Abstract
Protection from infections in early life relies extensively on innate immunity, but it is unknown whether and how maternal infections modulate infants' innate immune responses, thereby altering susceptibility to infections. Plasmodium falciparum causes pregnancy-associated malaria (PAM), and epidemiological studies have shown that PAM enhances infants' susceptibility to infection with P. falciparum. We investigated how PAM-mediated exposures in utero affect innate immune responses and their relationship with infection in infancy. In a prospective study of mothers and their babies in Benin, we investigated changes in Toll-like receptor (TLR)-mediated cytokine responses related to P. falciparum infections. Whole-blood samples from 134 infants at birth and at 3, 6, and 12 months of age were stimulated with agonists specific for TLR3, TLR4, TLR7/8, and TLR9. TLR-mediated interleukin 6 (IL-6) and IL-10 production was robust at birth and then stabilized, whereas tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) responses were weak at birth and then increased. In multivariate analyses, maternal P. falciparum infections at delivery were associated with significantly higher TLR3-mediated IL-6 and IL-10 responses in the first 3 months of life (P < 0.05) and with significantly higher TLR3-, TLR7/8-, and TLR9-mediated TNF-α responses between 6 and 12 months of age (P < 0.05). Prospective analyses showed that higher TLR3- and TLR7/8-mediated IL-10 responses at birth were associated with a significantly higher risk of P. falciparum infection in infancy (P < 0.05). Neonatal and infant intracellular TLR-mediated cytokine responses are conditioned by in utero exposure through PAM late in pregnancy. Enhanced TLR-mediated IL-10 responses at birth are associated with an increased risk of P. falciparum infection, suggesting a compromised ability to combat infection in early life.
INTRODUCTION
Chronic infections during pregnancy, including those of parasitic origin, are a frequent occurrence in low-income areas of the world in general and in sub-Saharan African countries in particular, and they affect fetal immunity such that infants' responses to vaccination are diminished and their susceptibility to infection is increased (1, 2). Maternal infection with Trypanosoma cruzi, for example, without vertical transmission, stimulates fetal innate and adaptive immune responses such that exposed but uninfected neonates produce higher concentrations of proinflammatory and anti-inflammatory cytokines than their unexposed counterparts (3, 4). Maternal infection with Schistosoma spp. has also been shown to be associated with fetal inflammation, characterized by increased levels of interleukin 1β (IL-1β) and tumor necrosis factor (TNF) receptor II in cord blood of those born to infected mothers (5). With an annual estimate of 50 million or more mothers at risk, pregnancy-associated malaria (PAM) due to Plasmodium falciparum is a well-recognized and well-described example of such an infection, representing a major public health burden measurable by the adverse pregnancy outcomes it causes (reviewed in references 6–10). Quite apart from the association between PAM and low birth weight and the poor prognosis of survival that goes with it, a number of studies have documented the increased susceptibility to malaria of infants born to mothers with P. falciparum infection detected at delivery (11–15). PAM has also recently been shown to be associated with an increased risk of fever episodes of nonmalarial causes in infancy (16). Taken together, these findings suggest that exposure to P. falciparum in utero alters fetal and/or neonatal immune development, resulting in enhanced susceptibility to malaria in infancy, and, furthermore, that such alterations may also affect susceptibility to infections other than malaria.
Evidence of altered fetal/neonatal cellular immunological activity comes from reports of cord blood cell frequencies, their activation status, and their antigen-specific proliferative and/or cytokine responses resulting from placental infection with P. falciparum and consequent exposure of the fetal immune system to parasite-derived antigens in utero (reviewed in reference 17). At the level of induction and acquisition of specific T cell activity, the conclusion from several of these studies is that regulatory T cells (Treg) producing the immunosuppressive cytokine IL-10 play a pivotal role in downmodulating cord blood Th1-type responses to P. falciparum antigens (18–23). The latter studies, however, examined only the downstream nature of the altered immunological responses, giving little, if any, information on the character of the upstream innate immune interactions, for example, that may have led to the observed changes in T cell activity.
Innate immunity involves the family of so-called “professional” antigen-presenting cells (APC), a family that includes myeloid and plasmacytoid dendritic cells (mDC and pDC) as well as monocytes and B cells. These cells play a pivotal role in recognition of pathogens and orchestrating the subsequent adaptive immune response (24). A major component of pathogen recognition by APC comprises a family of pattern recognition receptors referred to as Toll-like receptors (TLR) that interact with a range of highly conserved microbial components, leading, conventionally, to a burst of proinflammatory cytokine activity. In early life, the components of the adaptive immune system, although present, are insufficiently mature, and an infant's response to infection therefore relies extensively on innate immunity (reviewed in reference 25). At all times, the balance between production of pro- and anti-inflammatory mediators is tightly regulated to allow efficient, protective immune responses to develop while preventing the pathological consequences of excessive inflammation (26). In the particular context of control of inflammatory activity in early life, a pivotal role for neonatal B cells producing IL-10 as a result of TLR9 activation has been reported (27), a role that is consistent with reports of robust TLR ligand-mediated IL-10 responses present at birth both in non-African and in African populations (28–30).
In the context of pregnancy, we and others have shown that placental infection with P. falciparum at delivery is associated with altered fetal innate immune responses, modified frequency of cord blood DC (31), partial activation of cord blood APC (32), and modulated cord blood cytokine responses to TLR ligation (33). Two independent studies in nonpregnant individuals have reported the capacity of P. falciparum infection to cause proinflammatory priming of responses to subsequent TLR ligation, a capacity that distinguishes the parasite from most other microbial pathogens (34, 35). Thus, although the detailed mechanisms remain to be characterized, with controversy remaining over the precise nature of the parasite-derived TLR ligands, it is clear that P. falciparum generates an array of responses through interaction with different TLR, including at least TLR2, -4, and -9 (36). The above-mentioned cross-sectional studies present a limitation, i.e., the role of PAM in influencing early-life cellular immunological responses has been investigated only at delivery, leaving unknown the potential impact of in utero exposure to P. falciparum on the normal profile of maturation of the innate immune system in infancy.
Here, we evaluated the development of TLR-mediated cytokine responses both at birth and during the first 12 months of life in a large cohort of children born to mothers with different malaria histories, following the hypothesis that PAM and, potentially, infection and/or malaria episodes in early life would affect the development of infants' innate immune responses. Since the timing of occurrence of PAM during pregnancy is associated with fetal and infant malaria outcomes (37, 38), parasitological data were collected from all mothers starting with inclusion, during the second trimester, and thereafter throughout pregnancy up to and including delivery. These data were then combined with measures of innate immune activity at birth and during infancy. Through prospective evaluation during the first year of life, we also evaluated the independent influence of being born premature and of infection and/or malaria episodes during infancy on the same TLR-mediated responses.
MATERIALS AND METHODS
Ethics statement.
The Strategies to Prevent Pregnancy-Associated Malaria (STOPPAM) study was approved by the ethics committee of the Health Science Faculty of the University of Abomey-Calavi, Benin, and by the ethics committees of the Research Institute for Development (IRD) in France. Written informed consent was obtained from all mothers, who additionally provided consent for their babies, prior to inclusion in the study. All procedures adhered to Declaration of Helsinki principles.
Study design.
The STOPPAM project was conducted in parallel in Benin and in Tanzania from 2008 to 2011. In Benin, the study took place in the district of Come, Mono Province, located 70 km west of the economical capital, Cotonou. Malaria transmission in this area can be characterized as hyperendemic, with two peaks during the rainy seasons (April to July, September to November), and an entomological inoculation rate estimated at 35 to 60 infective bites per person per year (13). The STOPPAM study design has been described in detail elsewhere (37). In total, 1,037 women less than 24 weeks pregnant were enrolled in 3 clinics (Come, Akodeha, and Ouedeme Pedah) in the Come district. Clinical and parasitological data were subsequently collected at monthly follow-up visits and at delivery. According to the existing national policy for malaria, women received intermittent preventive treatment during pregnancy (IPTp) with the antimalarial drug combination sulfadoxine-pyrimethamine (SP) on two separate occasions during scheduled antenatal visits (ANV) spaced at least 1 month apart in the second/third trimesters. Women diagnosed with malaria (positive malaria rapid diagnostic test [RDT]; see below) received a standard treatment regimen of quinine unless the diagnosis coincided with a scheduled IPTp dose, in which case SP was given instead of quinine. Women were encouraged to present at the maternity clinic to receive care whenever necessary between ANV. Such visits are referred to as emergency visits.
