Abstract
The development of vaccine candidates against Plasmodium vivax—the most geographically widespread human malaria species—is challenged by technical difficulties, such as the lack of in vitro culture systems and availability of animal models. Chimeric rodent Plasmodium parasites are safe and useful tools for the preclinical evaluation of new vaccine formulations. We report the successful development and characterization of chimeric Plasmodium berghei parasites bearing the type I repeat region of P. vivax circumsporozoite protein (CSP). The P. berghei-P. vivax chimeric strain develops normally in mosquitoes and produces highly infectious sporozoites that produce patent infection in mice that are exposed to the bites of as few as 3 P. berghei-P. vivax-infected mosquitoes. Using this transgenic parasite, we demonstrate that monoclonal and polyclonal antibodies against P. vivax CSP strongly inhibit parasite infection and thus support the notion that these antibodies play an important role in protective immunity. The chimeric parasites we developed represent a robust model for evaluating protective immune responses against P. vivax vaccines based on CSP.
INTRODUCTION
The global burden of malaria due to Plasmodium vivax is approximately 70 to 390 million cases annually (1), with around 2.5 billion people living at risk of infection (2). Even though Plasmodium falciparum remains the leading cause of malaria in Africa, P. vivax malaria is the most geographically widespread of the human malarias (2, 3). It is extensively distributed throughout the tropics, in the Middle East, Asia, the western Pacific, and Latin America (1–4).
P. vivax malaria was long considered a benign and non-life-threatening disease; however, recent publications report an increasing number of severe, complicated cases due to P. vivax infections (5, 6). Efforts toward the development of antimalaria vaccines are still mainly focused on P. falciparum, although P. vivax malaria is “harder to prevent, diagnose, and treat, and both species are coendemic” (7). While the importance of a vaccine targeting P. vivax is recognized, the development of P. vivax malaria vaccines is hampered by insufficient funding and technical difficulties (2, 8, 9). The lack of in vitro culture systems and suitable animal models, for example, imposes a significant limitation on testing novel vaccine candidates (7, 10).
The circumsporozoite protein (CSP) is the most abundant protein on the surfaces of sporozoites and is responsible for eliciting both T-cell and antibody responses. The plasmodial CSP is one of the best studied antigens and is considered to be a major malaria vaccine candidate. RTS,S, the most effective malaria vaccine candidate to date, is based on P. falciparum CSP, thus underscoring CSP's immunogenic properties. CSP is comprised of an immunodominant central repeat region, diverse among Plasmodium species, flanked by two conserved domains: region I at the N terminus of the repeats and a type I thrombospondin repeat (TSR) motif C terminal to the repeat region. Early studies in animals have indicated that antibodies against the repeat region neutralize the infectivity of sporozoites and confer sterile immunity (11, 12). Importantly, recent studies in humans vaccinated with RTS,S indicated that protection among vaccinees correlates with the titer of anti-CSP repeat antibodies (13).
Plasmodium berghei parasites bearing the repeat region of P. falciparum CSP have been developed and characterized by Persson et al. (14). These parasites, “antigenically P. falciparum but functionally rodent malaria” (14), are a practical tool for evaluating the efficacy of human preerythrocytic P. falciparum CSP-based vaccine candidates. On the basis of the study by Persson et al., we developed and characterized a chimeric P. berghei parasite strain expressing the repeat region of P. vivax (VK210) CSP as a first step in evaluating vaccine candidates based on the CSP of P. vivax. We demonstrate that these chimeric parasites are recognized by a monoclonal antibody (MAb), 2F2, specific for the P. vivax VK210 CSP repeats (15) and that the in vivo infectivity of chimeric sporozoites is strongly inhibited by this monoclonal antibody, as well as by polyclonal antibodies raised against a recombinant P. vivax CSP construct (16).
MATERIALS AND METHODS
Plasmid construction and parasite transfection.
