Abstract
Borrelia burgdorferi, the bacterium that causes Lyme disease, has a unique segmented genome consisting of numerous linear and circular plasmids and a linear chromosome. Many of these genetic elements have been found to encode factors critical for B. burgdorferi to complete the infectious cycle. However, several plasmids remain poorly characterized, and their roles during infection with B. burgdorferi have not been elucidated. To more fully characterize the role of one of the four 28-kb linear plasmids, lp28-3, we generated strains specifically lacking lp28-3 and assayed the contribution of genes carried by lp28-3 to B. burgdorferi infection. We found that lp28-3 does not carry any genes that are strictly required for infection of a mouse or tick and that lp28-3-deficient spirochetes are competent at causing a disseminated infection. Interestingly, spirochetes containing lp28-3 were at a selective advantage compared to lp28-3-deficient spirochetes when coinjected into a mouse, and this advantage was reflected in the population of spirochetes acquired by feeding ticks. Our data demonstrate that genes carried by lp28-3, although not essential, contribute to the fitness of B. burgdorferi during infection.
INTRODUCTION
Lyme disease is the most common tick-transmitted disease in the United States and is caused by infection with the pathogen Borrelia burgdorferi (1–3). This bacterium survives in a complex enzootic cycle transmitted by Ixodes ticks and infects a wide range of vertebrate hosts (4–8). B. burgdorferi must adapt to markedly different environments within the arthropod vector and mammalian host to ensure successful colonization, persistence, and transmission throughout the natural infectious cycle.
The unique genome of the B. burgdorferi type strain B31 consists of a small 0.9-Mbp linear chromosome and a collection of 12 linear and 9 circular plasmids (9–12). Together, the plasmids comprise 0.533 Mbp and range in size from 5 kb to 56 kb (13–16). With the genetic tools now available to manipulate B. burgdorferi, a number of chromosomal and plasmid genes have been identified that encode factors required by this spirochete at particular stages of the infectious cycle.
Plasmid-borne genes in B. burgdorferi have been shown to encode proteins required for viability, virulence, and fundamental metabolic processes (see reviews in references 17, 18, 19, 20, and 21). However, identification of such genes in B. burgdorferi by means of sequence homology alone has been difficult. Cumulatively, the strain B31 plasmids encode 706 putative proteins, 58% of which have no database match and 26% of which are conserved hypothetical proteins. In contrast, the B31 chromosome has 815 predicted open reading frames (ORFs), with only 29% lacking a database match and 12% annotated as conserved hypothetical proteins (14, 22). Although a growing number of plasmid-borne genes have defined roles in the infectious cycle, most remain poorly characterized.
Several B. burgdorferi plasmids have been shown to be required for or to contribute to survival in the tick vector or mammalian host. One of the four 28-kb linear plasmids of strain B31, designated lp28-3, has not been fully investigated for its role in the B. burgdorferi life cycle. Analysis of the nucleotide sequence of lp28-3 indicates that this plasmid is highly conserved except at the very distal ends (22), and the predicted ORFs encode mostly hypothetical or conserved hypothetical proteins with no known function (14, 22). Interestingly, some of the annotated protein-coding sequences with predicted functions are suggestive of a role in the infectious cycle. These include cspZ, encoding complement regulator-acquiring surface protein 2 (CRASP2), a factor H-binding protein produced during mammalian infection and thought to play a role in the resistance of B. burgdorferi to complement-mediated killing (23–25), although it is dispensable in a mouse infection model (26–28). In addition, lp28-3 genes bbh13, bbh15, bbh16 and bbh18 encode hypothetical lipoproteins, and bbh12 and bbh14, while not homologous to genes encoding proteins of known function, are differentially regulated in response to conditions mimicking those found in a mammal relative to those found in a tick (29–32).
In this study, we directly investigate the role of lp28-3 in the B. burgdorferi infectious cycle. We characterized lp28-3-deficient (lp28-3−) strains obtained by two independent methods and analyze these B. burgdorferi variants during in vitro growth and the entire mouse-tick infectious cycle. Our data suggest that although lp28-3 is not strictly required for completion of the infectious cycle, lp28-3 confers a selective advantage to spirochetes harboring the plasmid during mammalian infection.
MATERIALS AND METHODS
B. burgdorferi strains and growth conditions.
All B. burgdorferi strains were grown in Barbour-Stoenner-Kelly II (BSKII) medium as previously described (33) and plated in solid BSK medium. Wild-type infectious B. burgdorferi clone B31-A3 (termed A3) was previously isolated and described (34). Clone B31-A3-C33 (termed C33), which lacks lp28-3, is a derivative of a B31-A3 mouse isolate. C33 was identified by a PCR screen of individual colonies using primers specific for lp28-3 (primers 9 and 10 in Table S1 in the supplemental material). Additional B31-A3 derivatives lacking lp28-3 were obtained by selectively displacing lp28-3 with an incompatible shuttle vector, as described below. One such clone, termed C12, was used in subsequent experiments. The complete plasmid contents of clones C33 and C12 were determined as previously described (35) and found to be identical to that of B31-A3, other than the loss of lp28-3.
Construction of pBSV28-3G.
