosa wspF/pJN105 on agar plates without (C) or with (D) l-arabinose.
Overexpression of yhjH causes dispersal of the majority of the bacteria in in vitro P. aeruginosa biofilms.
Subsequently, we investigated the effect of overexpression of the YhjH phosphodiesterase in P. aeruginosa on in vitro biofilm development and dispersal. In agreement with previous studies (27, 28), biofilm formation in microtiter dishes with PAO1/pPBAD-yhjH was significantly reduced when the yhjH gene was induced with arabinose in comparison to when it was uninduced, and in comparison to the PAO1/pJN105 strain under inducing or noninducing conditions (Fig. 2). We then investigated the effect of induction of the YhjH phosphodiesterase in established microtiter dish biofilms. To this end, PAO1/pPBAD-yhjH and PAO1/pJN105 were grown in microtiter dishes with minimal medium for 24 h, upon which the medium was changed to contain 0.05% l-arabinose or 0.9% NaCl as a control. Liquid samples from the wells were then collected at time points 0, 15, 30, 60, 120, and 180 min, and the CFU/ml was determined for each sample. We found a significant (P < 0.0001) increase in the CFU/ml in the liquid phase surrounding the biofilms of PAO1/pPBAD-yhjH induced with l-arabinose compared to the same strain uninduced or PAO1/pJN105 induced or uninduced (Fig. 3). No significant difference was observed between the other three groups.
Fig 2.
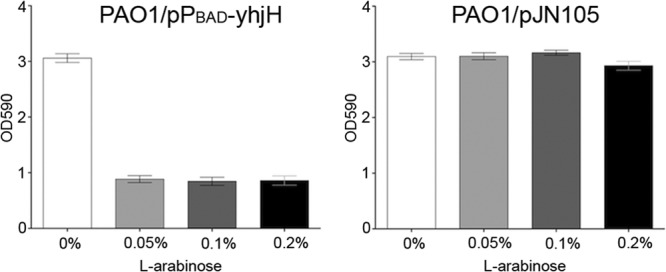
Biofilm formation of PAO1/pPBAD-yhjH (left) and PAO1/pJN105 (right) in microtiter trays. l-Arabinose in different concentrations (0, 0.05, 0.1, or 0.2%) was added to the medium before inoculation, and biofilm growth was allowed for 24 h, after which the adherent biomass was quantified by crystal violet staining. The values are averages of three experiments with 12 replicates in each, and the bars indicate standard deviations (SD). There was a significant (P < 0.0001) decrease in the biomass of PAO1/pPBAD-yhjH when induced with l-arabinose compared to the same strain uninduced (evaluated by analysis of variance [ANOVA] test). No significant difference was observed when comparing PAO1/pJN105 induced with uninduced samples (evaluated by ANOVA test).
Fig 3.
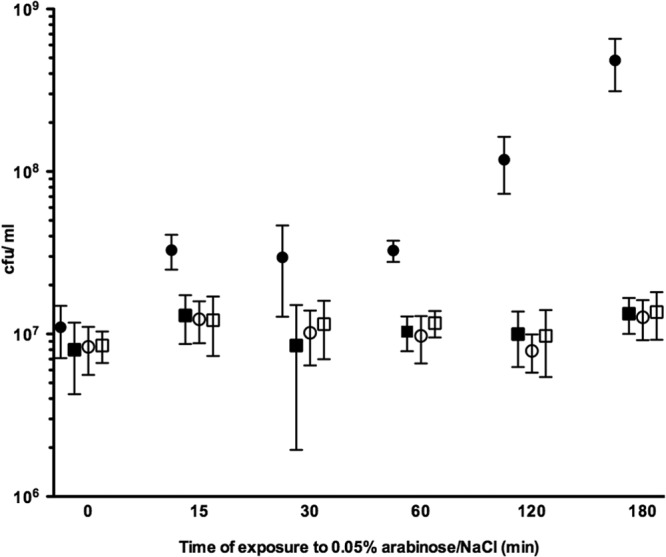
Quantification of P. aeruginosa bacteria dispersing from microtiter tray biofilms. Biofilms were allowed to develop for 24 h before inducing PBAD-driven yhjH expression with l-arabinose. Liquid culture samples were collected at intervals after yhjH induction and were plated to obtain CFU/ml. Circles represent PAO1/pPBAD-yhjH with l-arabinose (filled) or sodium chloride (open) in the medium. Squares represent PAO1/pJN105 with l-arabinose (filled) or sodium chloride (open) in the medium. There was a significant (P < 0.0001) increase in the CFU/ml in the liquid phase surrounding the biofilms of PAO1/pPBAD-yhjH induced with l-arabinose compared to the same strain uninduced or PAO1/pJN105 induced or uninduced, evaluated by ANOVA test. Error bars represent the SD.