The transfection plasmid, pR-CSPv, carrying the repeat region of P. vivax, was derived from plasmid pR-CSwt (17), which carries the CS gene of P. berghei (wild type [WT]) and results from the combination of plasmids pIC-CSwt (18) and pPv (Retrogen Inc., CA) encoding the VK210 repeat motif based on the Salvador I (SalI) strain of P. vivax. Briefly, a PmlI-SexAI 567-bp fragment was excised from pIC-CSwt and replaced with a PmlI-SexAI 675-bp fragment comprising the P. vivax VK210 CSP repeat region, which was codon harmonized to optimize protein synthesis and folding in the P. berghei transgenic host (19) and was excised from the engineered pPv. From the resulting intermediate plasmid, pIC-CSPv, the entire CSP gene was excised as a KpnI-XhoI fragment and inserted into the transfection plasmid, pR-CSPv. KpnI and SacI were used to linearize pR-CSPv prior to transfection; WT P. berghei (strain ANKA) parasites were transfected as previously described (20).
Chimeric parasites were selected in Swiss Webster mice (NCI, Frederick, MD) by treatment with pyrimethamine in drinking water (0.07 mg/ml). Parasites surviving drug treatment were then cloned by means of a limiting-dilution assay (21). Recombination at the 5′ and 3′ ends of the locus was verified by PCR. The primers used to verify integration at the 5′ end of the construct are 5′F (TCACCCTCAAGTTGGGTAAAA) and 5′R (CCTGTCCCCTGGTTGCTTA); the primers to verify integration at the 3′ end are 3′F (TGTAAAAATGTGTATGTTGTGTGC) and 3′R (GTGCCCATTACGACTTTGCT). To verify that the isolated clone did not have contaminating WT P. berghei parasites, a PCR product flanking the SexAI restriction site was digested with SexAI. The primers used for this PCR are PbCS-F (AAGAAAGCAGAAGATTTGACCTT) and PbCS-R (AAGGTCAAATCTTCTGCTTTCTT). Finally, DNA isolated from the cloned transgenic parasites was sequenced (Macrogen Inc., USA) to confirm the replacement of the P. berghei repeat region with the P. vivax repeat region for the generation of P. berghei-P. vivax chimeric parasites.
Mosquito infection and parasite development.
Anopheles stephensi mosquitoes were fed on Swiss Webster mice (NCI, Frederick, MD) infected with blood stages of cloned P. berghei-P. vivax CSRepeat (P. berghei-P. vivax) parasites. Development of viable male gametocytes in blood was qualitatively assessed by means of an exflagellation assay. Briefly, 1.5 μl of blood from a mouse with patent blood parasitemia was incubated at room temperature for 20 min with 1.5 μl of heparin (40 U/ml in 1× phosphate-buffered saline [PBS]) and 7 μl of complete ookinete medium (RPMI 1640 medium with 10% fetal calf serum, 25 mM HEPES, 2 mM glutamine, 0.2 mM hypoxanthine, 12 mM sodium bicarbonate, 1 μM xanthurenic acid). After incubation, the mixture was placed on a slide and observed under a light microscope (×100 magnification).
Development of oocysts in mosquito midguts was assessed 14 days post-blood meal. The midguts were dissected in sterile PBS and stained in a 0.1% mercury chromate solution.
Infectivity of P. berghei-P. vivax sporozoites.
The in vivo infectivity of chimeric P. berghei-P. vivax sporozoites was assessed in 5- to 8-week-old C57BL/6 mice both by assessing the liver parasite load, measured by quantitative PCR (qPCR) (22), in mice that were challenged intravenously (i.v.) with 1 × 104 P. berghei-P. vivax sporozoites and by the development of blood-stage parasites after feeding with 3 P. berghei-P. vivax-infected A. stephensi mosquitoes for 3 min.
Antibodies.
Development of 3D11, a monoclonal antibody directed against the repeat region of CSP of P. berghei (23), and 2F2, a monoclonal antibody directed against the repeat region of P. vivax CSP (24), has been previously described.
Polyclonal antibodies against P. vivax CSP were generated by immunizing New Zealand White rabbits with 25 μg VMP001 (16) in complete Freund's adjuvant, followed by 2 booster immunizations with 25 μg VMP001 in incomplete Freund's adjuvant. Serum obtained post-3rd immunization was pooled and used in the present study.
IFA.