For an immunological substudy conducted as part of the STOPPAM project in Benin, a subgroup of 217 pregnant women from the enrolled cohort were selected at delivery on the basis of their recorded history of infection with P. falciparum (uninfected during pregnancy, n = 99; infected during pregnancy but uninfected at delivery, n = 71; infected at delivery, n = 47). The cohort of infants of these selected women were actively followed up at home from birth to 12 months of age, with clinical assessments every 2 weeks and parasitological assessments (see below) every month. Cellular immunological studies were performed with cord blood and the infants' peripheral blood that was collected at 3, 6, and 12 months of age. Infants from whom fewer than 3 blood samples were collected during the follow-up period were excluded from the study, as were infants either of mothers who subsequently tested seropositive for HIV or for whom the HIV serostatus was unknown.
P. falciparum infection status.
To assess the impact of P. falciparum infection on neonatal immunity, clinical and parasitological data were collected from mothers at each ANV and emergency visit and from infants as described above. RDT (Parascreen; Zephyr Biomedical Systems) were used for mothers at all routine ANV and emergency visits and for infants whenever fever was detected during active surveillance. Retrospective confirmation of infection comprised parasitological diagnosis via standard microscopic examination of thick blood smears (TBS) that were prepared at monthly intervals from both mothers and infants. Briefly, smears were stained with Giemsa and examined by two experienced laboratory technicians for the presence and density of parasites. Smears were considered negative if no asexual-stage Plasmodium parasite was detected by counting high-power fields containing the equivalent of 500 leukocytes. Parasites were counted against 200 leukocytes, and parasite density was calculated based on an estimate of 8,000 leukocytes/μl of blood. At delivery, TBS were prepared with maternal peripheral and cord blood samples, and an impression smear of placental blood was also examined. Infection of mothers at delivery was thus defined by the presence of parasites in placental and/or in peripheral blood, while infections earlier in pregnancy and during infancy were defined either by a positive RDT or by a positive TBS result.
Blood collection and cell stimulation with TLR agonists.
Cord (10 ml) and peripheral (2 ml) venous blood samples were collected in tubes containing citrate phosphate dextrose adenine (CPDA) as an anticoagulant. All samples were transported within 4 h to the laboratory of the Research Center for Malaria during Pregnancy and Infancy (CERPAGE) in Cotonou, where stimulation assays were performed the same day. Whole-blood samples were diluted 1:1 with RPMI, and separate aliquots (200 μl) of diluted whole blood distributed in fluorescence-activated cell sorting (FACS) tubes were either left unstimulated or stimulated either with poly(I·C) (TLR3 ligand; 20 μg/ml; Sigma-Aldrich, Schnelldorf, Germany), with ultrapure lipopolysaccharide (LPS) from Escherichia coli (TLR4 ligand; 100 ng/ml; Sigma-Aldrich, Schnelldorf, Germany), with resiquimod (R848; TLR7/8 ligand; 1 mg/ml; Sigma-Aldrich), or with CpG oligonucleotide type A (CpG ODN2216; TLR9 ligand; 3 μg/ml; Metabion, Martinsried, Germany). After 24 h of incubation at 37°C in 5% CO2, culture supernatants were collected by centrifugation and stored at −80°C for cytokine determination.
The different TLR ligands used in these assays exert their effects on distinct immune cell subsets: poly(I·C) interacts with TLR3 and RIG-like receptors expressed within mDC (39), natural killer cells, and lymphocytes (40, 41); LPS activates TLR4 expressed on monocytes and mDC (42); R848 activates TLR7 and TLR8, with TLR7 being expressed mainly within pDC and B cells (40, 43), whereas TLR8 is found within monocytes and mDC (44, 45); the type A CpG 2216 interacts with TLR9 expressed within pDC but not B cells (46). The choice of stimulants reflects a desire, based on our own knowledge and experience (32), to focus as much as possible on DC-mediated activity.
Cytokine measurement.
We quantified the cytokines IL-6, IL-10, gamma interferon (IFN-γ), and TNF-α in 50 μl of sampled supernatants through the use of the commercially available cytometric bead array (CBA soluble protein Flex set assay; BD Biosciences, Grenoble, France), conducted according to the manufacturer's instructions on a FACsCalibur 4-color cytometer. The assay sensitivity was 1.6 pg/ml for IL-6, 0.13 pg/ml for IL-10, 0.8 pg/ml for IFN-γ, and 1.2 pg/ml for TNF-α. When the cytokine concentration in a sample was below the detection level of the test, an arbitrary value was assigned that corresponded to half of the sensitivity value for the specific cytokine concerned. Results were formatted using the BD CBA analysis software. Spontaneous cytokine production in unstimulated samples was assessed independently of that in stimulated samples, while the latter was analyzed after the corresponding unstimulated sample values were subtracted. Pilot studies of cytokine kinetics following stimulation with TLR agonists showed the 24-h incubation time to be optimal for the selected cytokines but suboptimal for pDC-specific IFN-α (maximal at 8 h), which was therefore not included in the panel.
Statistical analysis.
The association between TLR-mediated cytokine responses and infant age was analyzed using the nonparametric Kruskal-Wallis test. To determine changes in TLR-mediated cytokine production as a function of malaria during pregnancy and/or infancy, the analysis proceeded as follows: for each cytokine response, we built a multivariate linear mixed model (LMM), which allows to take into account the correlation between the repeated measurements as well as the potential confounders in the relation between malaria and the TLR level. The analysis was performed in two steps: first a univariate model which aimed to select potential confounders, followed by a multivariate model. In the univariate step, we investigated the association between baseline characteristics and cytokine levels upon TLR stimulation. The baseline characteristics included in the analysis were gravidity, maternal anemia (Hb < 11 g/dl), prematurity (<37 weeks), low birth weight (<2,500 g), and infant gender.
All variables related to P. falciparum infection were included in the final multivariate model, while infant age and baseline characteristics were used to adjust for confounding of the association between TLR-mediated cytokine responses and either P. falciparum infection during pregnancy (segregated into three periods according to the time of occurrence; see below), P. falciparum infection during infancy (also segregated into three time periods; see below), or other selected variables. To graphically illustrate the predicted effect of maternal infection on the TLR responses of infants, we then computed the mean predicted TLR-mediated cytokine levels of infants born to uninfected and infected mothers at each time point.
Maternal infections were segregated into the following intervals: (i) infection before the third trimester of pregnancy, (ii) infection during the third trimester of pregnancy but more than 10 days before delivery, (iii) infection from 10 days prior to delivery up to and including delivery. Designation of the latter group was based on the premise that infections detected during an emergency visit occurring 10 days or less before delivery, and therefore treated, were too close in time to delivery to be separable from it, and because most of those concerned were also found to be infected at delivery.
During the first year of life, infection/malaria episodes were also segregated into 3 intervals, (i) those occurring before 3 months of age, (ii) those occurring between 3 and 6 months of age, and (iii) those occurring between 7 and 12 months of age, and were separately assessed for associations both with spontaneous (unstimulated) and with TLR stimulation-mediated cytokine production.
In a separate analysis, we determined whether TLR-mediated cytokine responses at birth were associated with the occurrence of malaria during the first 12 months of age. For this purpose, we employed a logistic mixed model; risk factors were first analyzed in a univariate model and then, after adjusting for potential confounders, in a multivariate model. TLR responses in cord blood were considered a baseline for prediction of the development of P. falciparum infection during infancy. Malaria episodes recorded each month during the follow-up of infants were considered the dependent variables and coded as “infected” or “not infected.” The model allowed estimation of the predictive values of the TLR-mediated cytokine levels (for any set of covariates) of all infants at each time point.
Statistical significance in all multivariate analyses was considered if P values were <0.05. All analyses were performed using the R statistical package (R Development Core Team; R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org), and graphs were made with GraphPad (Prism 5.0).
RESULTS
Characteristics of the study population.
Between November 2008 and April 2011, 217 mother-infant pairs were enrolled in the study, but of these, 83 were excluded due to insufficient numbers of blood samples (80) and HIV serostatus (3 either HIV+ or unknown). Mother and infant characteristics of the 134 remaining pairs are presented in Table 1. Mothers' mean age (±standard deviation [SD]) was 26.3 (±0.2) years, 18.7% were primigravid, and 13.4% had anemia at delivery. Babies' mean birth weight was 3,020 ± 410 g (mean ± SD). Fifteen newborns (11.2%) had a low birth weight (≤2,500 g), and 9 (6.7%) were born premature.
Table 1.