The genes putatively responsible for replication and partitioning of lp28-3, bbh26, bbh27, bbh28, and bbh29, were amplified from A3 genomic DNA by PCR using Phusion high-fidelity polymerase and primers 1 and 2 (see Table S1 in the supplemental material). The PCR product was purified using a PCR purification kit (Qiagen, Valencia, CA) and digested with the restriction enzyme NheI. Plasmid pOG1 (36) was digested with XbaI and SpeI and subsequently purified using a PCR purification kit (Qiagen). The 3.6-kb fragment containing bbh26, bbh27, bbh28, and bbh29 was ligated with digested pOG1 using T4 DNA ligase, creating pBSV28-3G (Fig. 1). The nucleotide sequence of the region of pBSV28-3G containing bbh26, bbh27, bbh28, and bbh29 was confirmed by DNA sequencing. All enzymes used were from New England Biolabs (Ipswich, MA) unless otherwise noted.
Fig 1.
Schematic diagram depicting the construction of pBSV28-3G. The relevant restriction enzyme recognition sites used for cloning have been indicated, as well as the paralogous gene family designations for bbh26, bbh27, bbh28, and bbh29 in boxes above the genes. The family 62, 50, 32, and 49 genes are responsible for the replication and partitioning functions required for plasmid maintenance in B. burgdorferi (11).
Transformation of A3 with pBSV28-3G.
To enhance the transformation efficiency of infectious A3 with pBSV28-3G, the shuttle vector was first methylated in vitro using Mss1 methyltransferase (Zymo Research), as described previously (37, 38). Strain A3 was grown to 5 × 107 spirochetes/ml and transformed by electroporation with 10 μg of methylated pBSV28-3G (34, 39). Electroporated B. burgdorferi spirochetes were immediately resuspended in 5 ml of BSKII medium and allowed to recover for 24 h before being plated in solid BSK medium containing 40 μg/ml gentamicin. The resulting gentamicin-resistant colonies were screened by PCR for the aacC1 gene, using primers 3 and 4 (see Table S1 in the supplemental material), to identify A3 transformants carrying pBSV28-3G, and positive colonies were picked for subsequent analyses.
Southern blot analysis.
B. burgdorferi strains A3 and C33 and A3 transformants carrying pBSV28-3G were grown in BSKII to the late exponential phase, and DNA was isolated using a total genomic DNA purification kit (Qiagen). DNA was resolved by field-inversion electrophoresis on a 0.6% agarose gel for 24 h at 150 V, using a PPI-200 power inverter (program 2), as set by the manufacturer (MJ Research, Hercules, CA). Agarose gels were depurinated and denatured, and the genomic DNA was transferred to a nylon membrane (GE Biosciences, Piscataway, NJ) by capillary transfer and UV cross-linked to the nylon membrane using a StrataLinker 1800 (StrataGene, Indianapolis, IN). Probes for Southern blots were generated by PCR using primers 3 and 4 and 12 and 13 (see Table S1 in the supplemental material), for aacC1 and bbh17, respectively, with a digoxigenin (DIG) PCR labeling kit (Roche, Indianapolis, IN). Membranes containing genomic DNA were incubated with denatured probes and washed as previously described (40). Probe hybridization was detected using a DIG luminescent detection kit (Roche) and X-ray film.
Experimental mouse-tick infectious cycle.
Mouse infection studies were carried out in accordance with guidelines of the National Institutes of Health. All animal work was done according to protocols approved by the Rocky Mountain Laboratories Animal Care and Use Committee. The Rocky Mountain Laboratories are accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Studies were done with 6- to 8-week-old female RML mice, an outbred strain of Swiss-Webster mice reared at the Rocky Mountain Laboratories breeding facility. The B. burgdorferi inoculum was enumerated using a Petroff-Hausser chamber. A portion of the inoculum was also plated in solid BSK medium, and CFU were subsequently determined to confirm the number of viable spirochetes injected. Spirochetes were injected intraperitoneally (i.p.) (4 × 103 spirochetes) and subcutaneously (s.c.) (1 × 103 spirochetes), and mice were bled 3 weeks postinoculation to assess seroreactivity to B. burgdorferi cell lysates. Approximately 100 Ixodes scapularis larvae were fed to repletion on mice infected with wild-type B. burgdorferi strain A3 or the lp28-3− strain C33. A subset of fed larvae were mechanically disrupted and plated in solid BSK medium 8 to 10 days postfeeding to assess the acquisition of B. burgdorferi from infected mice and to enumerate spirochetes. Mice were euthanized 4 weeks postinoculation, and the ear, bladder, and ankle joint were harvested and incubated in BSKII medium. The presence of spirochetes in cultures derived from these organs was assessed by dark-field microscopy. The remaining I. scapularis larvae were allowed to molt to nymphs, and 3 ticks per mouse were fed on naive RML mice to assess persistence of the B. burgdorferi clones in ticks and transmission to naive mice. Fed nymphs were mechanically disrupted 10 days postfeeding, and dilutions of the homogenate were plated in BSK medium to determine the number of spirochetes present in fed ticks. Mice fed upon by infected nymphs were analyzed 3 weeks after tick feeding for seroreactivity to B. burgdorferi whole-cell lysates, and the ear, bladder, and ankle joints were harvested and incubated in BSKII to assess the presence of spirochetes in those tissues.
In vitro growth rate analysis and CI evaluation.
To compare the growth rate of the lp28-3-deficient C12 and C33 clones to that of the wild-type A3 strain, spirochetes were grown to ∼1 × 107 spirochetes/ml and then diluted in triplicate to a culture density of 2 × 105 spirochetes/ml in 7 ml of BSKII medium. Culture densities were monitored every 24 h by dark-field microscopy using a Petroff-Hausser chamber. A portion of the mixed cultures containing A3 and C12 or A3 and C33 were plated in solid BSK medium, and the resulting colonies were screened using lp28-3-specific primers 9 and 10 (see Table S1 in the supplemental material) to determine the ratio of A3 to lp28-3-deficient spirochetes when mixed and grown in vitro.