An indirect immunofluorescence assay (IFA) was used to characterize P. berghei-P. vivax sporozoties using monoclonal antibodies. Briefly, 10 μl of a sporozoite suspension (4 × 105 to 6 × 105 sporozoites/ml) was air dried on poly-l-lysine-covered slides (Tekdon Inc., Myakka City, FL) and incubated for 30 min at room temperature with 1 μg/ml of 3D11 or 2F2 monoclonal antibody against the P. berghei (23) or the P. vivax (15, 24, 25) CSP repeat region, respectively. The slides were then washed with PBS-1% bovine serum albumin (BSA) and incubated for 30 min at room temperature with a secondary-antibody solution [Alexa Fluor 488 F(ab′)2 fragment of goat anti-mouse IgG(H+L); 2 mg/ml; Invitrogen]. Green-fluorescent sporozoites were visualized under an upright fluorescence microscope (Nikon Eclipse 90i).
Inhibition of sporozoite infectivity by passive transfer of monoclonal antibodies or polyclonal rabbit sera directed against P. vivax CSP.
Three hundred micrograms of 2F2 antibody specific for the repeat region of P. vivax (15) or 0.5 ml pooled sera from rabbits immunized with VMP001, a recombinant P. vivax CSP (16), were passively transferred into 5- to 8-week-old C57BL/6 mice i.v. immediately prior to sporozoite challenge. Following antibody transfer, the mice were challenged with 1 × 104 sporozoites delivered by tail vein injection into each mouse. Forty-two hours after challenge, livers were harvested to assess the parasite burden by qPCR. In some experiments, mice receiving monoclonal antibody were challenged by mosquito bites. For this purpose, 5- to 8-week-old C57BL/6 mice were anesthetized by intraperitoneal injection of 250 μl of 2% avertin, and then 400 μg of 2F2 antibody was passively transferred i.v. prior to feeding P. berghei-P. vivax-infected A. stephensi mosquitoes. For increased stringency, we allowed 4 mosquitoes to feed for 5 min and then visually examined them for a blood feed. The presence of sporozoites was then assessed in those mosquitoes that fed to ensure that mice were exposed to the bite of at least 1 infected mosquito.
Data analysis.
Most of the data were plotted using Prism 4 software (GraphPad). Unless otherwise stated, data were compared for significance using a Mann-Whitney test.
RESULTS
Generation and biological characterization of P. berghei-P. vivax chimeric parasites.
Using a plasmid containing the sequences of the P. berghei CSP gene and a modified version of the dihydrofolate reductase (DHFR) gene, which confers resistance to pyrimethamine on transfected parasites, we generated P. berghei-P. vivax chimeric parasites. Briefly, we replaced the repeat region of P. berghei CSP with the repeat domain of P. vivax CSP. The P. vivax replacement represents the VK210 CSP sequence of the P. vivax SalI strain, from nucleotides 268 to 825 (amino acids 90 to 275). P. berghei ANKA blood stages were transfected in vitro with a plasmid linearized using KpnI and XhoI restriction enzymes, as described previously (21) (Fig. 1A). The transfected parasites were injected intravenously into Swiss Webster mice, and transgenic parasites were selected under pyrimethamine treatment. Ten days later, drug-resistant parasites were recovered and cloned by limiting dilution.
Fig 1.
Generation of P. berghei-P. vivax (Pb-Pv) chimeric parasites. (A) Schematic representation of the approach used for replacing the CSP gene of P. berghei ANKA with the P. vivax SalI repeat region. The locations of the primers used to verify recombination by PCR are indicated at the bottom. The restriction sites shown are KpnI (K), PmlI (P), SexAI (Se), XhoI (Xh), and SacI (S). (B) Integration at the 5′ and 3′ ends of the replacement fragment was verified by PCR using genomic DNA from P. berghei-P. vivax parasites. Primers 5′F and Pb-Pv R yield a 1,020-bp product; primers 3′F and 3′R yield a 1,000-bp product. (C) To demonstrate that the parasite population was clonal, a 908-bp PCR fragment was amplified from the CSP gene using primers PbCS-F and PbCS-R. The PCR product was then digested with SexAI, which does not cut the WT CSP gene. Genomic DNA from P. berghei ANKA was used as a control. (D) Comparison of CSP amino acid sequences of P. berghei ANKA, P. berghei-P. vivax, and P. vivax strain Salvador I; the P. berghei-P. vivax amino acid sequence was derived from DNA sequencing of clonal transgenic parasites. (E) In vivo infectivity of P. berghei-P. vivax sporozoites compared to WT P. berghei ANKA sporozoites, both injected intravenously. ns, not significant; horizontal bars, geometric means.