Next, we examined the effect of inducing YhjH in P. aeruginosa biofilms grown under continuous culture conditions in flow chambers. We grew PAO1/pPBAD-yhjH and PAO1/pJN105 in flow chambers irrigated with minimal medium; after 24 h the medium was changed to contain 0.05% l-arabinose, and confocal laser scanning micrographs were acquired at time points 0, 3, 10, and 15 h after the shift to l-arabinose-containing medium. As shown in Fig. 4, induction of the phosphodiesterase led to dispersal of the microcolonies and inter-microcolony biomass in the flow-chamber-grown PAO1/pPBAD-yhjH biofilm, but a thin layer of cells remained at the glass surface. In contrast, the PAO1/pJN105 vector control biofilm continued to grow after the shift to l-arabinose-containing medium (Fig. 4). In order to prevent loss of the pPBAD-yhjH and pJN105 plasmids the medium irrigated to the biofilms contained gentamicin. However, aminoglycoside antibiotics has little affect on the bacteria deep inside P. aeruginosa biofilms (e.g., see reference 3), and it was therefore possible that the bacteria that remained in the flow cell after the induced biofilm dispersal had lost the plasmid. It would be expected that plasmid-free cells would be killed by the gentamicin after dispersal of the protective biofilm. However, propidium iodide staining of the thin layer of bacteria that remained 15 h after the induction of biofilm dispersal suggested that the majority of the cells were alive (see Fig. S1 in the supplemental material). Moreover, plating of bacteria from the remaining biofilm on LB plates and replica plating to gentamicin plates suggested that all of the viable bacteria still contained the pPBAD-yhjH plasmid (data not shown). Furthermore, the pPBAD-yhjH fusion in the bacteria recovered from the remaining biofilm was active, since the experiment shown in Fig. 2 (left part) could be repeated with these bacteria (data not shown). We also performed experiments where we collected effluent from the flow chambers for 5 min initiated at time points 0, 15, 30, 60, 120, 180, and 240 min after the shift to l-arabinose-containing medium and subsequently determined CFU/ml for each sample. We found a significant (P < 0.0001) increase in the CFU/ml effluent for PAO1/pPBAD-yhjH induced with l-arabinose compared to the same strain uninduced or PAO1/pJN105 induced or uninduced (Fig. 5). No significant difference was found between the other three groups.
Fig 4.
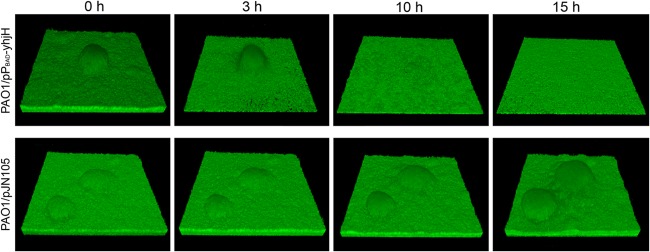
Dispersal of flow-chamber-grown P. aeruginosa biofilms. Biofilms of GFP-labeled PAO1/pPBAD-yhjH (top) and PAO1/pJN105 (bottom) were allowed to develop in flow chambers for 24 h before PBAD-driven yhjH expression was induced. CLSM micrographs were acquired at times t = 0, 3, 10, and 15 h after yhjH induction. The images were acquired at the same location in the PAO1/pPBAD-yhjH biofilm (top) and PAO1/pJN105 biofilm (bottom). The microscope field corresponds to 220 by 220 μm.
Fig 5.

Quantification of P. aeruginosa bacteria dispersing from flow-chamber biofilms. Biofilms were allowed to develop for 24 h before PBAD-driven yhjH expression was induced. Effluent was collected at intervals after yhjH induction, and the CFU/ml were determined. Circles represent PAO1/pPBAD-yhjH with l-arabinose (filled) or sodium chloride (open) in the medium. Squares represent PAO1/pJN105 with l-arabinose (filled) or sodium chloride (open) in the medium. There was a significant (P < 0.0001) increase in the CFU/ml of effluent for PAO1/pPBAD-yhjH induced with l-arabinose compared to the same strain uninduced or to PAO1/pJN105 induced or uninduced, evaluated by ANOVA. Error bars represent the SD.
From the experiments described above we conclude that induction of the YhjH phosphodiesterase in P. aeruginosa functions as anticipated and leads to dispersal of the majority of the biomass in in vitro-grown P. aeruginosa biofilms, although a thin layer of cells remain surface attached.
Overexpression of yhjH causes dispersal of in vivo P. aeruginosa biofilms.