To determine the competitive indices (CIs) of strains lacking lp28-3, we conducted mouse infection studies as described above with minor modifications. Initially, we coinjected wild-type A3 and lp28-3− (C12) spirochetes intraperitoneally (i.p.) and subcutaneously (s.c.) at a ratio of 1:1, with a combined inoculum of 9.2 × 103 spirochetes, as confirmed by CFU. In a second experiment, we coinjected wild-type A3 and lp28-3− (C33) spirochetes with a combined inoculum of 1.3 × 104 spirochetes and experimentally confirmed the inoculum ratio of A3 to C33 as 1.25:1 by colony PCR with lp28-3-specific primers. Seroreactivity and isolation of spirochetes from mouse tissues were performed as described above, with minor modifications; ear punch biopsy specimens were taken at the 2- and 4-week time points in the second experiment, to attempt spirochete isolation, and the mice were euthanized at either 4 weeks or 8 weeks postinoculation (CI experiments 1 and 2, respectively), at which point the ear, bladder, and tibiotarsal joint tissues were incubated in BSKII medium to attempt isolation of spirochetes from these tissues. Spirochete isolates were plated in solid BSK medium when the density of the outgrowth cultures reached ∼5.0 × 107/ml. To determine spirochete acquisition by ticks, cohorts of I. scapularis larvae were fed upon mice 4 weeks after coinjection, in the second competitive index experiment. Ten days after drop-off, a subset of these ticks was crushed and the homogenate plated as described above. The relative proportion of lp28-3-deficient to wild-type B. burgdorferi was determined by PCR screening of the resulting colonies using lp28-3-specific primers 9 and 10 (see Table S1 in the supplemental material). The numbers of colonies screened per tissue isolate are listed in Tables S2 and S3 in the supplemental material. Wild-type and lp28-3-deficient colonies were included with each set of PCRs as positive and negative controls. The CIs of clones lacking lp28-3 relative to wild-type B. burgdorferi were calculated as previously described (41–43). In our experimental design, the CI is the change in the ratio of 28-3-deficient spirochetes relative to wild-type spirochetes from the point of inoculation to spirochete isolation following infection.
Quantitative analysis of spirochete burden in tissues by qPCR.
Spirochete burden in tissues was determined as previously described (44). Briefly, ear tissues were harvested at 4 or 8 weeks postinoculation and stored at −80°C until processed. Tissues were digested with collagenase A (Roche) and proteinase K (Life Technologies) solutions, and DNA was extracted using a series of phenol-chloroform and chloroform extractions, followed by ethanol precipitation and a cleanup step using a QIAquick column (Qiagen). Equivalent amounts of DNA were used in each quantitative PCR (qPCR) with primer-probe sets specific for the B. burgdorferi chromosomal flaB gene or the mouse nid gene (see Table S1 in the supplemental material). Reactions were performed in triplicate, and the mean values were plotted using PRISM software and analyzed using a Student two-tailed t test. To determine the relative proportion of lp28-3-deficient spirochetes to wild-type spirochetes in coinfected mice, infected tissues were treated as described above with minor modifications. flaB- and bbh35-specific primers and probes were used to quantitate the number of copies of the chromosome and lp28-3, respectively, based on standard curves generated from A3 genomic DNA (gDNA). We then calculated the ratio of flaB to bbh35 gene copies in mouse tissues infected with wild-type or lp28-3-deficient spirochetes or mice that had been coinoculated with both strains.
Determination of ID50.
The dose required to infect 50% of the mice inoculated (50% infective dose [ID50]) was determined for strains A3 and C12. Groups of 5 mice were inoculated with 10-fold-increasing doses of spirochetes from 1 × 102 to 1 × 105 for A3 and from 1 × 102 to 1 × 106 for C12. The inoculum was confirmed by plating a fraction of the inoculum in BSK and enumerating viable spirochetes. Mouse infection status was assessed as described above. The ID50 value for each clone was determined as previously described (45), and the two dose-response curves generated were analyzed using a Student's paired t test and logit analysis.
RESULTS
Construction of pBSV28-3G.
Genes encoding factors critical for Borrelia burgdorferi survival throughout the infectious cycle have been identified on the chromosome as well as on several plasmids (see reviews in references 17, 18, 19, 20, and 21). However, the role of the 28-kb linear plasmid lp28-3 has not been fully examined. We sought to determine if any genes carried by lp28-3 provide a critical function for B. burgdorferi in mice or ticks by isolating clones derived from an infectious wild-type strain that have lost only lp28-3. Such strains allowed analysis of the discrete contribution of lp28-3 to the phenotype of B. burgdorferi in vivo.
Shuttle vectors harboring the genes required for autonomous replication of an endogenous B. burgdorferi plasmid can selectively displace the cognate plasmid when stably introduced into B. burgdorferi (36, 46–48). In order to use this strategy to isolate a B. burgdorferi clone lacking lp28-3 (lp28-3−), we constructed a shuttle vector containing the genes putatively responsible for the autonomous replication of lp28-3. These genes, bbh26, bbh27, bbh28, and bbh29, which belong to the paralogous gene families 62, 50, 32, and 49, respectively, were amplified by PCR using primers 1 and 2 (see Table S1 in the supplemental material) and cloned into pOG1, an Escherichia coli plasmid containing a gentamicin resistance cassette that confers resistance in both E. coli and B. burgdorferi (36, 49), thus yielding the shuttle vector pBSV28-3G (Fig. 1).