PCR analysis was used to confirm recombination at the 5′ and 3′ ends for gene replacement (Fig. 1B), and the cloned parasites were verified by PCR-restriction fragment length polymorphism (RFLP) (Fig. 1C). The cloned parasites were sequenced to further confirm that the P. berghei-P. vivax parasites have the correct insertion in the CSP gene (Fig. 1D).
Mosquito infection.
A cloned P. berghei-P. vivax parasite isolate was used to infect mice, which were then used to feed A. stephensi mosquitoes. Transgenic parasites were evaluated by using standard methods, such as exflagellation and oocyst and sporozoite counts, to compare them to the wild-type P. berghei.
Exflagellation of male P. berghei-P. vivax gametocytes, assessed prior to feeding female A. stephensi mosquitoes on infected mice, was normal and comparable to that of WT P. berghei ANKA parasites (data not shown). Twelve to 14 days after mosquito feeding, P. berghei-P. vivax-infected mosquito midguts had approximately 100 to 150 healthy-looking oocysts per midgut, which was also comparable to WT P. berghei ANKA parasites. Finally, on day 21, mosquito salivary glands were dissected, and the parasite load was found to be comparable to that of WT P. berghei ANKA (Table 1).
Table 1.
Developmental characteristics and infectivity of P. berghei-P. vivax parasites in A. stephensi mosquitoesa
| Parasite | % of midgut infected | No. of oocysts/midgut | % of salivary gland infected | No. of sporozoites/salivary gland (103) | % of mice infected after mosquito bitesb |
|---|---|---|---|---|---|
| P. berghei-P. vivax | 80.72 | 54.76 | 75 | 8.5 | 100 |
| P. berghei ANKA | 75.14 | 42.25 | 66 | 10 | 100 |
Mean of 3 experiments, with at least 20 mosquitoes examined in each experiment.
Three infected mosquitoes were allowed to feed on C57BL/6 mice for 3 min. Blood-stage parasitemia was assessed 5 days later.
Characterization of P. berghei-P. vivax sporozoites with monoclonal antibodies.
Sporozoites obtained from salivary glands of P. berghei-P. vivax-infected mosquitoes were used for IFA with antibodies specific for the repeat domain of P. vivax (2F2) CSP. An antibody specific for the repeat region of P. berghei (3D11) served as a control. The IFA results clearly demonstrated that the chimeric P. berghei-P. vivax sporozoites were all uniformly positive for the anti-P vivax repeat antibodies and negative for the antibody against P. berghei repeats (Fig. 2).
Fig 2.
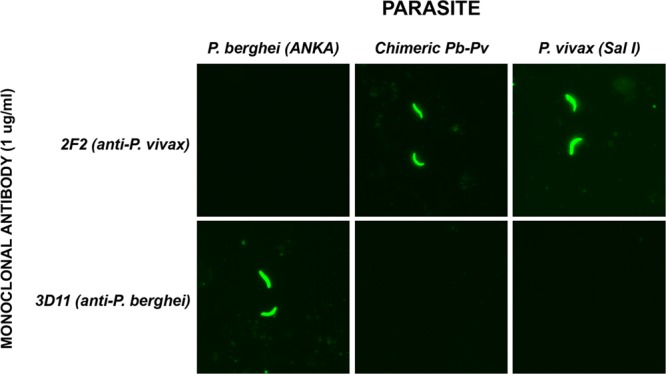
Characterization of P. berghei-P. vivax sporozoites using anti-repeat monoclonal antibodies. Monoclonal antibody 2F2, targeting the repeat region of P. vivax CSP VK210, specifically recognizes P. berghei-P. vivax chimeric sporozoites and P. vivax SalI sporozoites. No cross-reactivity was observed against WT P. berghei ANKA sporozoites. Monoclonal antibody 3D11, specific for the repeat region of P. berghei, reacts to wild-type P. berghei, but not to P. vivax or P. berghei-P. vivax chimeric sporozoites.
Infectivity of P. berghei-P. vivax chimeric parasites in mice.