Characteristics of the mother-infant pair study population (n = 134 pairs)
| Characteristic | No. of individuals (%) |
|---|---|
| Mother | |
| Age (yrs) | |
| ≤20 | 29 (21.6) |
| 21–25 | 43 (32.2) |
| 26–30 | 29 (21.6) |
| ≥31 | 33 (24.6) |
| Gravidity status | |
| Primigravid | 25 (18.7) |
| Multigravid | 109 (81.3) |
| Anemia at delivery (Hb < 11 g/dl) | 18 (13.4) |
| Infected before 3rd trimester of pregnancy | 41 (30.6) |
| Infected during 3rd trimester of pregnancy up until 11 days before delivery | 24 (17.9) |
| Infected 10 days or less prior to or at delivery | 29 (21.6) |
| Infected placenta | 21 (15.7) |
| No sign of infection | 40 (29.9) |
| Infant | |
| Female | 64 (47.8) |
| Premature (gestational age of ≤37 weeks) | 9 (6.7) |
| Residence | |
| Rural | 35 (26.1) |
| Semirural | 99 (73.9) |
| Low birth wt (<2,500 g) | 15 (11.2) |
| Infected before 3 mo of age | 7 (5.2) |
| Infected between 4 and 6 mo of age | 13 (9.7) |
| Infected between 7 and 12 mo of age | 34 (25.4) |
| No sign of infection | 80 (59.7) |
There were 41 (30.6%) women in whom infections before the third trimester of pregnancy were identified, while 24 (17.9%) were infected during the third trimester of pregnancy (but not within 10 days of delivery), and 29 (21.6%) were infected in the 10 days prior to or at delivery. Twenty-one women (15.7%) had an infected placenta, as determined by placental impression smear. Seven infants (5.2%) were infected during the first 3 months of life, 13 (9.7%) were infected between 4 and 6 months of age, and 34 (25.4%) were infected between 7 and 12 months of age.
Age-dependent maturation of TLR-mediated cytokine responses.
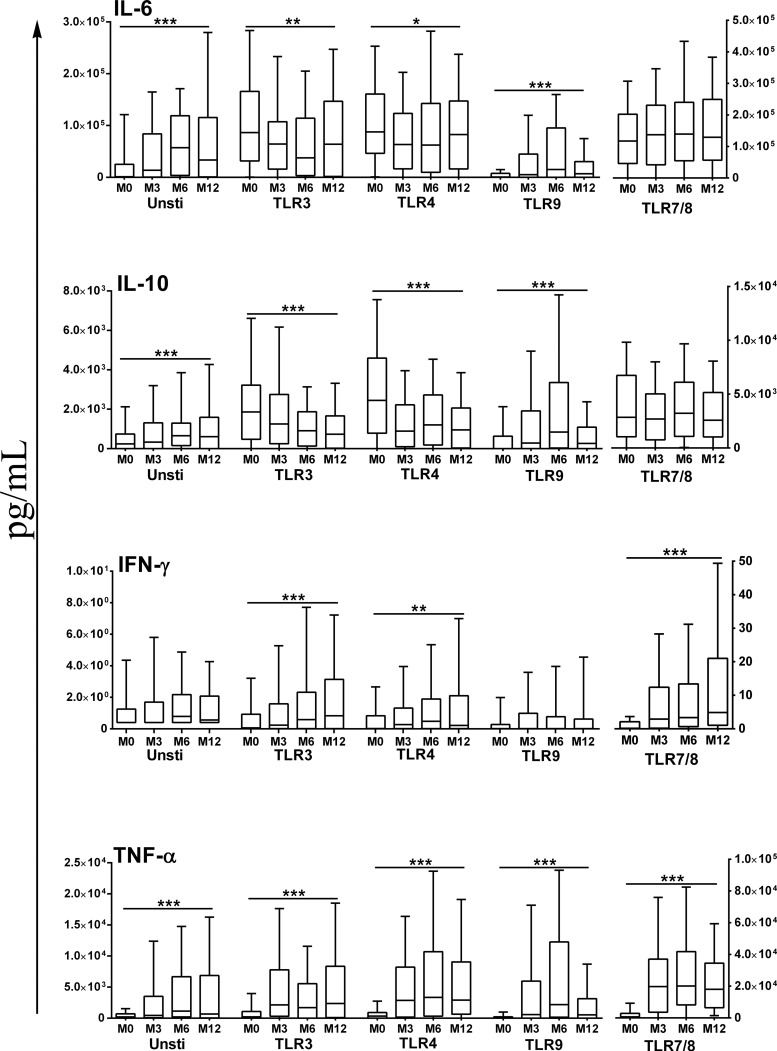
Whole-blood samples from 134 infants were stimulated with TLR agonists at birth (cord blood) and at 3, 6, and 12 months of age (peripheral venous blood). At all time points, the production of IL-6, IL-10, and TNF-α by agonist-stimulated cells was higher than the amounts spontaneously secreted by unstimulated control samples (Table 2, Fig. 1). Conversely, only very limited TLR-mediated IFN-γ production above unstimulated levels was observed regardless of time point, with the highest values seen in response to the TLR7/8 agonist R848 (Table 2, Fig. 1). Overall, the latter agonist induced the highest levels of all cytokines, while the TLR9 agonist, CpG type A, induced comparatively the weakest cytokine responses at all time points.
Table 2.
Univariate analysis of spontaneous and TLR agonist-mediated cytokine responses in cord/infant whole blood as a function of P. falciparum infection detected either in the mother at delivery or during infancya
| Cytokine and P. falciparum infectionb | TLR3 |
TLR4 |
TLR7/8 |
TLR9 |
Unstimulated |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient (SD) | P | Coefficient (SD) | P | Coefficient (SD) | P | Coefficient (SD) | P | Coefficient (SD) | P | |
| IL-6 | ||||||||||
| Mother (at delivery) | 39,697 (18,685) | 0.02 | 23,266 (17,497) | 0.04 | 15,335 (22,503) | NS | −8,615 (9,930) | NS | −0.2 (0.4) | NS |
| Infant | ||||||||||
| M0-M3 | −7,969 (35,165) | NS | −18,425 (32,309) | NS | 64,449 (40,690) | 0.06 | −16,961 (17,797) | NS | −0.7 (0.8) | NS |
| M4-M6 | −13,092 (26,431) | NS | −31,224 (24,277) | 0.07 | −34,977 (30,934) | NS | −9,364 (13,902) | NS | −0.3 (0.6) | NS |
| M7-M12 | −15,297 (17,943) | NS | −1,602 (16,736) | NS | −8,256 (21,346) | NS | −11,586 (9,569) | NS | −0.2 (0.4) | NS |
| IL-10 | ||||||||||
| Mother (at delivery) | 623 (283) | <0.01 | 325 (274) | NS | −191 (520) | NS | 98 (278) | 0.02 | 0.1 (0.4) | NS |
| Infant | ||||||||||
| M0-M3 | 136 (534) | NS | −438 (499) | NS | 64 (948) | 0.06 | −759 (497) | 0.05 | −0.1 (0.8) | NS |
| M4-M6 | 191 (402) | NS | 31 (379) | NS | 596 (715) | NS | −301 (389) | NS | 0.5 (0.6) | NS |
| M7-M12 | 300 (272) | NS | 297 (261) | NS | 1,133 (483) | 0.03 | 159 (267) | NS | −0.4 (0.4) | NS |
| TNF-α | ||||||||||
| Mother (at delivery) | 1,772 (941) | <0.01 | 2,005 (1,207) | 0.06 | 4,023 (2,807) | 0.08 | −334 (921) | NS | 0.1 (0.4) | NS |
| Infant | ||||||||||
| M0-M3 | 1,747 (1,741) | NS | −1,347 (2,202) | NS | 992 (5,080) | NS | −1,016 (1,652) | NS | −0.3 (0.7) | NS |
| M4-M6 | −298 (1,312) | NS | −1,194 (1,671) | NS | −3,363 (3,854) | NS | −1,777 (1,288) | NS | 0.5 (0.5) | NS |
| M7-M12 | 535 (891) | NS | 1,268 (1,151) | 0.09 | 1,739 (2,666) | NS | 1,029 (885) | 0.08 | 0.2 (0.4) | NS |
Positive/negative coefficients indicate cytokine concentrations above/below control (uninfected) levels. SD, standard deviation; NS, not significant.
“Mother (at delivery)” denotes the influence of infection at delivery or in the time period ≤10 days prior to delivery on neonatal/infant cytokine responses in the first 12 months of life. “Infant” denotes the influence of infection during different periods of early life on spontaneous or TLR stimulation-mediated cytokine responses over the whole 12-month period. M0, cord blood; M3, M6, M12, blood drawn at 3, 6, 12 months of age, respectively.
Fig 1.