Isolation and confirmation of B. burgdorferi clones specifically lacking lp28-3.
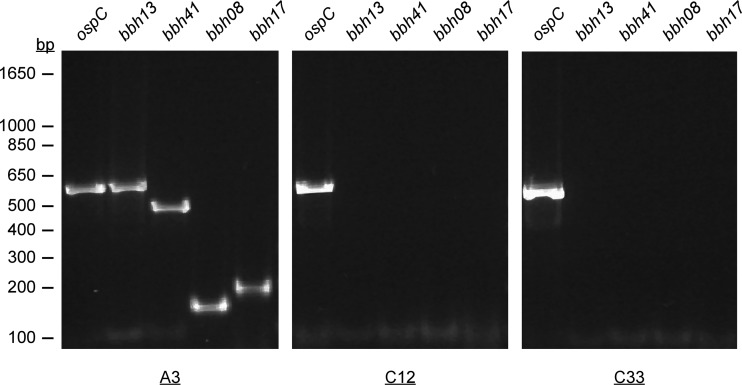
To attempt displacement of the endogenous lp28-3 plasmid from B. burgdorferi and thereby generate lp28-3-deficient clones, we transformed the low-passage-number infectious clone A3 with pBSV28-3G. Transformants were selected in solid BSK medium containing gentamicin, and individual colonies were analyzed by PCR for the presence of aacC1, encoding gentamicin resistance, located on pBSV28-3G. We also screened for distal portions of lp28-3 by assaying with PCR primers specific for lp28-3 genes bbh08, bbh13, bbh17, and bbh41 (see Table S1 in the supplemental material). Using this approach, we confirmed the presence of pBSV28-3G and were unable to detect any lp28-3 genes other than bbh26, bbh27, bbh27, and bbh29 in all A3/pBSV28-3G transformants analyzed. PCR amplification data are shown for one such derived clone, C12, which was used in subsequent infection studies (Fig. 2). Plasmid content analysis of total genomic DNA isolated from several A3/pBSV28-3G transformants demonstrated that all A3 plasmids (excluding lp28-3) had been retained; transformation of E. coli with Borrelia plasmid DNA allowed rescue of pBSV28-3G from these clones (data not shown). These results demonstrate that the pBSV28-3G shuttle vector is capable of autonomous replication in B. burgdorferi and can selectively displace lp28-3 from A3.
Fig 2.
PCR screen confirming the absence of lp28-3 in C12 and C33. Shown are results from screening of genomic DNA isolated from A3, C12, and C33 for lp28-3 genes bbh13, bbh41, bbh08, and bbh17 using primer sets 5 and 6, 7 and 8, 14 and 15, and 12 and 13, respectively (see Table S1 in the supplemental material). The ospC locus on cp26 was included as a positive control using primers 16 and 17 (see Table S1), and the relative migrations of size standards are indicated in bp on the left.
B. burgdorferi plasmids are frequently lost during growth in vitro, necessitating a careful analysis of the plasmid contents of strains to ensure that a full complement of plasmids is maintained (34, 35, 50–52). We used spontaneous plasmid loss as a second method to independently isolate clones specifically lacking lp28-3. To this end, we isolated spirochetes from a mouse infected with A3, grew the isolate to the late log phase, and plated dilutions into solid BSK medium. We then conducted a PCR-based screen (primers 9 and 10; see Table S1 in the supplemental material) to identify lp28-3-deficient clones. We screened 66 colonies and isolated only one clone, C33, which lacked lp28-3 but retained the remainder of the A3 plasmids. We were unable to amplify lp28-3-specific genes bbh08, bbh13, bbh17, and bbh41, indicating that C33 had spontaneously lost lp28-3 (Fig. 2).
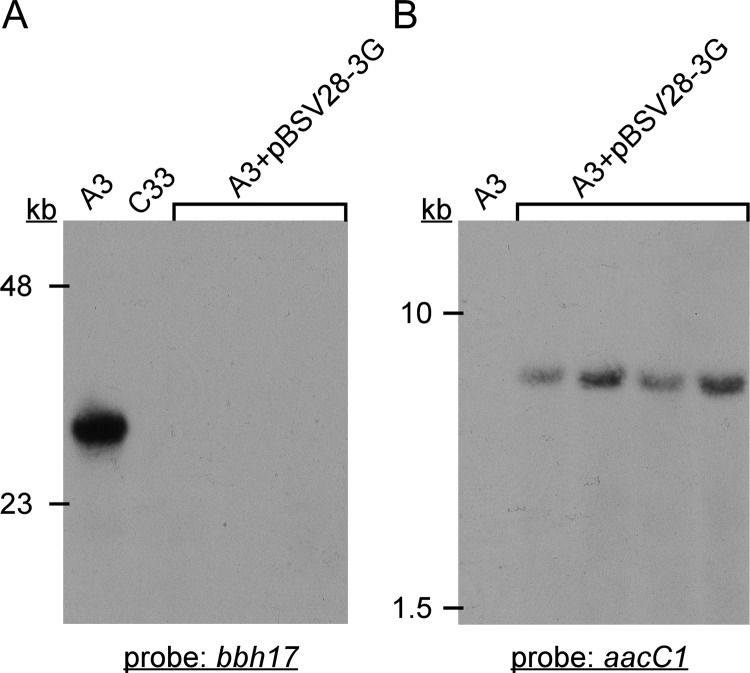
To further characterize the C33 and A3/pBSV28-3G clones (including C12), total genomic DNA was subjected to Southern blot analysis with probes specific for the bbh17 and aacC1 loci on lp28-3 and pBSV28-3G, respectively. As expected, the bbh17 probe hybridized to a band of ∼28 kb in wild-type A3 genomic DNA, whereas no hybridization was detected with C33 or any A3/pBSV28-3G transformants (Fig. 3A). Conversely, the aacC1 probe hybridized to a band present only in A3/pBSV28-3G transformants, consistent with the size of the supercoiled shuttle vector plasmid (Fig. 3B). These data confirmed and extended the results of the PCR screens (Fig. 2) and demonstrate displacement of lp28-3 by autonomously replicating pBSV28-3G in A3 transformants and spontaneous loss of lp28-3 in clone C33.