To evaluate the in vivo fitness of the transgenic parasites, we challenged C57BL/6 mice with 1 × 104 sporozoites, delivered by i.v. injection, from either WT P. berghei ANKA or P. berghei-P. vivax parasites. Forty-two hours after infection, the parasite load in the liver was measured by qPCR specific for the P. berghei 18S rRNA. The data indicated that the infectivities of WT P. berghei ANKA and P. berghei-P. vivax sporozoites were comparable, and there was no statistically significant difference between the parasites.
The infectivity of the transgenic parasites was further confirmed by delivering the parasites via the natural route, i.e., using mosquito bites. Mice exposed to the bites of 3 infected mosquitoes demonstrated patent blood-stage infection by day 5 postchallenge.
Anti-P. vivax antibodies inhibit the development of parasite liver stages.
In order to evaluate the utility of the transgenic parasites as a tool to assess the efficacy of future vaccines, we tested the neutralization capabilities of anti-P. vivax antibodies in vivo. The anti-repeat monoclonal antibody 2F2 was passively transferred into mice immediately prior to an intravenous challenge with 1 × 104 chimeric P. berghei-P. vivax sporozoites. As seen in Fig. 3, mice that received 2F2 antibody showed a significant reduction in parasite load in the liver (99% reduction compared to naive controls). In contrast, the group that received the irrelevant antibody had a parasite load comparable to that of naive controls, confirming the specificity of the results observed with the anti-P. vivax MAb 2F2. To determine the effects of polyclonal antibodies that contain a mixture of specificities (as opposed to the repeat-specific monoclonal antibody 2F2), we evaluated the neutralization capability of rabbit polycolonal serum that was generated against a recombinant P. vivax CSP protein, VMP001. Rabbit anti-VMP001 antibodies recognized CSP on the surfaces of P. vivax sporozoites and reacted to the whole protein (VMP001), in addition to the N-terminal and C-terminal proteins, as well as a synthetic type 1 peptide. As shown in Fig. 3B, similar to MAb 2F2, polyclonal rabbit sera also reduced the liver parasite burden in comparison to mice that either were naive or received preimmune rabbit serum. When mice receiving 400 μg of monoclonal antibody 2F2 were exposed to the bites of 4 P. berghei-P. vivax-infected mosquitoes for 5 min, we did not observe sterile protection. However, control mice (n = 5) receiving an irrelevant monoclonal antibody developed parasitemia at day 4, while mice receiving anti-parasite monoclonal antibody (n = 5) became patent at day 5. Taken together, these results demonstrate that P. berghei-P. vivax sporozoites are highly infectious and a stringent model to evaluate immune responses that inhibit parasite development in the liver, as well as the acquisition of sterile immunity after immunization with vaccine candidates.
Fig 3.
Protection against P. berghei-P. vivax sporozoite challenge by passive transfer of monoclonal antibodies or hyperimmune sera. Following passive transfer of monoclonal antibodies or sera, mice were challenged intravenously with 1 × 104 P. berghei-P. vivax sporozoites. Forty hours after challenge, livers were harvested to determine parasite burdens by qPCR. Passive transfer of monoclonal antibody 2F2 (A) or rabbit polyclonal sera (B) had a significant protective effect against P. berghei-P. vivax sporozoites compared to mice that received irrelevant antibody (ab.) or preimmune sera or to naive controls. The horizontal bars show the geometrical means. *, P < 0.05; **, P < 0.01. The percent parasite burden inhibition in the liver is shown in parentheses. The results are representative of at least two independent experiments. Each group consisted of 4 or 5 mice.
DISCUSSION
Studies on assessing protective immune responses against malaria in general, and P. vivax in particular, are difficult due to the inability to culture the parasite and to the lack of a suitable animal model. Human studies are expensive, and therefore, this is not a practical first step to evaluate vaccine candidates. Over the last decade or so, transfection of Plasmodium has revolutionized the ways in which protective immune responses can be evaluated using transgenic rodent parasites that have been transfected with the gene encoding a specific human malaria antigen (14, 26–29). Active- and passive-immunization studies directed toward these antigens are more indicative of a biologically relevant immune response than the evaluation of immunogenicity alone. Transgenic parasites carrying the CSP, MSP, and P25 genes of P. falciparum have been developed and are being used to evaluate vaccine candidates and/or platforms (14, 26–29). To date, only one transgenic P. berghei sporozoite expressing P25 from P. vivax has been developed as a tool for evaluating the efficacy of vaccine candidates (29).