Spontaneous and TLR agonist-mediated cytokine production in neonatal/infant whole blood. In each case, agonist-mediated responses presented are those following subtraction of the corresponding cytokine concentrations in supernatants of unstimulated cells. Box plots illustrate medians with 25th and 75th percentiles and whiskers for 10th and 90th percentiles. M0, cord blood; M3, M6, M12, peripheral venous blood at 3, 6, 12 months of age, respectively; Unsti, unstimulated samples. The statistical significance of differences in cytokine activity detected at different ages was determined using the nonparametric Kruskal-Wallis test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The concentrations of cytokines spontaneously released by unstimulated cells increased with age: univariate analyses showed that the amounts of IL-6 and TNF-α increased significantly from birth to 6 months of age (P < 0.001), and the amounts of IL-10 increased from birth to 3 months of age (P < 0.001) (Table 2, Fig. 1). Consequently, analyses of the age-dependent maturation of TLR agonist-mediated cytokine responses were conducted after subtraction of cytokine concentrations in supernatants of unstimulated cells from those in supernatants of stimulated cells.
The cytokine responses to the TLR3, TLR4, and TLR7/8 agonists exhibited similar age-related patterns, characterized by robust secretion of IL-6 and IL-10 at birth that either remained stable over time (TLR7/8) or diminished significantly with increasing age (TLR3 and -4; P < 0.04 by Kruskal-Wallis; Fig. 1). In contrast, TNF-α and IFN-γ production in response to these three agonists was weak at birth and then increased significantly to reach plateau levels at 3 to 6 months of age (P < 0.001 by Kruskal-Wallis; Fig. 1). In all cases, TLR9 agonist-mediated cytokine responses in cord blood were low but subsequently increased with age, with significantly enhanced production of IL-6, IL-10, and TNF-α (P < 0.001 by Kruskal-Wallis; Fig. 1).
In multivariate analyses that adjusted for potential confounders (maternal anemia, premature birth, mother/infant infection), the various profiles of age dependency of TLR agonist-mediated cytokine responses described above were confirmed (Table 3).
Table 3.
Multivariate (LMM) analyses of alterations in spontaneous and TLR-mediated cytokine responses in whole blood as a function of infants' age and of maternal P. falciparum infection at deliverya
| Cytokine and P. falciparum infection | TLR3 |
TLR4 |
TLR7/8 |
TLR9 |
Unstimulated |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regression coefficient (SD) | P | Regression coefficient (SD) | P | Regression coefficient (SD) | P | Regression coefficient (SD) | P | Regression coefficient (SD) | P | |
| IL-6 | ||||||||||
| Infant age (mo) | ||||||||||
| 3 | −31,335 (15,138) | <0.05 | −1.32 (0.76) | <0.05 | 14,919 (22,093) | NS | 1.79 (0.69) | <0.01 | 2.00 (0.54) | <0.01 |
| 6 | −38,365 (15,232) | <0.05 | −2.33 (0.77) | <0.01 | 31,892 (21,825) | NS | 2.84 (0.70) | <0.01 | 3.16 (0.54) | <0.01 |
| 12 | −7,910 (14,822) | NS | −1.60 (0.75) | <0.05 | 34,536 (21,579) | NS | 1.38 (0.68) | <0.05 | 2.00 (0.53) | <0.01 |
| Mother at delivery and: | ||||||||||
| M0 | 75,243 (27,539) | <0.05 | 1.25 (0.99) | NS | −6,932 (36,838) | NS | 1.03 (1.03) | NS | 0.11 (0.81) | NS |
| M3 | 58,659 (28,714) | <0.05 | −0.75 (1.15) | NS | 9,411 (38,250) | NS | 0.10 (1.07) | NS | −0.66 (0.85) | NS |
| M6 | 33,481 (28,406) | NS | 0.98 (1.19) | NS | 40,462 (37,592) | NS | −1.83 (1.07) | NS | −1.19 (0.83) | 0.05 |
| M12 | 8,772 (27,539) | NS | 0.64 (1.19) | NS | −16,047 (36,470) | NS | 0.63 (1.02) | NS | 0.82 (0.84) | NS |
| IL-10 | ||||||||||
| Infant age (mo) | ||||||||||
| 3 | −547 (268) | <0.05 | −2.05 (0.67) | <0.01 | −1,428 (518) | <0.05 | 2.04 (0.67) | <0.01 | 0.62 (0.40) | NS |
| 6 | −1,064 (269) | <0.01 | −1.48 (0.67) | <0.05 | −664 (512) | NS | 3.44 (0.67) | <0.01 | 1.81 (0.40) | <0.01 |
| 12 | −1,217 (262) | <0.01 | −2.34 (0.66) | <0.01 | −1,197 (506) | <0.05 | 0.94 (0.66) | NS | 1.04 (0.40) | <0.05 |
| Mother at delivery and: | ||||||||||
| M0 | 652 (454) | NS | −0.84 (1.01) | NS | −772 (858) | NS | 0.85 (1.00) | NS | 1.01 (0.69) | 0.05 |
| M3 | 1,300 (476) | <0.05 | 1.84 (1.05) | NS | 462 (891) | NS | 0.24 (1.03) | NS | −0.10 (0.72) | NS |
| M6 | 328 (470) | NS | 0.22 (1.05) | NS | 56 (876) | NS | −0.29 (1.03) | NS | −0.65 (0.70) | NS |
| M12 | 411 (454) | NS | 0.89 (1.01) | NS | −337 (850) | NS | 2.11 (0.98) | <0.05 | 0.69 (0.71) | NS |
| TNF−α | ||||||||||
| Infant age (mo) | ||||||||||
| 3 | 4,529 (1,129) | <0.01 | 2.15 (0.76) | <0.05 | 21,850 (3,398) | <0.01 | 3.62 (0.64) | <0.01 | 1.99 (0.46) | <0.01 |
| 6 | 2,254 (1,135) | <0.05 | 2.70 (0.76) | <0.01 | 21,817 (3,361) | <0.01 | 5.07 (0.64) | <0.01 | 2.83 (0.46) | <0.01 |
| 12 | 5,171 (1,107) | <0.01 | 2.32 (0.74) | <0.01 | 23,242 (3,327) | <0.01 | 2.42 (0.62) | <0.01 | 1.81 (0.45) | <0.01 |
| Mother at delivery and: | ||||||||||
| M0 | 7.4 (1,827.3) | NS | 0.38 (1.14) | NS | 92 (5,147) | NS | −0.79 (0.95) | NS | 1.12 (0.74) | 0.05 |
| M3 | 1,004 (1,918) | NS | 0.99 (1.18) | NS | −1,908 (5,360) | NS | −0.56 (0.98) | NS | −0.71 (0.78) | NS |
| M6 | 5,238 (1,894) | <0.05 | 0.45 (1.18) | NS | 15,856 (5,262) | <0.01 | −2.10 (0.98) | <0.05 | −0.36 (0.76) | NS |
| M12 | 491 (1,827) | NS | 1.36 (1.14) | NS | −679 (5,092) | NS | 2.85 (0.93) | <0.05 | 0.77 (0.77) | NS |
The reference values used for comparison are those recorded in cord blood (M0). “Mother at delivery” denotes the influence of infection at delivery or in the time period of ≤10 days prior to delivery on neonatal/infant responses measured at designated time points. M0, cord blood; M3, M6, M12, blood drawn at 3, 6, 12 months of age, respectively. Positive/negative coefficients indicate cytokine concentrations above/below control (uninfected) levels. SD, standard deviation.
Maternal P. falciparum infection at delivery affects the profile of TLR agonist-mediated cytokine responses in infants.
We next examined whether P. falciparum infection during pregnancy influenced TLR-mediated cytokine responses in the offspring using segregation into 3 gestational age-related intervals: (i) infection before the third trimester of pregnancy, (ii) infection during the third trimester of pregnancy but more than 10 days before delivery, and (iii) infection from 10 days before delivery up to delivery.
Univariate analyses revealed no differences in spontaneous cytokine release by cells from infants born to mothers with different infection histories (Table 2) but did show that TLR ligand-mediated cytokine release by infants' cells was modulated by maternal infection occurring either during the 3rd trimester or close to/at delivery. The changes related to infection close to/at delivery concerned significantly increased production of (i) IL-6, IL-10, and TNF-α upon TLR3 stimulation, (ii) IL-6 upon TLR4 stimulation, and (iii) IL-10 upon TLR9 stimulation, in comparison with cells of children born to mothers who were uninfected at delivery (Table 2). There were also associations of borderline significance between infections late in pregnancy and increased TNF-α production upon TLR4 and TLR7/8 stimulation (Table 2), as well as between infections earlier during the 3rd trimester and decreased IL-10 production following TLR4, TLR7/8, and TLR9 stimulation (data not shown). The concentration of IFN-γ in supernatants of cells stimulated with TLR3, TLR4, and TLR9 agonists were too low to identify differences in secretion of this cytokine, while no differences were observed in infants' TLR7/8-induced production of IFN-γ when segregated according to maternal infection history (data not shown).