Fig 3.
Southern blot analysis of genomic DNA isolated from clones used in this study. Probes specific for bbh17 (A) or aacC1 (B) were used to determine the presence of lp28-3 or pBSV28-3G, respectively. Genomic DNA in lanes labeled “A3+pBSV28-3G” are from individual transformant clones, including C12. Lanes containing genomic DNA from wild-type A3 and lp28-3-deficient strain C33 are also indicated. Relative mobilities of size standards in kilobases (kb) are indicated on the left.
Phenotypic characterization of strains lacking lp28-3.
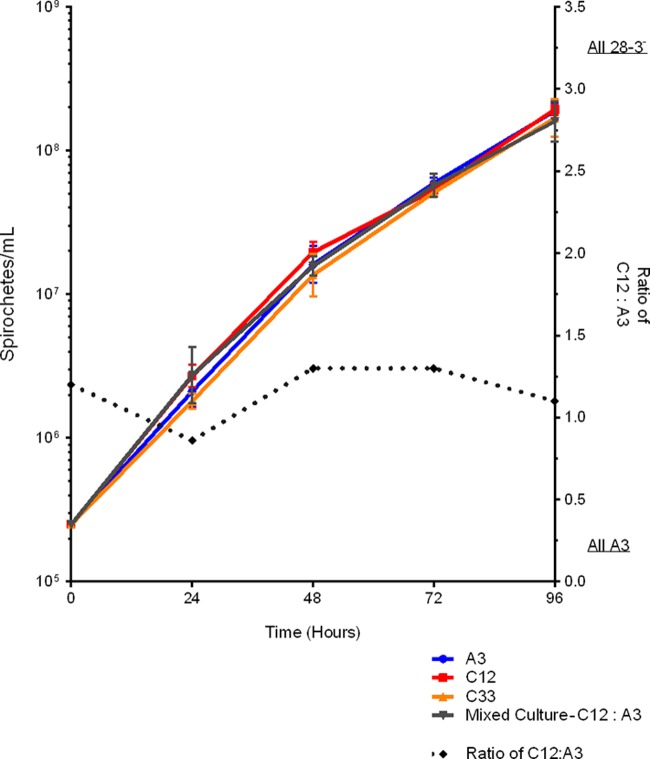
Having established that two independently derived clones, the A3 transformant C12 carrying pBSV28-3G and clone C33, lacked lp28-3, we sought to identify any phenotypic differences from the progenitor A3 wild-type clone. We analyzed the growth of all strains in BSKII medium at 35°C and were unable to detect any differences in growth rates between the wild-type and lp28-3-deficient clones (Fig. 4). We also analyzed the growth rates of mixed cultures containing A3 and C12 or C33. We found that when grown together, the wild-type and lp28-3-deficient strains grew at similar rates and maintained approximately a 1:1 ratio throughout all growth stages (Fig. 4 and data not shown). Whole-cell lysates prepared from these strains exhibited comparable protein profiles when separated by SDS-PAGE and visualized by Coomassie staining or immunoblot analysis using pooled sera from B. burgdorferi-infected mice (see Fig. S1 in the supplemental material). Finally, wild-type and lp28-3-deficient clones had similar plating efficiencies, ranging between 80 and 100% for both wild-type and mutant colony morphologies in solid media and spirochetal shape and motility when analyzed by dark-field microscopy (data not shown). We conclude that there is no obvious phenotypic change during in vitro growth associated with the loss of lp28-3.
Fig 4.
Analysis of the in vitro growth rates of B. burgdorferi strains in BSKII medium at 35°C. Strains were grown to the mid-exponential phase and diluted in triplicate to 2.5 × 105 spirochetes/ml. Spirochetes were enumerated by dark-field microscopy, and the mean and standard deviation are displayed as calculated by PRISM software. Spirochetes from the mixed culture (C12 plus A3) were plated and screened using lp28-3-specific primers. The ratio of lp28-3-deficient to wild-type spirochetes grown in the mixed culture remained constant throughout all growth phases and is plotted on the right y axis.
Characterization of lp28-3-deficient B. burgdorferi in an experimental infectious cycle.