Here, we describe the successful development, characterization, and applicability of chimeric P. berghei parasites expressing the repeat region of P. vivax CSP. We successfully replaced the P. berghei repeat region with the VK210 repeat region of P. vivax. The resulting parasites produce viable gametocytes and successfully infect A. stephensi mosquitoes, in which their development is comparable to that of WT P. berghei. The in vivo infectivity of P. berghei-P. vivax sporozoites is comparable to that of WT P. berghei in C57BL/6 mice. Moreover, feeding of P. berghei-P. vivax-infected mosquitoes on naive C57BL/6 mice results in the development of patent blood-stage parasitemia. Following immunization, the true potential of a vaccine needs to be evaluated using a natural route of sporozoite inoculation, which allows the participation of all components of the immune response in protection.
We used the transgenic parasites to evaluate their applicability as a tool for assessing the efficacies of antibodies in protection. Passive transfer of monoclonal antibodies or hyperimmune sera into naive recipients conferred significant protection against P. berghei-P. vivax sporozoite challenge. Mice that received 2F2 antibody or hyperimmune rabbit sera both showed above 95% inhibition of the parasite burden in the liver compared to the naive controls, thus proving that the transgenic parasites can serve as an important tool in evaluating protective anti-repeat immune response. Given that sporozoites rapidly migrate through the circulatory system (30), the availability and affinity of circulating anti-sporozoite antibodies determine their protective capacity. While the monoclonal antibody 2F2 was able to decrease liver infection by 90% of the sporozoites—as shown by measurement of the parasite load in the liver—and delayed the prepatent period by 1 day, it does not appear to be efficient enough to confer sterile immunity. Further comparative studies will be necessary to define the dosage, avidity, and fine specificity of different monoclonal and polyclonal antibodies capable of conferring sterile immunity. The possibility that sterile immunity was not achieved due to generation of mutants or revertant parasites is unlikely; however, this is an issue worth considering for future studies. Taken together, these results demonstrate that the P. berghei-P. vivax parasites are robust and that a natural mosquito bite challenge can be used as a stringent test to assess the full potential of vaccine candidates. Passive-transfer experiments in mice using serum samples from volunteers immunized with novel vaccine candidates could potentially be used to assess humoral protective capacity against P. berghei-P. vivax sporozoites. Such studies should also be useful for determining more accurate correlates between antibody titers and protective efficacy against P. vivax infection.
Chimeric rodent malaria parasites are a simple, robust, and practical tool for the in vivo evaluation of vaccine candidates against malaria. This is the first report of the development of a rodent parasite that can be used to evaluate the efficacy of the circumsporozoite protein central repeat region of P. vivax. As the replacement of the entire CSP gene could result in parasites with reduced infectivity in both mice and mosquitoes (31), we chose to first develop chimeric parasites in which we replaced only the P. berghei CSP repeat domain with that of P. vivax, which is a well-defined target of protective antibody responses. The successful generation of parasites that are able to reproducibly infect mice opens up the road to develop additional transgenic parasites expressing the N-terminal and C-terminal regions and, if possible, the full-length CSP to evaluate T-cell-mediated immune-protective mechanisms.
Such parasites will help select future vaccine candidates for clinical development. Over the last decade, the utility of chimeric rodent parasites has been highlighted by an increasing number of studies that report their use for the assessment of novel protein constructs, recombinant viral vectors, and synthetic adjuvant formulations (32–37). The P. berghei-P. vivax parasites we have generated should also facilitate the appraisal of new vaccine candidates and represent a practical platform for evaluating CSP-based protective immune responses against P. vivax.
ACKNOWLEDGMENTS
F.Z. is supported by National Institutes of Health grant AI44375. We are grateful for the support of the Bloomberg Family Foundation.