Although induction of the YhjH phosphodiesterase did not lead to complete dispersal of in vitro-grown P. aeruginosa biofilms, the effect of a decrease in the c-di-GMP level might be different in in vivo-growing P. aeruginosa biofilms, since the extracellular matrix components, whose synthesis is under c-di-GMP control, protects the biofilm bacteria against the activities of the immune system. We hypothesized that a stoppage of extracellular matrix synthesis in combination with immune system attack might lead to complete dispersal of in vivo-grown biofilms. To investigate this hypothesis, we examined induced dispersal of P. aeruginosa in in vivo biofilms using a foreign-body infection model. We precolonized silicone implants with either PAO1/pPBAD-yhjH or PAO1/pJN105 and inserted them in the peritoneal cavity of mice. After allowing the biofilms to further develop for 24 h in the mice, we induced yhjH expression by two consecutive, intraperitoneal injections of 0.2 ml of 2% l-arabinose administered at 24 h and 26.5 h postinfection. The mice were then euthanized 29 h postinfection, the implants were removed from the mice, and the numbers of CFU/implant were determined. In addition, the peritoneal cavity was flushed with 0.9% NaCl, and the spleen was removed and homogenized, and the CFU/ml of flush and CFU/spleen were determined. Two and a half hours after the second induction of biofilm dispersal, significantly (P < 0.001) fewer bacteria were recovered from the implants removed from mice infected with PAO1/pPBAD-yhjH compared to PAO1/pJN105 (Fig. 6). At this time point, the median number of PAO1/pPBAD-yhjH bacteria on the implants was ∼10-fold lower than the median number of PAO1/pJN105 bacteria on the implants (Fig. 6). In contrast, significantly more bacteria were found in both the fluid from the peritoneal cavity and in the spleens from mice infected with PAO1/pPBAD-yhjH compared to the control group (P < 0.0009 and P < 0.01, respectively) (Fig. 6).
Fig 6.
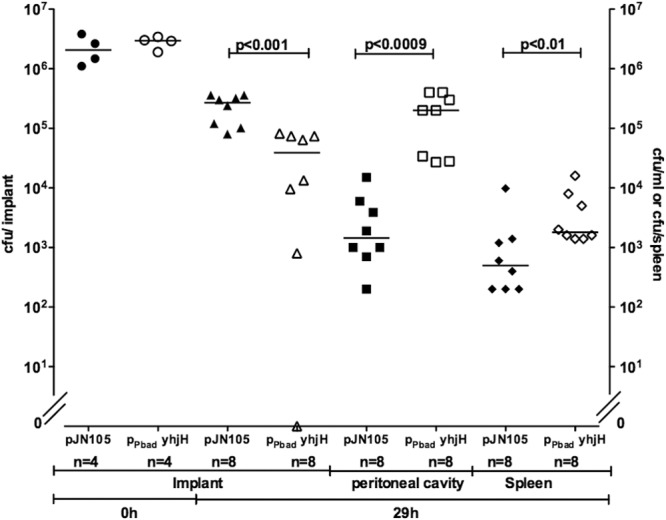
Dispersal of P. aeruginosa biofilms in vivo. Mice had silicone implants, precolonized with either PAO1/pPBAD-yhjH (open circles) or PAO1/pJN105 (filled circles), inserted in the peritoneal cavity. At 24 and 26.5 h postinsertion, the mice received intraperitoneal injections of 2% arabinose. The mice were euthanized 2.5 h after the last injection, the implants were removed, and the CFU/implant were determined (filled and open triangles). The peritoneal cavity was then flushed with 0.9% NaCl, and the CFU/ml was determined (filled and open squares). Lastly, the spleens were removed, and the CFU/spleen was determined (filled and open diamantes). Lines represent medians. P values are shown in the figure.
Subsequently, we followed the quantity of bacteria on the implants and in the mouse spleens over time to further investigate the outcome of induced dispersal. Again, implants precolonized with either PAO1/pPBAD-yhjH or PAO1/pJN105 were inserted in the peritoneal cavity of mice, and intraperitoneal injections of 0.2 ml of 2% l-arabinose were given 24 h and 26.5 h postinfection. Immediately after the second l-arabinose injection (t = 0), four mice from each group were euthanized, and the implants and spleens were removed for quantification of the bacteria. Subsequently, implants and spleens were removed from mice for quantification of the bacteria 1, 2.5, 5, 10, and 15 h after the second injection. The results shown in Fig. 7 and 8 are based on two experiments. Because the average initial number of bacteria per implant differed between the two experiments the pooled results were normalized to the average initial number of bacteria per implant of both experiments. Significantly (P < 0.006) fewer bacteria were present on the implants removed from mice infected with PAO1/pPBAD-yhjH compared to PAO1/pJN105 (Fig. 7). At 15 h after the induction of biofilm dispersal, we could not detect bacteria on six of seven of the implants that had been coated with PAO1/pPBAD-yhjH bacteria, whereas none of the nine PAO1/pJN105-colonized implants were cleared (Fig. 7). Bacterial counts in the spleens of mice infected with PAO1/pPBAD-yhjH increased the first 5 h after induction of biofilm dispersal and then decreased to reach a level similar to that of the mice infected with PAO1/pJN105 (Fig. 8). The CFU/spleen over time were significantly different (P < 0.0004) when comparing PAO1/pPBAD-yhjH to PAO1/pJN105 (Fig. 8).