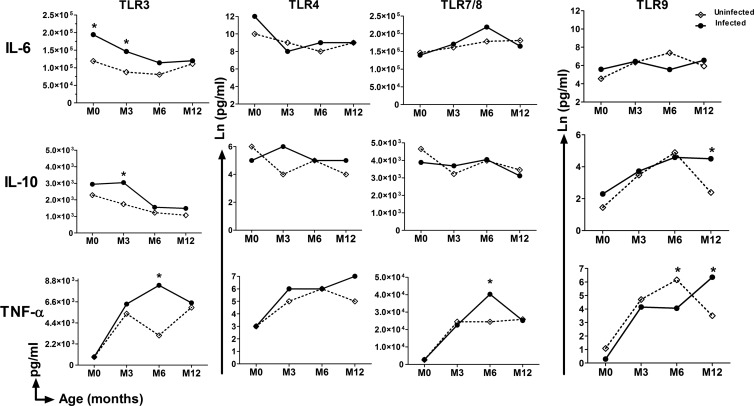
The multivariate analyses of infants' TLR-mediated cytokine secretion as a function of maternal infection history were designed to evaluate whether maternal infection affected either spontaneous or TLR-mediated cytokine responses in infancy while adjusting for potential confounding covariables identified in the univariate analyses, i.e., maternal anemia, premature birth, and age of the infant. Using the profiles obtained from those born to uninfected mothers as reference values, the associations that remained significant following these multivariate analyses concerned only infections occurring close to/at delivery and not those earlier in pregnancy. In terms of spontaneous release of cytokines, the production of both IL-10 and TNF-α was enhanced in cord blood, while that of IL-6 was reduced at 6 months of age (Table 3). In the context of TLR3 stimulation, the association between maternal infection at delivery and significantly enhanced production of IL-6, IL-10, and TNF-α found in univariate analyses remained, with higher production (i) of IL-6 at birth and at 3 months, (ii) of IL-10 at 3 months, and (iii) of TNF-α at 6 months of age than that in babies born to mothers who were uninfected at delivery (Table 3). Similarly, for TLR7/8 stimulation, the association between maternal infection at delivery and significantly enhanced production of TNF-α (at 6 months of age) remained, as did the association for increased TLR9 agonist-mediated IL-10 production, at 12 months of age, in parallel with increased TNF-α (Table 3), although production of the latter cytokine was significantly lower in infants 6 months of age. In marked contrast to the altered responses to TLR3, -7/8, and -9 stimulation, multivariate analyses revealed no significant differences in TLR4-mediated cytokine responses of infants when segregated according to maternal infection history.
A graphical depiction of the multivariate model-derived predicted, i.e., coefficient-derived TLR-mediated cytokine responses of infants as a function maternal infection close to/at delivery is shown in Fig. 2. The overall picture this gives is one of maternal infection-related enhancement of TLR3-mediated production of both IL-6 and IL-10 at birth and at 3 months of age and enhancement of TLR3-, TLR7/8-, and TLR9-mediated TNF-α and IL-10 production at 6 or 12 months of age.
Fig 2.
GLMM-based predictive profiles of TLR agonist-mediated cytokine production by neonatal/infant whole blood as a function of the presence or absence of PAM at delivery. M0, cord blood; M3, M6, M12, peripheral venous blood at 3, 6, 12 months of age, respectively. The statistical significance of differences was determined using the GLMM model. *, P < 0.05.
TLR-mediated cytokine production in cord blood as a predictor of P. falciparum infection in infants.
We next wished to ascertain whether the TLR-mediated cord blood cell cytokine responses shown to be altered or not as a result of maternal infection were predictive of P. falciparum infections during infancy. Univariate analyses showed that high IL-10 production, following stimulation of TLR3, TLR4, or TLR7/8 in cord blood, as well as high TNF-α production following TLR7/8 stimulation, was associated with an increased risk of malaria during the first year of life (Table 4). Multivariate analyses confirmed the independent associations for an increased risk of P. falciparum infection in infancy with high TLR3- and TLR7/8-mediated cord blood cell IL-10 production (Table 4).
Table 4.
Prospective assessment of the predictive value of TLR agonist-mediated cytokine production in cord blood for infection with P. falciparum in the first year of lifec
| TLR | Cytokine | ORb | Univariate |
Adjusted ORb | Multivariatea |
||
|---|---|---|---|---|---|---|---|
| CI 95% | P value | CI 95% | P value | ||||
| TLR3 | IL-6 | 1.17 | 0.97–1.42 | 0.09 | 1.07 | 0.88–1.30 | 0.50 |
| IL-10 | 1.24 | 1.03–1.49 | 0.02 | 1.22 | 1.00–1.50 | 0.05 | |
| TNF-α | 1.04 | 0.94–1.16 | 0.44 | 0.98 | 0.88–1.09 | 0.75 | |
| TLR4 | IL-6 | 1.23 | 0.96–1.59 | 0.09 | 1.12 | 0.84–1.48 | 0.43 |
| IL-10 | 1.24 | 1.01–1.54 | 0.04 | 1.19 | 0.93–1.54 | 0.16 | |
| TNF-α | 1.00 | 0.90–1.12 | 0.93 | 0.95 | 0.85–1.06 | 0.37 | |
| TLR7/8 | IL-6 | 1.13 | 0.95–1.35 | 0.15 | 0.94 | 0.79–1.12 | 0.46 |
| IL-10 | 1.40 | 1.06–1.86 | 0.01 | 1.38 | 1.00–1.90 | 0.04 | |
| TNF-α | 1.18 | 1.03–1.35 | 0.01 | 1.11 | 0.97–1.27 | 0.11 | |
| TLR9 | IL-6 | 1.00 | 0.89–1.12 | 0.95 | 0.94 | 0.80–1.11 | 0.48 |
| IL-10 | 1.02 | 0.90–1.14 | 0.80 | 0.96 | 0.82–1.14 | 0.66 | |
| TNF-α | 1.10 | 0.97–1.24 | 0.12 | 1.17 | 0.99–1.38 | 0.06 | |
Adjusted on P. falciparum infection history of mother and gravidity and infant age and low birth weight.
Relative risk of P. falciparum infection in the infant as a function of a log-fold increase in the cord blood TLR-mediated cytokine response.
The concentration of IFN-γ in supernatants of cells stimulated with TLR agonists was too low to allow appropriate analyses.
P. falciparum infection in infants influences TLR agonist-mediated cytokine response profiles.
Infants' innate immune responses, while having been “conditioned” in utero as a result of maternal P. falciparum infection, will also be potentially altered by P. falciparum when the infant itself is infected. Our next step was therefore to incorporate the prospectively collected data on P. falciparum infections in the first year of life into the multivariate model of TLR-mediated cytokine responses. In order to control for the possible influence of passively acquired (maternal antibody-mediated) antiplasmodial immunity, infection and/or malaria episodes in infants were segregated into three different time periods for the assessments of independent associations with either spontaneous or TLR agonist-mediated cytokine responses. Regardless of age, infection arising during infancy had no observable effect on spontaneous cytokine release (Table 2), but univariate analyses did show significantly increased TLR7/8-mediated IL-10 production as a function of infection after 6 months of age and significantly decreased TLR9-mediated IL-10 production related to infection between birth and 3 months of age (Table 2). Multivariate analyses confirmed both those associations, while also revealing independent associations with infection between birth and 3 months of age for increased TLR7/8-mediated IL-6 production and for decreased TLR9-mediated TNF-α production (data not shown).
Influence of maternal anemia and gravidity and baby gender and prematurity on the profile of TLR-mediated cytokine responses over time.
In univariate analyses, maternal anemia at delivery was associated with increased spontaneous release of IL-6 and with decreased spontaneous release of TNF-α (both with P values of <0.05) but not with any change in TLR-mediated cytokine production (data not shown). In multivariate analyses, neither of the associations with anemia remained. In univariate analyses, no cytokine production of any type was affected either by gravidity status (primi- versus multigravid) or by the baby's gender (data not shown).
Univariate analyses of data from those born premature (delivery at <37 weeks) revealed significantly elevated levels of IL-6, IL-10, and TNF-α in response to TLR9 ligation (data not shown). Multivariate analyses confirmed the independent influence of being born premature on increased TLR9-mediated TNF-α production, increased TLR4-mediated IL-10 production, and increased TLR7/8-mediated IFN-γ production compared to the production levels of full-term babies (P < 0.05; data not shown).