B. burgdorferi persists in nature by colonizing and transiting between the disparate environments of its tick vector and small vertebrate hosts. Plasmid-encoded factors have been shown to be essential for survival at distinct stages of this cycle. To assess the contribution of lp28-3 to survival and replication in the vector and the host, we evaluated the competency of B. burgdorferi clones lacking lp28-3 in a complete experimental infectious cycle. We conducted three individual experiments in which groups of mice were challenged with wild-type A3 or lp28-3-deficient clones C12 or C33 by needle inoculation with a target inoculum of 5 × 103 spirochetes per mouse. Typically, the infection status of mice was assessed 3 weeks later by seroreactivity to B. burgdorferi cell lysates by immunoblotting and subsequently confirmed by attempted isolation of spirochetes from several tissues (cumulative data in Table 1). In our first experiment, we found that all 3 mice inoculated with A3 became infected, while 1 of 3 and 2 of 3 mice inoculated with C12 or C33, respectively, became infected. Interestingly, the lp28-3-deficient clones C12 and C33 did not appear to infect at wild-type levels, but the number of mice used in this experiment was small (n = 3). To further investigate the ability of spirochetes lacking lp28-3 to infect mice, we inoculated another set of mice with A3 or C12 spirochetes and assessed the infection status as previously described. We found that 4 out of 5 mice inoculated with A3 became infected and 3 out of 5 mice inoculated with C12 became infected (cumulative data summarized in Table 1). In a third experiment, we analyzed the ability of lp28-3-deficient spirochetes to persist in a mouse to an extended time point. We needle inoculated 3 sets of 5 mice with A3, C12, or C33 and attempted spirochete isolation from the ear, bladder, and tibiotarsal joint tissues 8 weeks postinoculation. We found that all of the mice inoculated with either A3 or C12 and two of the five mice inoculated with C33 became infected, and the spirochetes persisted in a mouse to this later time point (cumulative data summarized in Table 1). Thus, all strains established a disseminated infection in mice that persisted following development of the acquired immune response. Having observed that lp28-3-deficient spirochetes appeared slightly attenuated, we next determined the infectious dose of lp28-3-deficient spirochetes required to infect 50% of the mice inoculated (ID50). Sets of 5 mice were needle inoculated with A3 or C12 spirochetes over a range from 1 × 102 to 1 × 106 spirochetes per mouse, and the ID50 was calculated as previously described (45). The ID50 was determined as 1.44 × 102 versus 2.40 × 102 spirochetes for A3 and C12, respectively (see Table S4 in the supplemental material). Although the ID50 of C12 is slightly increased compared to that of the wild type, the difference is not significant when the dose-response curves are analyzed using Student's paired t test or logit analysis. Taken together, these data suggest that lp28-3 does not encode any factors essential to the spirochete's ability to establish infection, disseminate, or persist during the mammalian host phase of the infectious cycle. Furthermore, the loss of lp28-3 does not lead to a significant increase in the infectious dose of spirochetes required for infection by needle inoculation.
Table 1.
Infection of mice with lp28-3-deficient strains by needle inoculation or tick transmission
| B. burgdorferi straina | Route of infectionb | No. of mice seropositive/totalc | No. of infected mice/total inoculatedd |
|---|---|---|---|
| A3 | i.p./s.c. | 12/13 | 12/13 |
| C12 | i.p./s.c. | 9/13 | 9/13 |
| C33 | i.p./s.c. | 4/8 | 4/8 |
| A3 | Tick bite | 3/3 | 3/3 |
| C33 | Tick bite | 2/3 | 2/3 |