Fig 7.
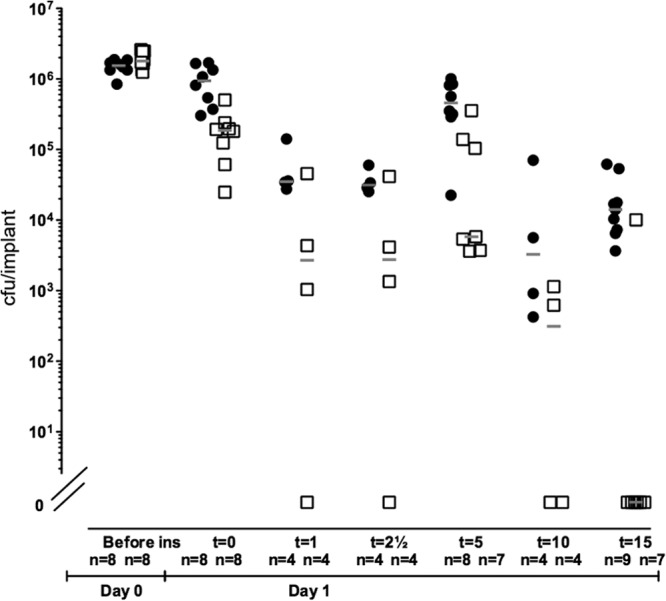
Number of implant-associated P. aeruginosa evaluated over time. Mice had silicone implants, precolonized with either PAO1/pPBAD-yhjH or PAO1/pJN105, inserted in the peritoneal cavity. At 24 and 26.5 h postinsertion, the mice received intraperitoneal injections of 2% arabinose. The mice were euthanized over a time period of 15 h after the last arabinose injection. Implants were removed from the mice at 0, 1, 2.5, 5, 10, and 15 h after the last arabinose injection. The results shown in the figure were pooled from two experiments, and the CFU obtained at the different time points were normalized to the average initial CFU/implant of both experiments. The open squares represent PAO1/pPBAD-yhjH CFU/implant, and the closed circles represent PAO1/pJN105 CFU/implant. The number of mice assessed to each time point from each group is indicated in the figure. There was a significant difference between the recovered bacterial amounts from mice infected with PAO1/pPBAD-yhjH compared to mice infected with the vector control strain PAO1/pJN105 over time, P < 0.006, evaluated by ANOVA. Gray lines represent the medians.
Fig 8.
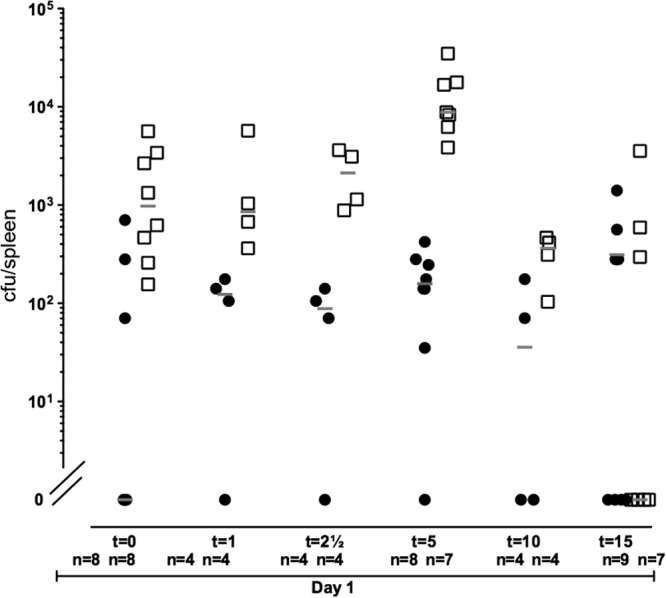
Number of P. aeruginosa in the spleen followed over time. Mice had silicone implants, precolonized with either PAO1/pPBAD-yhjH or PAO1/pJN105, inserted in the peritoneal cavity. At 24 and 26.5 h postinsertion the mice received intraperitoneal injections of 2% arabinose. The mice were euthanized over a time period of 15 h after the last arabinose injection. Spleens were removed from the mice at 0, 1, 2.5, 5, 10, and 15 h after the last arabinose injection. The results shown in the figure were pooled from two experiments, and the CFU obtained at the different time points were normalized to the average initial CFU/implant of both experiments. The open squares represent PAO1/pPBAD-yhjH CFU/spleen, and the closed circles represent PAO1/pJN105 CFU/spleen. The number of mice assessed to each time point from each group is indicated in the figure. There was a significant difference between the recovered bacterial amounts from mice infected with PAO1/pPBAD-yhjH compared to mice infected with the vector control strain PAO1/pJN105 over time, P < 0.0004, evaluated by ANOVA. Gray lines represent the medians.