DISCUSSION
The published evidence of in utero exposure to P. falciparum resulting in long-term changes to the immunological responses of infants is limited, to the best of our knowledge, to a single prospective birth cohort study that documented altered parasite antigen-specific responses that persisted through childhood (23). In parallel, epidemiological studies, including our own, have clearly documented the altered susceptibility of infants to P. falciparum infection or disease (11–16). It has not been documented whether and how specific components of the innate immune system of such “tolerant” infants may contribute to their apparent inability to control the parasite. The study reported here is thus the first prospective birth cohort study to address this issue by investigating in detail the effect of both the presence and timing of maternal P. falciparum infection on an essential component of innate immunity in early life, namely, TLR-mediated cytokine responses.
The overall age-related patterns of cytokine responses to TLR ligands we observed here in infancy are consistent with those reported in the only study of its kind published to date, in sub-Saharan African (Gambian) infants, that used whole blood and a short-term (18- to 24-h) culture period, i.e., conditions almost identical to those we used (28). The principal similarities include the presence of robust IL-6 and IL-10 responses at birth and of proinflammatory TNF-α and IFN-γ responses that increased markedly from birth through the first 3 months of life. In contrast to the Gambian study, we did not detect appreciable TLR7/8-mediated production of IFN-γ at birth. This difference most likely relates to the fact that we used just a single dual TLR7/8 agonist (R848) compared to the panel of 3 different agonists used in the Gambian study that allowed the identification of fine differences in TLR7- and TLR8-mediated activity, differences our study could not distinguish. Also, in the same context of TLR7/8-mediated activity, we did not find the same dramatic age-related increase in the amounts of IFN-γ produced, again a difference that may be explained by the choice of agonist. Nevertheless, and as seen both in Caucasian (47, 48) and Gambian infants (28), we did find that R848 was the agonist that consistently induced the strongest pro- and anti-inflammatory cytokine responses in Beninese infants, while responses induced by the TLR9 agonist were relatively weak. Cytokine production in response to the TLR9 agonist nevertheless increased from birth up to 6 months of age, a pattern not seen in the comparable studies of European or African infants (28, 49). These disparate findings may be explained (i) by the comparatively broad target populations of R848 that can stimulate pDC, mDC, monocytes, and B cells (50) and (ii) by the TLR9 ligand we used (ODN CpG 2216) that exerts selective activity only on pDC (46) as opposed to on both pDC and B cells (28, 49). Our data also confirm published observations of a decrease in the production of IL-10 in response to TLR4 agonists postnatally (29).
In the context of the principal focus of our study, i.e., maternal infection-related changes to neonatal/infant TLR-mediated responses, the most notable finding is that exposure to P. falciparum in utero just prior to or at delivery significantly modulated infants' subsequent cytokine responses arising specifically from ligation of the endosomally expressed TLR3, -7/8, and -9, whereas responses arising from ligation of the surface-expressed TLR4 were unaffected. All of the observed changes involved increased production, either early or late in the first year of life, of both proinflammatory (IL-6, TNF-α) and anti-inflammatory (IL-10) cytokines. The same exposure in utero also led to significantly increased spontaneous secretion of both TNF-α and IL-10 by cord blood cells but reduced IL-6 secretion by 6-month-old infants' cells. As has been noted by others (51), the relative predominance of the Th17 cell-promoting cytokine IL-6 along with IL-10 in infants' TLR-mediated cytokine repertoire almost certainly contributes to the well-documented deficiency in Th1 cell-type (IFN-γ-led) responses (25). The latter are responses that are commonly considered to be an essential component of antimalarial immunity (52). Thus, maternal infection and consequent fetal exposures that reinforce TLR-mediated IL-6 and IL-10 responses in early life, rather than inducing a switch to a “mature” Th1 type of cytokine response, could quite plausibly be implicated in enhancing infants' susceptibility to P. falciparum infection. That enhanced IL-6 activity may indeed have such effects is supported by the fact that (i) P. falciparum-induced IL-6 is known to be associated with susceptibility to malaria in children (53) and (ii) several studies have reported the circulating concentration of IL-6 to be significantly higher in children with severe or uncomplicated malaria than in healthy controls (54). The results of the prospective assessment of cord blood cells' TLR-mediated cytokine profiles we conducted here provide support for a similarly detrimental effect of IL-10 (Table 4).
Three recent studies have substantially expanded the knowledge of the interactions between P. falciparum and TLR. All revealed that, somewhat unexpectedly, exposure to the parasite in vivo or in vitro leads to a form of proinflammatory priming of TLR-mediated responses, although, notably in the context of the findings we report here, no significant change in parasite-induced TLR3-mediated cytokine responses was found (34, 35, 55). Paradoxically, in this context, our data clearly identify enhanced TLR3-mediated cytokine responses in early life as one of the major modified outcomes of exposure to P. falciparum in utero. Current knowledge nevertheless gives no indication of a parasite-derived TLR3-specific ligand that may be at the origin of this effect (56, 57). These findings are particularly striking, because the significantly increased TLR3-mediated IL-6 and IL-10 production by exposed infants' cells at birth and/or at 3 months of age runs exactly counter to the significant age-related decline in production of these same two cytokines occurring over precisely the same time period (Fig. 1). We also observed significantly increased TLR3-mediated TNF-α production by “exposed” 6-month-old infants' cells, but this, in contrast to IL-6 and IL-10, is on a background of an increasing age-related production profile. Pertinent perhaps here is the fact that TLR3, among the panel of TLR that we investigated, is the only one expressed within T lymphocyte populations as well as in a prominent APC population (mDC), possibly implicating maternal infection-induced upregulation of TLR3 expression by fetal T cells. Further, we employed a TLR3 ligand, poly(I·C), that is also known to stimulate non-TLR pattern recognition receptors in mDC (39). We did not detect the pattern of TLR3/4-mediated reduced TNF-α and enhanced IFN-γ production by cord blood cells associated with placental infection at delivery reported in a Gabonese study by Adegnika and colleagues (33). Although the agonists used were the same in both studies, the substantially different culture conditions used (purified cord mononuclear cells cultured for 3 days versus whole blood for 24 h) possibly explain the different outcomes.
Our findings relating to infants' TLR7/8-mediated cytokine responses, although comparatively less pronounced in scope, echo those for TLR3 in the sense that, here again, none of the relevant published studies have thus far identified a P. falciparum-derived ligand for either TLR7 or -8. Despite this, maternal infection at delivery was associated with enhanced TLR7/8-mediated TNF-α responses in 6-month-old infants, as was also the case for TLR3. In the case of TLR9, for which there is a recognized parasite-derived ligand (57), the enhancing effects of maternal infection on cytokine responses in early life were even more prolonged, with significantly stronger IL-10 and TNF-α production apparent in 12-month-old infants.
The fact that only maternal P. falciparum infections occurring close to or at delivery affected the innate immune responses we measured, while infections during the second or third trimester of pregnancy appeared to have no such influence, is intriguing for two main reasons. First, in cord blood innate immune (γδ) T lymphocyte subsets, we have previously documented distinct “activated” phenotypes ex vivo associated with maternal infections that were successfully treated earlier in pregnancy but not with infections detected at delivery (58). These findings suggest that different components of the fetal innate immune response may be affected differently according to the timing, type, or duration of exposure to parasite-derived molecules. Second, our data imply that plasmodial infections acquired by mothers very late in pregnancy can have sustained effects on infants' innate immune responses, raising the question of the possible implication of such changes in the increased frequency of nonplasmodial febrile illnesses with which such infants present (16).
Two other aspects of our results also deserve mention. First, we identified the impacts of P. falciparum infections occurring early (0 to 3 months of age) or late (6 to 12 months of age) during infancy that included, respectively, enhanced IL-6 or enhanced IL-10 responses after TLR7/8 ligation but, for infections occurring early in infancy, reduced IL-10 and TNF-α responses after TLR9 ligation. Inappropriately elevated IL-10 activity may contribute to sustaining infants' susceptibility to infection by suppressing Th1-type responses, and, similarly, impaired responses to TLR9 activation may lead to increased susceptibility to pathogens that bind TLR9, such as DNA viruses (59–62). Second, we observed for the first time that premature newborns, upon TLR4 stimulation, produced more IL-10 and, upon TLR7/8 or TLR9 stimulation, produced more IFN-γ or TNF-α. These data suggest upregulation of the MyD88-dependent pathway in preterm infants, which contrasts with previous findings in a non-African population (63).