The opinions or assertions contained herein are our private views and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
Published ahead of print 28 May 2013
REFERENCES
- 1. Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. 2007. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg. 77:79–87 [PMC free article] [PubMed] [Google Scholar]
- 2. Gething PW, Elyazar IRF, Moyes CM, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HFL, Price RN, Mueller I, Baird JK, Hay SI. 2012. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl. Trop. Dis. 6:e1814. 10.1371/journal.pntd.0001814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arévalo-Herrera M, Chitnis C, Herrera S. 2010. Current status of Plasmodium vivax vaccine. Hum. Vaccin. 6:124–132 [DOI] [PubMed] [Google Scholar]
- 4. Mendis K, Sina BJ, Marchesini P, Carter R. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97–106 [DOI] [PubMed] [Google Scholar]
- 5. Baird JK. 2007. Neglect of Plasmodium vivax. Trends Parasitol. 23:533–539 [DOI] [PubMed] [Google Scholar]
- 6. Baird JK. 2013. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin. Microbiol. Rev. 26:36–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carlton JM, Sina BJ, Adams JH. 2011. Why is Plasmodium vivax a neglected tropical disease? PLoS Negl. Trop. Dis. 5:e1160. 10.1371/journal.pntd.000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pigott DM, Atun R, Moyes CL, Hay SI, Gething PW. 2012. Funding for malaria control 2006–2010: a comprehensive global assessment. Malar. J. 11:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Battle KE, Gething PW, Elyazar IR, Moyes CL, Sinka ME, Howes RE, Guerra CA, Price RN, Baird KJ, Hay SI. 2012. The global public health significance of Plasmodium vivax. Adv. Parasitol. 80:1–111 [DOI] [PubMed] [Google Scholar]
- 10. Herrera S, Corradin G, Arévalo-Herrera M. 2007. An update on the search for a Plasmodium vivax vaccine. Trends Parasitol. 23:122–128 [DOI] [PubMed] [Google Scholar]
- 11. Tam JP, Clavijo P, Lu YA, Nussenzweig V, Nussenzweig R, Zavala F. 1990. Incorporation of T and B epitopes of the circumsporozoite protein in a chemically defined synthetic vaccine against malaria. J. Exp. Med. 171:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zavala F, Tam JP, Romero PJ, Ley V, Nussenzweig RS, Nussenzweig V. 1987. Synthetic peptide vaccine confers protection against murine malaria. J. Exp. Med. 66:1591–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, Juompan L, Williams J, Dowler M, Stewart VA, Wirtz RA, Dubois MC, Lievens M, Cohen J, Ballou WR, Heppner DG, Jr, RTS,S. Vaccine Evaluation Group 2009. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 200:337–346 [DOI] [PubMed] [Google Scholar]
- 14. Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, Nardin EH. 2002. Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J. Immunol. 169:6681–6685 [DOI] [PubMed] [Google Scholar]
- 15. Romero P, Heimer EP, Herrera S, Felix AM, Nussenzweig RS, Zavala F. 1987. Antigenic analysis of the repeat domain of the circumsporozoite protein of Plasmodium vivax. J. Immunol. 139:1679–1682 [PubMed] [Google Scholar]
- 16. Yadava A, Sattabongkot J, Washington MA, Ware LA, Majam V, Zheng H, Kumar S, Ockenhouse CF. 2007. A novel chimeric Plasmodium vivax circumsporozoite protein induces biologically functional antibodies that recognize both VK210 and VK247 sporozoites. Infect. Immun. 75:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Q, Fujioka H, Nussenzweig V. 2005. Exit of Plasmodium sporozoites from oocysts is an active process that involves the circumsporozoite protein. PLoS Pathog. 1:e9. 10.1371/journal.ppat.0010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cockburn IA, Tse S-W, Radtke AJ, Srinivasan P, Chen Sinnis Y-CP, Zavala F. 2011. Dendritic cells and hepatocytes use distinct pathways to process protective antigen from Plasmodium in vivo. PLoS Pathog. 7:e1001318. 10.1371/journal.ppat.1001318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Angov E, Hillier CJ, Kincaid RL, Lyon JA. 2008. Heterologous protein expression is enhanced by harmonizing the codon usage frequencies of the target gene with those of the expression host. PLoS One 3:e2189. 10.1371/journal.pone.0002189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janse CJ, Ramesar J, Waters AP. 2006. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat. Protoc. 1:346–356 [DOI] [PubMed] [Google Scholar]
- 21. Menard R, Janse CJ. 1997. Gene targeting in malaria parasites. Methods 13:148–157 [DOI] [PubMed] [Google Scholar]