DISCUSSION
Interference with the c-di-GMP levels in bacteria has been suggested as a new approach to biofilm control (51). Previous studies have shown that a constant low level of c-di-GMP maintained by overexpression of phosphodiesterases prevents in vitro biofilm formation by Salmonella enterica, Pseudomonas aeruginosa, Burkholderia cenocepacia, Pseudomonas putida, and Shewanella oneidensis (24, 27–29, 43). Moreover, in the case of P. putida and S. oneidensis, it was shown that a sudden decrease in the c-di-GMP level, mediated by the induction of a phosphodiesterase, led to rapid dispersal of established in vitro biofilms (24, 43). In addition, nitric oxide has been shown to induce dispersal of P. aeruginosa biofilms via activation of a phosphodiesterase (26). Here, we demonstrate that induction of phosphodiesterase activity in P. aeruginosa can disperse biofilms formed on a foreign-body located in the peritoneal cavity of mice.
We initially demonstrated that expression of the YhjH phosphodiesterase prevented biofilm formation by P. aeruginosa in microtiter dishes. Subsequently, we showed that the induction of YhjH led to the dispersal of bacteria from biofilms in both static and hydrodynamic systems. We then investigated the effects of induction of YhjH activity in biofilms established on foreign bodies located in the peritoneal cavity of mice. Fifteen hours after the induction of the phosphodiesterase, we could not detect bacteria on six of seven of the implants that had been colonized with PAO1/pPBAD-yhjH, whereas none of the nine PAO1/pJN105-colonized implants were cleared. The inducible yhjH gene is located on a plasmid, and it is possible that the population of bacteria on the single implant in the PAO1/pPBAD-yhjH group that was still colonized 15 h after arabinose injection consists of bacteria that have lost the plasmid, although we have not addressed this issue in the present study. After the induction of biofilm dispersal the number of PAO1/pPBAD-yhjH bacteria in the spleens of the mice increased during the first 5 h. This is a concern since the presence of liberated bacteria could lead to conditions of sepsis and a fatal outcome for the host. However, after 5 h, the number of bacteria in the spleens then decreased to the level of the PAO1/pJN105 bacteria. Furthermore, the mice did not show any signs of discomfort during the duration of the experiments. Our experiments show that dispersal of the silicone implant-associated biofilms occurred in response to yhjH induction, and even though the bacteria had dispersed to the peritoneal cavity and thereafter to the bloodstream and accumulated in the spleen, induction of biofilm dispersal did not show adverse effects on the mice. Thereby we have provided proof of the concept that manipulation of the c-di-GMP level in bacteria is a viable treatment strategy against biofilm-based infections.
We have previously presented evidence that P. putida flow-chamber biofilms disperse rapidly and completely after the induction of the YhjH phosphodiesterase (24). P. putida biofilm formation in flow chambers and microtiter dishes is mainly governed by the large adhesive protein LapA, whereas exopolysaccharides play minor roles (52). A decrease in the c-di-GMP level in P. putida cells leads to the activation of the LapG protease, which specifically cleaves off LapA from the cell surface enabling rapid and complete biofilm dispersal (50). In the case of P. aeruginosa, however, biofilm formation is dependent mainly on the Psl and Pel polysaccharides (46, 53, 54), and for some clinical isolates the alginate polysaccharide (40). Biosynthesis of all of these exopolysaccharides is at some level regulated by c-di-GMP. Hickman et al. performed microarray analysis on a P. aeruginosa wild-type strain and its isogenic wspF mutant (which has elevated c-di-GMP levels) and found increased expression of both the pel and psl genes in the wspF mutant (28). In addition, Hickman and Harwood presented data showing that c-di-GMP binds to the transcriptional regulator FleQ and thereby derepresses transcription of the pel genes (55). Furthermore, evidence has been presented that c-di-GMP binding allosterically activates the transmembrane protein PelD, which is involved in synthesis of the Pel polysaccharide (56). In the case of alginate expression, c-di-GMP regulation occurs at the posttranslational level, as c-di-GMP-binding allosterically activates the transmembrane protein Alg44, which is required for the synthesis of alginate (57). Due to this c-di-GMP dependency of exopolysaccharide synthesis in P. aeruginosa, it is expected that a decrease in the c-di-GMP level will lead to ceasing of exopolysaccharide synthesis. A halt of exopolysaccharide synthesis was expected to lead to some biofilm dispersal in in vitro systems, but it could have more dramatic effects under in vivo conditions where the exopolysaccharides contribute to persistence of the biofilm against the host immune system. Accordingly, we found that induction of the yhjH gene led to dispersal of the majority of the cells in P. aeruginosa biofilms grown in flow chambers, but to even more pronounced dispersal of biofilms in our implant infection model.