In conclusion, we report the first prospective study to examine the influence of pregnancy-associated malaria on the maturation of TLR responses in infants. Notably, our results show the profound effects of maternal infection close to or at delivery on an infant's innate immune responses and indicate that some of the immunological consequences of PAM we measured here are long-lasting. Further studies are needed to better define the consequences of the increased production of the proinflammatory cytokines TNF-α and IL-6 on adaptive T cell-mediated immunity and of the suppressive cytokine IL-10 on the development of regulatory T cells during the first year of life. In this same STOPPAM study, we have found that the frequency of circulating Treg during infancy of those born to mothers with placental infection at delivery is consistently higher than in infants born to uninfected mothers (N. Fievet, O. Nouatin, and S. Ibitokou, unpublished observations), suggesting but not proving causality vis-à-vis enhanced susceptibility to P. falciparum infection. How specific cells and the cytokines they produce in early life may also separately influence infants' responses to routine infant and/or candidate malaria vaccines is an area that needs to be urgently addressed.
ACKNOWLEDGMENTS
We are grateful to all women who participated in the study. We thank all the field and administration staffs of Akodeha, Comé Central, and Ouedèmé Pedah health centers for their contribution. We particularly thank Jacqueline Affedjou, Jean-Claude Sagbo, Bernadette Gandounou, Gildas Gbaguidi, and all field staff members at the STOPPAM site for their hard work. We thank Bich-Tram Huynh, Sebastien Dechavanne, and Valérie Briand for database management and Carine Agbowaï, Aurax Fernando, Charles Ahouansou, Pépin Kounou, Honoré Kounou, and Darius Sossou for their lab contribution.
This paper describes work undertaken in the context of the STOPPAM project (www.stoppam.org), a small- and medium-scale collaborative project supported by the European 7th Framework Programme under contract number 200889. This work was also supported by the Ministère des Affaires Etrangères of France (project reference no. 2006-22), SIDA/SAREC (Swedish International Development Cooperation Agency; grant to S.V.), and the Institut de Recherche pour le Développement, which contributed to the study financially and with research material. Financial support was also provided by the AIRD-DPF to S.I. (Ph.D. research scholarship).
K.G., S.V., S.I., A.M., P.D., M.T.-B., N.F., and A.J.F.L. conceived, designed, and coordinated the study. K.G., S.I., S.E., and O.N. participated in the sample collection and processing. K.G., S.I., S.V., N.F., and A.J.F.L. designed and supervised the immunoassays. P.H. and G.C. performed statistical analysis. K.G., S.I., O.N., S.E., and A.A. carried out the immunoassays. K.G., S.I., N.F., and S.V. drafted the first version of the manuscript. All authors read and approved the final manuscript.
Footnotes
Published ahead of print 20 May 2013
REFERENCES
- 1. Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. 2012. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect. Dis. 12:330–340 [DOI] [PubMed] [Google Scholar]
- 2. Labeaud AD, Malhotra I, King MJ, King CL, King CH. 2009. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl. Trop. Dis. 3:e442. 10.1371/journal.pntd.0000442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cuna WR, Choque AG, Passera R, Rodriguez C. 2009. Proinflammatory cytokine production in chagasic mothers and their uninfected newborns. J. Parasitol. 95:891–894 [DOI] [PubMed] [Google Scholar]
- 4. Vekemans J, Truyens C, Torrico F, Solano M, Torrico MC, Rodriguez P, Alonso-Vega C, Carlier Y. 2000. Maternal Trypanosoma cruzi infection upregulates capacity of uninfected neonate cells to produce pro- and anti-inflammatory cytokines. Infect. Immun. 68:5430–5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kurtis JD, Higashi A, Wu HW, Gundogan F, McDonald EA, Sharma S, PondTor S, Jarilla B, Sagliba MJ, Gonzal A, Olveda R, Acosta L, Friedman JF. 2011. Maternal Schistosomiasis japonica is associated with maternal, placental, and fetal inflammation. Infect. Immun. 79:1254–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brabin BJ, Kalanda BF, Verhoeff FH, Chimsuku LH, Broadhead RL. 2004. Risk factors for fetal anaemia in a malarious area of Malawi. Ann. Trop. Paediatr. 24:311–321 [DOI] [PubMed] [Google Scholar]
- 7. Cot M, Deloron P. 2003. Malaria prevention strategies. Brit. Med. Bull. 67:137–148 [DOI] [PubMed] [Google Scholar]
- 8. Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. 2010. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 7:e1000221. 10.1371/journal.pmed.1000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. 2007. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 7:93–104 [DOI] [PubMed] [Google Scholar]
- 10. Rogerson SJ, Boeuf P. 2007. New approaches to pathogenesis of malaria in pregnancy. Parasitology 134:1883–1893 [DOI] [PubMed] [Google Scholar]
- 11. Bardaji A, Sigauque B, Sanz S, Maixenchs M, Ordi J, Aponte JJ, Mabunda S, Alonso PL, Menendez C. 2011. Impact of malaria at the end of pregnancy on infant mortality and morbidity. J. Infect. Dis. 203:691–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le Hesran JY, Cot M, Personne P, Fievet N, Dubois B, Beyeme M, Boudin C, Deloron P. 1997. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am. J. Epidemiol. 146:826–831 [DOI] [PubMed] [Google Scholar]
- 13. Le Port A, Watier L, Cottrell G, Ouedraogo S, Dechavanne C, Pierrat C, Rachas A, Bouscaillou J, Bouraima A, Massougbodji A, Fayomi B, Thiebaut A, Chandre F, Migot-Nabias F, Martin-Prevel Y, Garcia A, Cot M. 2011. Infections in infants during the first 12 months of life: role of placental malaria and environmental factors. PLoS One 6:e27516. 10.1371/journal.pone.0027516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mutabingwa TK, Bolla MC, Li JL, Domingo GJ, Li X, Fried M, Duffy PE. 2005. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2:e407. 10.1371/journal.pmed.0020407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwarz NG, Adegnika AA, Breitling LP, Gabor J, Agnandji ST, Newman RD, Lell B, Issifou S, Yazdanbakhsh M, Luty AJ, Kremsner PG, Grobusch MP. 2008. Placental malaria increases malaria risk in the first 30 months of life. Clin. Infect. Dis. 47:1017–1025 [DOI] [PubMed] [Google Scholar]
- 16. Rachas A, Le Port A, Cottrell G, Guerra J, Choudat I, Bouscaillou J, Massougbodji A, Garcia A. 2012. Placental malaria is associated with increased risk of nonmalaria infection during the first 18 months of life in a Beninese population. Clin. Infect. Dis. 55:672–678 [DOI] [PubMed] [Google Scholar]
- 17. Broen K, Brustoski K, Engelmann I, Luty AJ. 2007. Placental Plasmodium falciparum infection: causes and consequences of in utero sensitization to parasite antigens. Mol. Biochem. Parasitol. 151:1–8 [DOI] [PubMed] [Google Scholar]
- 18. Bisseye C, van der Sande M, Morgan WD, Holder AA, Pinder M, Ismaili J. 2009. Plasmodium falciparum infection of the placenta impacts on the T helper type 1 (Th1)/Th2 balance of neonatal T cells through CD4(+)CD25(+) forkhead box P3(+) regulatory T cells and interleukin-10. Clin. Exp. Immunol. 158:287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brustoski K, Moller U, Kramer M, Hartgers FC, Kremsner PG, Krzych U, Luty AJ. 2006. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J. Infect. Dis. 193:146–154 [DOI] [PubMed] [Google Scholar]
- 20. Brustoski K, Moller U, Kramer M, Petelski A, Brenner S, Palmer DR, Bongartz M, Kremsner PG, Luty AJ, Krzych U. 2005. IFN-gamma and IL-10 mediate parasite-specific immune responses of cord blood cells induced by pregnancy-associated Plasmodium falciparum malaria. J. Immunol. 174:1738–1745 [DOI] [PubMed] [Google Scholar]
- 21. Flanagan KL, Halliday A, Burl S, Landgraf K, Jagne YJ, Noho-Konteh F, Townend J, Miles DJ, van der Sande M, Whittle H, Rowland-Jones S. 2010. The effect of placental malaria infection on cord blood and maternal immunoregulatory responses at birth. Eur. J. Immunol. 40:1062–1072 [DOI] [PubMed] [Google Scholar]
- 22. Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. 2011. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J. Immunol. 186:2780–2791 [DOI] [PubMed] [Google Scholar]
- 23. Malhotra I, Dent Mungai AP, Wamachi A, Ouma JH, Narum DL, Muchiri E, Tisch DJ, King CL. 2009. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med. 6:e1000116. 10.1371/journal.pmed.1000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazzoni A, Segal DM. 2004. Controlling the Toll road to dendritic cell polarization. J. Leukoc. Biol. 75:721–730 [DOI] [PubMed] [Google Scholar]