A3, wild type; C12 and C33, lp28-3 deficient.
i.p./s.c., intraperitoneal and subcutaneous injection with a target inoculum of 5 × 103 spirochetes per mouse. For the tick bite route, three naturally infected nymphs were fed to repletion on each mouse.
Seroconversion was assessed by immunoblot analysis with B. burgdorferi lysates.
Infection status was determined 4 or 8 weeks postinoculation by the ability to isolate spirochetes from the ear, bladder, and tibiotarsal joint tissues; isolates were recovered from all tissues tested from infected mice.
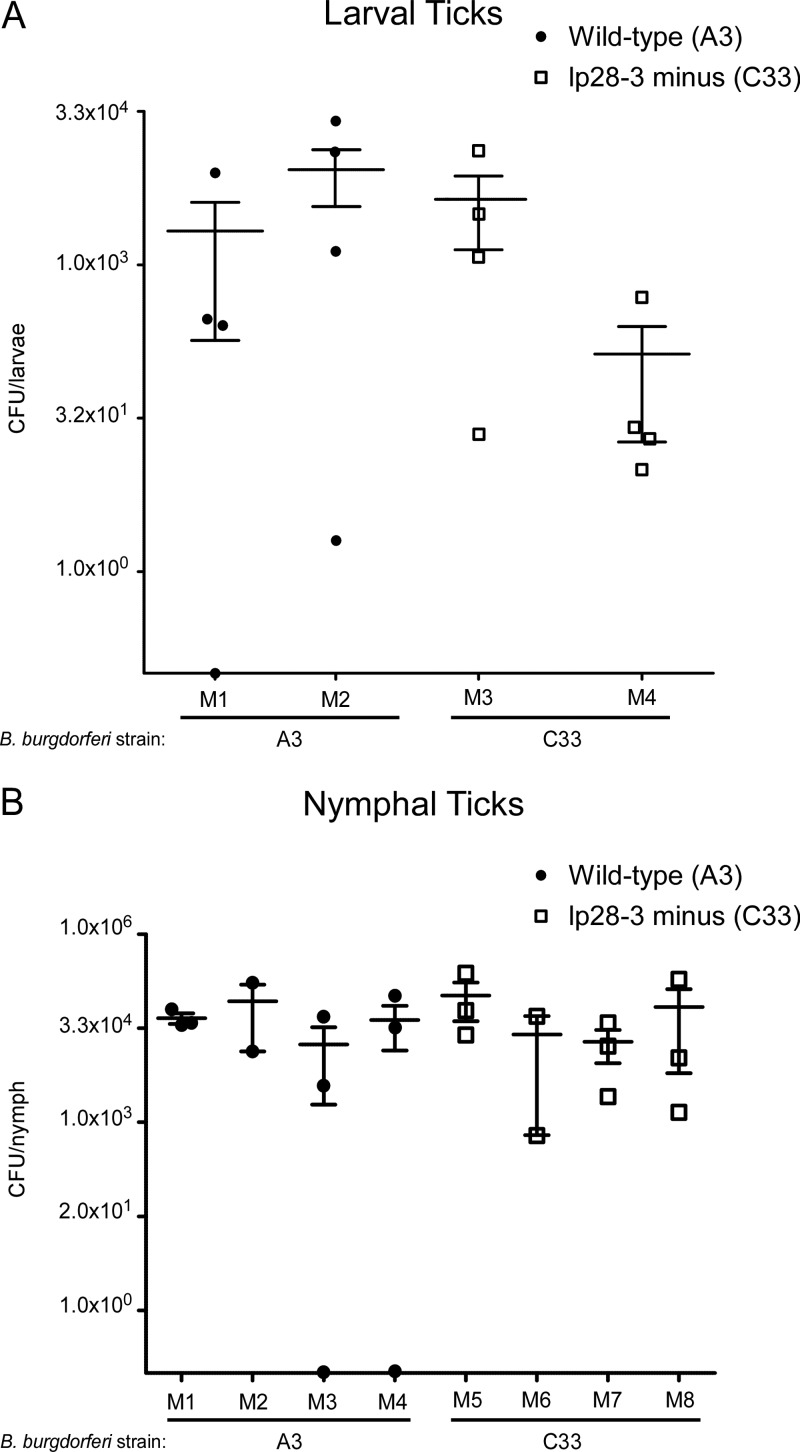
We next determined if any factors encoded by lp28-3 were required for acquisition of spirochetes by feeding ticks or for survival within the tick vector. To this end, a cohort of Ixodes scapularis larvae was fed to repletion upon seropositive mice infected with A3 or C33, described above. Spirochetes were enumerated from 4 fed larvae per infected mouse 10 days after feeding by plating disrupted tick material in solid medium (Fig. 5A). Analysis of the mean number of spirochetes present in fed larval ticks indicated no significant difference, using a Student's unpaired two-tailed t test, between ticks infected with either wild-type or lp28-3-deficient B. burgdorferi (Fig. 5A and data not shown). In addition, comparable numbers of infected larval ticks harboring wild-type or lp28-3-deficient spirochetes were recovered, suggesting that there is no defect in the ability of ticks to acquire these spirochetes from an infected mouse.
Fig 5.
Number of viable spirochetes in fed I. scapularis ticks as determined by colony formation. (A) Spirochete load in individual I. scapularis larvae 10 days after feeding on mice infected with wild-type B. burgdorferi A3 (closed symbols) or C33 (lp28-3-deficient [open symbols]). The designations M1, M2, M3, and M4 along the x axis represent the individual infected mice that were fed on by larval I. scapularis. The mean and standard deviation are shown as horizontal lines. (B) Spirochete load in individual I. scapularis nymphs infected with wild-type B. burgdorferi A3 (closed symbols) or C33 (lp28-3 deficient [open symbols]) 10 days postfeeding on naive mice. The designations M1 through M8 along the x axis represent individual naive mice that were fed on by infected I. scapularis nymphs. The mean and standard deviation are shown as horizontal lines.
Having demonstrated that lp28-3-deficient clones can establish infection in mice by needle inoculation and be acquired by feeding ticks, we next wanted to analyze the ability of the lp28-3-deficient strain to be transmitted by infected nymphal ticks and infect a new host by this more natural route. To this end, we allowed the larval ticks to molt into nymphs and feed on naive mice. Spirochetes were enumerated in fed nymphs at 10 days postfeeding by plating disrupted tick homogenate in solid medium. No significant difference was observed in the mean number of wild-type or lp28-3-deficient spirochetes present in infected ticks when analyzed using Student's two-tailed t test (Fig. 5B). These data indicate that B. burgdorferi does not require lp28-3 to persist in ticks through the molt or to replicate following a blood meal. Analysis of mice 3 weeks after tick feeding demonstrated that both wild-type and lp28-3-deficient spirochetes were efficiently transmitted by challenge with 3 infected ticks feeding per mouse. Spirochete isolation from mouse tissues demonstrated that B. burgdorferi established disseminated infections in naive hosts by this route (Table 1). Based on these data, we conclude that linear plasmid lp28-3 does not encode factors critical for B. burgdorferi survival at any stage of an experimental mouse-tick-mouse infectious cycle.
Quantitative analysis of lp28-3-deficient spirochetes in the skin.