In our studies we found that the mice did not show any signs of discomfort or illness after induction of dispersal of the P. aeruginosa biofilms in their peritoneal cavity. The bacterial counts in the spleens of the mice increased the first 5 h after induction of biofilm dispersal but then declined. However, it may be expected that patients with biofilm-based infections will be more sensitive toward systemic P. aeruginosa than the mice. Therefore, the dose of a biofilm dispersal-inducing drug should be considered carefully, and the drugs should be administered in combination with antibiotics in high concentrations, since conventional antibiotics will be effective against the planktonic bacteria. In some cases, it may also be useful to treat prophylactically with c-di-GMP signaling blockers in order to prevent biofilm formation.
The results of genetic manipulation of c-di-GMP levels indicates that compounds that affect c-di-GMP signaling in bacteria may be used to prevent or clear biofilm infections. c-di-GMP signaling involves (i) activation of the cyclase or phosphodiesterase enzymes that produce or degrade c-di-GMP, (ii) an effector component that binds and is allosterically modulated by c-di-GMP, and (iii) a target component that produces a molecular output in response to contact with the effector component (see reference 58 for a recent review). Because of the conserved nature of the GGDEF and EAL domains, it is expected that compounds that decreases the intracellular c-di-GMP level via diguanylate cyclase inhibition or phosphodiesterase activation will affect a large number of bacterial species (including the commensal gut flora), whereas compounds that target a specific c-di-GMP effector will exert its effect on a single bacterial species. The present work shows that interference with c-di-GMP signaling is a promising strategy for treatment of biofilm-based infections, and it may be expected that our increasing knowledge about c-di-GMP signaling will form the basis for the development of antibiofilm drugs in the future.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Danish Strategic Research Council (to M.G.), the Danish Council for Independent Research (to T.T.-N.), and the Novo Nordisk Foundation (to M.G.).
Footnotes
Published ahead of print 20 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00332-13.
REFERENCES
- 1. Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 2. Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Hoiby N, Givskov M. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjarnsholt T, Jensen PO, Burmolle M, Hentzer M, Haagensen JA, Hougen HP, Calum H, Madsen KG, Moser C, Molin S, Hoiby N, Givskov M. 2005. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 151:373–383 [DOI] [PubMed] [Google Scholar]
- 4. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711–745 [DOI] [PubMed] [Google Scholar]
- 5. Koch C, Hoiby N. 1993. Pathogenesis of cystic fibrosis. Lancet 341:1065–1069 [DOI] [PubMed] [Google Scholar]
- 6. Gristina AG, Dobbins JJ, Giammara B, Lewis JC, DeVries WC. 1988. Biomaterial-centered sepsis and the total artificial heart: microbial adhesion versus tissue integration. JAMA 259:870–874 [PubMed] [Google Scholar]
- 7. von Eiff C, Jansen B, Kohnen W, Becker K. 2005. Infections associated with medical devices: pathogenesis, management, and prophylaxis. Drugs 65:179–214 [DOI] [PubMed] [Google Scholar]
- 8. Kirketerp-Moller K, Jensen PO, Fazli M, Madsen KG, Pedersen J, Moser C, Tolker-Nielsen T, Hoiby N, Givskov M, Bjarnsholt T. 2008. Distribution, organization, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 46:2717–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Institutes of Health 1997. Minutes of the National Advisory Dental and Craniofacial Research Council, 153rd meeting. National Institute of Dental and Craniofacial Research, Bethesda, MD [Google Scholar]
- 10. Bjarnsholt T, Jensen PO, Rasmussen TB, Christophersen L, Calum H, Hentzer M, Hougen HP, Rygaard J, Moser C, Eberl L, Hoiby N, Givskov M. 2005. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 151:3873–3880 [DOI] [PubMed] [Google Scholar]
- 11. Christensen LD, Moser C, Jensen PO, Rasmussen TB, Christophersen L, Kjelleberg S, Kumar N, Hoiby N, Givskov M, Bjarnsholt T. 2007. Impact of Pseudomonas aeruginosa quorum sensing on biofilm persistence in an in vivo intraperitoneal foreign-body infection model. Microbiology 153:2312–2320 [DOI] [PubMed] [Google Scholar]
- 12. Christensen LD, van Gennip M, Jakobsen TH, Alhede M, Hougen HP, Hoiby N, Bjarnsholt T, Givskov M. 2012. Synergistic antibacterial efficacy of early combination treatment with tobramycin and quorum-sensing inhibitors against Pseudomonas aeruginosa in an intraperitoneal foreign-body infection mouse model. J. Antimicrob. Chemother. 67:1198–1206 [DOI] [PubMed] [Google Scholar]