- 22. Bruña-Romero O, Hafalla JC, González-Aseguinolaza G, Sano G, Tsuji M, Zavala F. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499–1502 [DOI] [PubMed] [Google Scholar]
- 23. Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. 1980. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 207:71–73 [DOI] [PubMed] [Google Scholar]
- 24. Nardin EH, Nussenzweig V, Nussenzweig RS, Collins WE, Harinasuta KT, Tapchaisri P, Chomcharn Y. 1982. Circumsporozoite protein of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J. Exp. Med. 156:20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zavala F, Cochrane AH, Nardin EH, Nussenzweig RS, Nussenzweig V. 1983. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J. Exp. Med. 157:1947–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao Y, Zhang D, Pan W. 2009. Construction of transgenic Plasmodium berghei as a model for evaluation of blood-stage vaccine candidate of Plasmodium falciparum chimeric protein 2.9. PLoS One 4:e6894. 10.1371/journal.pone.0006894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Koning-Ward TF, O'Donnell RA, Drew DR, Thomson R, Speed TP, Crabb BS. 2003. A new rodent model to assess blood stage immunity to the Plasmodium falciparum antigen merozoite surface protein 119 reveals a protective role for invasion inhibitory antibodies. J. Exp. Med. 198:869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mlambo G, Maciel J, Kumar N. 2008. Murine model for assessment of Plasmodium falciparum transmission-blocking vaccine using transgenic Plasmodium berghei parasites expressing the target antigen Pfs25. Infect. Immun. 76:2018–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramjanee S, Robertson JS, Franke-Fayard B, Sinha R, Waters AP, Janse CJ, Wu Y, Blagborough AM, Saul A, Sinden RE. 2007. The use of transgenic Plasmodium berghei expressing the Plasmodium vivax antigen P25 to determine the transmission-blocking activity of sera from malaria vaccine trials. Vaccine 25:886–894 [DOI] [PubMed] [Google Scholar]
- 30. Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, Menard R. 2006. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 12:220–224 [DOI] [PubMed] [Google Scholar]
- 31. Tewari K, Spaccapelo R, Bistoni F, Holder AA, Crisanti A. 2002. Function of region I and II adhesive motifs of Plasmodium falciparum circumsporozoite protein in sporozoite motility and infectivity. J. Biol. Chem. 277:47613–47618 [DOI] [PubMed] [Google Scholar]
- 32. Nardin EH, Oliveira GA, Calvo-Calle JM, Wetzel K, Maier C, Birkett AJ, Sarpotdar P, Corado ML, Thornton GB, Schmidt A. 2004. Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes. Infect. Immun. 72:6519–6527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calvo-Calle JM, Oliveira GA, Watta CO, Soverow J, Parra-Lopez C, Nardin EH. 2006. A linear peptide containing minimal T- and B-cell epitopes of Plasmodium falciparum circumsporozoite protein elicits protection against transgenic sporozoite challenge. Infect. Immun. 74:6929–6939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oliveira GA, Wetzel K, Calvo-Calle JM, Nussenzweig R, Schmidt A, Birkett A, Dubovsky F, Tierney E, Gleiter CH, Boehmer G, Luty AJ, Ramharter M, Thornton GB, Kremsner PG, Nardin EH. 2005. Safety and enhanced immunogenicity of a hepatitis B core particle Plasmodium falciparum malaria vaccine formulated in adjuvant Montanide ISA 720 in a phase I trial. Infect. Immun. 73:3587–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumar KA, Oliveira GA, Edelman R, Nardin EH, Nussenzweig V. 2004. Quantitative Plasmodium sporozoite neutralization assay (TSNA). J. Immunol. Methods 292:157–164 [DOI] [PubMed] [Google Scholar]
- 36. Palma C, Overstreet MG, Guedon JM, Hoiczyk E, Ward C, Karen KA, Zavala F, Ketner G. 2011. Adenovirus particles that display the Plasmodium falciparum circumsporozoite protein NANP repeat induce sporozoite-neutralizing antibodies in mice. Vaccine 29:1683–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kastenmüller K, Espinosa DA, Trager L, Stoyanov C, Salazar AM, Pokalwar S, Singh S, Dutta S, Ockenhouse CF, Zavala F, Seder RA. 2013. Full-length Plasmodium falciparum circumsporozoite protein administered with long-chain poly(I-C) or the Toll-like receptor 4 agonist glucopyranosyl lipid adjuvant-stable emulsion elicits potent antibody and CD4+ T cell immunity and protection in mice. Infect. Immun. 81:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]