- 25. Levy O. 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 7:379–390 [DOI] [PubMed] [Google Scholar]
- 26. Marshak-Rothstein A. 2006. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6:823–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang X, Deriaud E, Jiao X, Braun D, Leclerc C, Lo-Man R. 2007. Type I interferons protect neonates from acute inflammation through interleukin 10-producing B cells. J. Exp. Med. 204:1107–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burl S, Townend J, Njie-Jobe J, Cox M, Adetifa UJ, Touray E, Philbin VJ, Mancuso C, Kampmann B, Whittle H, Jaye A, Flanagan KL, Levy O. 2011. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS One 6:e18185. 10.1371/journal.pone.0018185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, Crabtree J, Rein-Weston A, Lavoie PM, Turvey SE, Hawkins NR, Self SG, Wilson CB, Hajjar AM, Fortuno ES, III, Kollmann TR. 2010. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One 5:e15041. 10.1371/journal.pone.0015041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lisciandro JG, Prescott SL, Nadal-Sims MG, Devitt CJ, Pomat W, Siba PM, Tulic MC, Holt PG, Strickland D, van den Biggelaar AH. 2012. Ontogeny of Toll-like and NOD-like receptor-mediated innate immune responses in Papua New Guinean infants. PLoS One 7:e36793. 10.1371/journal.pone.0036793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Breitling LP, Fendel R, Mordmueller B, Adegnika AA, Kremsner PG, Luty AJ. 2006. Cord blood dendritic cell subsets in African newborns exposed to Plasmodium falciparum in utero. Infect. Immun. 74:5725–5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fievet N, Varani S, Ibitokou S, Briand V, Louis S, Perrin RX, Massougbogji A, Hosmalin A, Troye-Blomberg M, Deloron P. 2009. Plasmodium falciparum exposure in utero, maternal age and parity influence the innate activation of foetal antigen presenting cells. Malar. J. 8:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adegnika AA, Kohler C, Agnandji ST, Chai SK, Labuda L, Breitling LP, Schonkeren D, Weerdenburg E, Issifou S, Luty AJ, Kremsner PG, Yazdanbakhsh M. 2008. Pregnancy-associated malaria affects toll-like receptor ligand-induced cytokine responses in cord blood. J. Infect. Dis. 198:928–936 [DOI] [PubMed] [Google Scholar]
- 34. Franklin BS, Parroche P, Ataide MA, Lauw F, Ropert C, de Oliveira RB, Pereira D, Tada MS, Nogueira P, da Silva LH, Bjorkbacka H, Golenbock DT, Gazzinelli RT. 2009. Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function. Proc. Natl. Acad. Sci. U. S. A. 106:5789–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCall MB, Netea MG, Hermsen CC, Jansen T, Jacobs L, Golenbock D, van der Ven AJ, Sauerwein RW. 2007. Plasmodium falciparum infection causes proinflammatory priming of human TLR responses. J. Immunol. 179:162–171 [DOI] [PubMed] [Google Scholar]
- 36. Erdman LK, Finney CA, Liles WC, Kain KC. 2008. Inflammatory pathways in malaria infection: TLRs share the stage with other components of innate immunity. Mol. Biochem. Parasitol. 162:105–111 [DOI] [PubMed] [Google Scholar]
- 37. Huynh BT, Fievet N, Gbaguidi G, Dechavanne S, Borgella S, Guezo-Mevo B, Massougbodji A, Ndam NT, Deloron P, Cot M. 2011. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am. J. Trop. Med. Hyg. 85:214–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kalilani L, Mofolo I, Chaponda M, Rogerson SJ, Meshnick SR. 2010. The effect of timing and frequency of Plasmodium falciparum infection during pregnancy on the risk of low birth weight and maternal anemia. Trans. R. Soc. Trop. Med. Hyg. 104:416–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perrot I, Deauvieau F, Massacrier C, Hughes N, Garrone P, Durand I, Demaria O, Viaud N, Gauthier L, Blery M, Bonnefoy-Berard N, Morel Y, Tschopp J, Alexopoulou L, Trinchieri G, Paturel C, Caux C. 2010. TLR3 and Rig-like receptor on myeloid dendritic cells and Rig-like receptor on human NK cells are both mandatory for production of IFN-gamma in response to double-stranded RNA. J. Immunol. 185:2080–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388–3393 [DOI] [PubMed] [Google Scholar]
- 41. Muzio M, Bosisio D, Polentarutti N, D'Amico G, Stoppacciaro A, Mancinelli R, van't Veer C, Penton-Rol G, Ruco LP, Allavena P, Mantovani A. 2000. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998–6004 [DOI] [PubMed] [Google Scholar]
- 42. Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166:249–255 [DOI] [PubMed] [Google Scholar]
- 43. Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S. 2002. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J. Exp. Med. 195:1507–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D'Cruz D, Casanova JL, Trouillet C, Geissmann F. 2010. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33:375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. 2005. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J. Immunol. 174:1259–1268 [DOI] [PubMed] [Google Scholar]
- 46. Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. 2001. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce large amounts of IL-12. Eur. J. Immunol. 31:3026–3037 [DOI] [PubMed] [Google Scholar]
- 47. Levy O, Suter EE, Miller RL, Wessels MR. 2006. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 108:1284–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. 2004. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 173:4627–4634 [DOI] [PubMed] [Google Scholar]
- 49. Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, Goldman M, Goriely S. 2010. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One 5:e10407. 10.1371/journal.pone.0010407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Desmet CJ, Ishii KJ. 2012. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat. Rev. Immunol. 12:479–491 [DOI] [PubMed] [Google Scholar]
- 51. PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA. 2011. Challenges in infant immunity: implications for responses to infection and vaccines. Nat. Immunol. 12:189–194 [DOI] [PubMed] [Google Scholar]
- 52. Langhorne J, Ndungu FM, Sponaas AM, Marsh K. 2008. Immunity to malaria: more questions than answers. Nat. Immunol. 9:725–732 [DOI] [PubMed] [Google Scholar]
- 53. Robinson LJ, D'Ombrain MC, Stanisic DI, Taraika J, Bernard N, Richards JS, Beeson JG, Tavul L, Michon P, Mueller I, Schofield L. 2009. Cellular tumor necrosis factor, gamma interferon, and interleukin-6 responses as correlates of immunity and risk of clinical Plasmodium falciparum malaria in children from Papua New Guinea. Infect. Immun. 77:3033–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. 2004. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun. 72:5630–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hartgers FC, Obeng BB, Voskamp A, Larbi IA, Amoah AS, Luty AJ, Boakye D, Yazdanbakhsh M. 2008. Enhanced Toll-like receptor responsiveness associated with mitogen-activated protein kinase activation in Plasmodium falciparum-infected children. Infect. Immun. 76:5149–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gowda DC. 2007. TLR-mediated cell signaling by malaria GPIs. Trends Parasitol. 23:596–604 [DOI] [PubMed] [Google Scholar]
- 57. Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, Gazzinelli RT, Golenbock DT. 2007. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Natl. Acad. Sci. U. S. A. 104:1919–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Engelmann I, Santamaria A, Kremsner PG, Luty AJ. 2005. Activation status of cord blood gamma delta T cells reflects in utero exposure to Plasmodium falciparum antigen. J. Infect. Dis. 191:1612–1622 [DOI] [PubMed] [Google Scholar]
- 59. Nordstrom I, Eriksson K. 2012. HHV-6B induces IFN-lambda1 responses in cord plasmacytoid dendritic cells through TLR9. PLoS One 7:e38683. 10.1371/journal.pone.0038683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sorensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. 2008. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J. Immunol. 181:8604–8612 [DOI] [PubMed] [Google Scholar]
- 61. Varani S, Cederarv M, Feld S, Tammik C, Frascaroli G, Landini MP, Soderberg-Naucler C. 2007. Human cytomegalovirus differentially controls B cell and T cell responses through effects on plasmacytoid dendritic cells. J. Immunol. 179:7767–7776 [DOI] [PubMed] [Google Scholar]
- 62. West JA, Gregory SM, Sivaraman V, Su L, Damania B. 2011. Activation of plasmacytoid dendritic cells by Kaposi's sarcoma-associated herpesvirus. J. Virol. 85:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sadeghi K, Berger A, Langgartner M, Prusa AR, Hayde M, Herkner K, Pollak A, Spittler A, Forster-Waldl E. 2007. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J. Infect. Dis. 195:296–302 [DOI] [PubMed] [Google Scholar]