- 13. Jakobsen TH, Bragason SK, Phipps RK, Christensen LD, van Gennip M, Alhede M, Skindersoe M, Larsen TO, Hoiby N, Bjarnsholt T, Givskov M. 2012. Food as a source for quorum sensing inhibitors: iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 78:2410–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jakobsen TH, van Gennip M, Phipps RK, Shanmugham MS, Christensen LD, Alhede M, Skindersoe ME, Rasmussen TB, Friedrich K, Uthe F, Jensen PO, Moser C, Nielsen KF, Eberl L, Larsen TO, Tanner D, Hoiby N, Bjarnsholt T, Givskov M. 2012. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 56:2314–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rybtke MT, Jensen PO, Hoiby N, Givskov M, Tolker-Nielsen T, Bjarnsholt T. 2011. The implication of Pseudomonas aeruginosa biofilms in infections. Inflamm. Allergy Drug Targets 10:141–157 [DOI] [PubMed] [Google Scholar]
- 16. Harmsen M, Yang L, Pamp SJ, Tolker-Nielsen T. 2010. An update on Pseudomonas aeruginosa biofilm formation, tolerance, and dispersal. FEMS Immunol. Med. Microbiol. 59:253–268 [DOI] [PubMed] [Google Scholar]
- 17. Jensen PO, Bjarnsholt T, Phipps R, Rasmussen TB, Calum H, Christoffersen L, Moser C, Williams P, Pressler T, Givskov M, Hoiby N. 2007. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 153:1329–1338 [DOI] [PubMed] [Google Scholar]
- 18. Alhede M, Bjarnsholt T, Jensen PO, Phipps RK, Moser C, Christophersen L, Christensen LD, van Gennip M, Parsek M, Hoiby N, Rasmussen TB, Givskov M. 2009. Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology 155:3500–3508 [DOI] [PubMed] [Google Scholar]
- 19. Allison DG, Evans DJ, Brown MR, Gilbert P. 1990. Possible involvement of the division cycle in dispersal of Escherichia coli from biofilms. J. Bacteriol. 172:1667–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sauer K, Cullen MC, Rickard AH, Zeef LA, Davies DG, Gilbert P. 2004. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J. Bacteriol. 186:7312–7326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gjermansen M, Ragas P, Sternberg C, Molin S, Tolker-Nielsen T. 2005. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms. Environ. Microbiol. 7:894–906 [DOI] [PubMed] [Google Scholar]
- 23. Schleheck D, Barraud N, Klebensberger J, Webb JS, McDougald D, Rice SA, Kjelleberg S. 2009. Pseudomonas aeruginosa PAO1 preferentially grows as aggregates in liquid batch cultures and disperses upon starvation. PLoS One 4:e5513. 10.1371/journal.pone.0005513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gjermansen M, Ragas P, Tolker-Nielsen T. 2006. Proteins with GGDEF and EAL domains regulate Pseudomonas putida biofilm formation and dispersal. FEMS Microbiol. Lett. 265:215–224 [DOI] [PubMed] [Google Scholar]
- 25. Barraud N, Hassett DJ, Hwang SH, Rice SA, Kjelleberg S, Webb JS. 2006. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 188:7344–7353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barraud N, Schleheck D, Klebensberger J, Webb JS, Hassett DJ, Rice SA, Kjelleberg S. 2009. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J. Bacteriol. 191:7333–7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simm R, Morr M, Kader A, Nimtz M, Romling U. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53:1123–1134 [DOI] [PubMed] [Google Scholar]
- 28. Hickman JW, Tifrea DF, Harwood CS. 2005. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci. U. S. A. 102:14422–14427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fazli M, O'Connell A, Nilsson M, Niehaus K, Dow JM, Givskov M, Ryan RP, Tolker-Nielsen T. 2011. The CRP/FNR family protein Bcam1349 is a c-di-GMP effector that regulates biofilm formation in the respiratory pathogen Burkholderia cenocepacia. Mol. Microbiol. 82:327–341 [DOI] [PubMed] [Google Scholar]
- 30. Goymer P, Kahn SG, Malone JG, Gehrig SM, Spiers AJ, Rainey PB. 2006. Adaptive divergence in experimental populations of Pseudomonas fluorescens. II. Role of the GGDEF regulator WspR in evolution and development of the wrinkly spreader phenotype. Genetics 173:515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kulasakara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, Lee DG, Neely AN, Hyodo M, Hayakawa Y, Ausubel FM, Lory S. 2006. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc. Natl. Acad. Sci. U. S. A. 103:2839–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim B, Beyhan S, Meir J, Yildiz FH. 2006. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol. Microbiol. 60:331–348 [DOI] [PubMed] [Google Scholar]
- 33. Ryan RP, Lucey J, O'Donovan K, McCarthy Y, Yang L, Tolker-Nielsen T, Dow JM. 2009. HD-GYP domain proteins regulate biofilm formation and virulence in Pseudomonas aeruginosa. Environ. Microbiol. 11:1126–1136 [DOI] [PubMed] [Google Scholar]
- 34. Rybtke MT, Borlee BR, Murakami K, Irie Y, Hentzer M, Nielsen TE, Givskov M, Parsek MR, Tolker-Nielsen T. 2012. Fluorescence-based reporter for gauging cyclic di-GMP levels in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 78:5060–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clark DJ, Maaløe O. 1967. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 23:99–112 [Google Scholar]
- 36. Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella, and type IV pilus mutants. Mol. Microbiol. 48:1511–1524 [DOI] [PubMed] [Google Scholar]
- 37. Newman JR, Fuqua C. 1999. Broad-host-range expression vectors that carry the l-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 227:197–203 [DOI] [PubMed] [Google Scholar]
- 38. West SE, Schweizer HP, Dall C, Sample AK, Runyen-Janecky LJ. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81–86 [DOI] [PubMed] [Google Scholar]
- 39. O'Toole GA, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295–304 [DOI] [PubMed] [Google Scholar]
- 40. Hoiby N. 1974. Pseudomonas aeruginosa infection in cystic fibrosis: relationship between mucoid strains of Pseudomonas aeruginosa and the humoral immune response. Acta Pathol. Microbiol. Scand. B Microbiol. Immunol. 82:551–558 [PubMed] [Google Scholar]
- 41. Crusz SA, Popat R, Rybtke MT, Camara M, Givskov M, Tolker-Nielsen T, Diggle SP, Williams P. 2012. Bursting the bubble on bacterial biofilms: a flow cell methodology. Biofouling 28:835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pamp SJ, Tolker-Nielsen T. 2007. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 189:2531–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thormann KM, Duttler S, Saville RM, Hyodo M, Shukla S, Hayakawa Y, Spormann AM. 2006. Control of formation and cellular detachment from Shewanella oneidensis MR-1 biofilms by cyclic di-GMP. J. Bacteriol. 188:2681–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rainey PB, Travisano M. 1998. Adaptive radiation in a heterogeneous environment. Nature 394:69–72 [DOI] [PubMed] [Google Scholar]
- 45. Spiers AJ, Kahn SG, Bohannon J, Travisano M, Rainey PB. 2002. Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. Genetic and phenotypic bases of wrinkly spreader fitness. Genetics 161:33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Friedman L, Kolter R. 2004. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 186:4457–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Friedman L, Kolter R. 2004. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 51:675–690 [DOI] [PubMed] [Google Scholar]
- 48. Branda SS, Vik S, Friedman L, Kolter R. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20–26 [DOI] [PubMed] [Google Scholar]
- 49. D'Argenio DA, Calfee MW, Rainey PB, Pesci EC. 2002. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 184:6481–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gjermansen M, Nilsson M, Yang L, Tolker-Nielsen T. 2010. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms: genetic elements and molecular mechanisms. Mol. Microbiol. 75:815–826 [DOI] [PubMed] [Google Scholar]
- 51. Sintim HO, Smith JA, Wang J, Nakayama S, Yan L. 2010. Paradigm shift in discovering next-generation anti-infective agents: targeting quorum sensing, c-di-GMP signaling and biofilm formation in bacteria with small molecules. Future Med. Chem. 2:1005–1035 [DOI] [PubMed] [Google Scholar]
- 52. Nilsson M, Chiang WC, Fazli M, Gjermansen M, Givskov M, Tolker-Nielsen T. 2011. Influence of putative exopolysaccharide genes on Pseudomonas putida KT2440 biofilm stability. Environ. Microbiol. 13:1357–1369 [DOI] [PubMed] [Google Scholar]
- 53. Matsukawa M, Greenberg EP. 2004. Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J. Bacteriol. 186:4449–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jackson KD, Starkey M, Kremer S, Parsek MR, Wozniak DJ. 2004. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 186:4466–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hickman JW, Harwood CS. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol. 69:376–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee VT, Matewish JM, Kessler JL, Hyodo M, Hayakawa Y, Lory S. 2007. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 65:1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Merighi M, Lee VT, Hyodo M, Hayakawa Y, Lory S. 2007. The second messenger bis-(3′-5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 65:876–895 [DOI] [PubMed] [Google Scholar]
- 58. Ryan RP, Tolker-Nielsen T, Dow JM. 2012. When the PilZ don't work: effectors for cyclic di-GMP action in bacteria. Trends Microbiol. 20:235–242 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