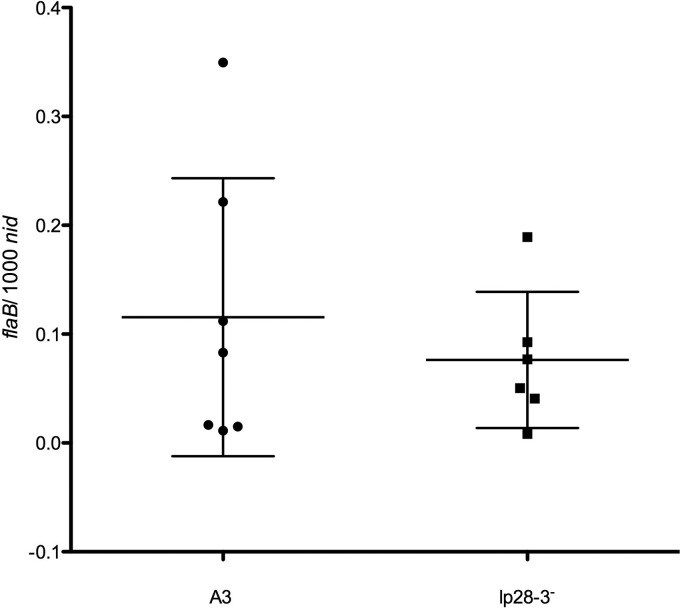
B. burgdorferi is acquired by ticks through a blood meal taken from the skin of an infected animal. Therefore, the skin plays a vital biological role in the transmission cycle of this pathogen. Although lp28-3-deficient and wild-type spirochetes were found at equivalent levels in fed ticks (Fig. 5), suggesting they are likely present at comparable levels in infected host tissues, we directly analyzed the numbers of wild-type and lp28-3-deficient spirochetes in the skin of infected mice. To this end, we performed quantitative PCR (qPCR) on DNA isolated from mouse ear tissues from 3 independent experiments (Table 1) and analyzed the levels of C12 or C33 and wild-type spirochetes in these samples. We found that wild-type and lp28-3-deficient spirochetes were present at equivalent levels in the skin of infected mice at 4 and 8 weeks postinoculation (Fig. 6 and data not shown).
Fig 6.
Quantitative determination by qPCR of spirochete load in ear tissues of infected mice 4 weeks post-needle inoculation with wild-type or lp28-3-deficient (lp28-3−) spirochetes. Total DNA was extracted from ear tissues of mice infected with A3, C12, or C33, and the numbers of copies of the flaB gene of B. burgdorferi and mouse nidogen (nid) gene were determined by qPCR using gene-specific primer probe sets (see Table S1 in the supplemental material). The mean value and standard deviation are depicted as horizontal lines for both the A3 and lp28-3-deficient strains. Using Student's two-tailed t test, the means were found to be not significantly different (P value of 0.509).
Analysis of the lp28-3-deficient spirochetes during coinfection with wild-type spirochetes.
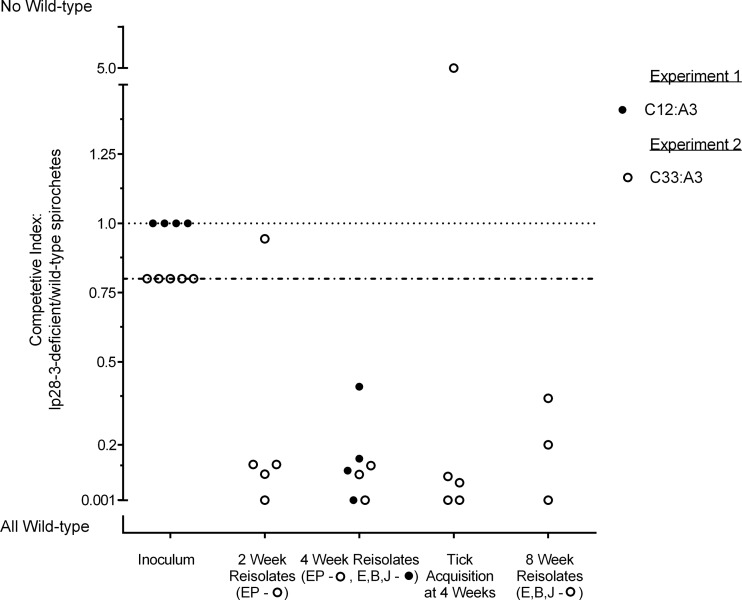
As described above, we did not detect any gross phenotypic changes or defects in B. burgdorferi lacking lp28-3, although by both needle inoculation and by tick challenge, lp28-3-deficient spirochetes may have a slightly lower infection rate than wild-type spirochetes (Table 1). This difference was not statistically significant, however, when analyzed using Fisher's exact test. To address a potentially subtle phenotype, we determined the competitive index (CI) of lp28-3-deficient spirochetes relative to wild-type organisms when coinfecting a mouse. CI is a measure of bacterial fitness, even when an obvious phenotype is not apparent (42, 43, 53). The CI is a measure of the change in the ratio of mutant bacteria relative to wild-type bacteria over a period of time, usually measured during coinfection with the two strains. Typically, the mutant and wild type are provided at a 1:1 ratio in the inoculum and the ratio of mutant to wild type is experimentally determined upon isolation at a later time point. The calculated change in the ratio of mutant to wild type from inoculation to isolation is the CI. If the mutant and wild type are isolated in equal proportions, there is no change from the initial ratio, and therefore the CI is 1, suggesting that the mutant and wild type are equally fit in that environment. A CI of <1, in which fewer mutant than wild-type bacteria are present, suggests that the wild type is better fit for survival in a particular environment. To determine if lp28-3 confers a fitness advantage, we determined the CI of the lp28-3-deficient strains versus the wild type. To this end, a group of five mice were injected with a mixed inoculum of A3 and C12 at an initial target ratio of 1:1. Mouse infectivity was assessed as previously described, and we found that four of the five mice inoculated had become infected, based on both seroconversion and isolation of spirochetes from all tissues tested (data not shown). To determine the ratio of A3 to C12 spirochetes in coinfected mice at the 4-week time point, isolated spirochetes from infected tissues were plated in solid BSK medium, and individual colonies were screened by PCR for the presence of lp28-3. We found that the proportion of C12 (lp28-3-deficient) to A3 (wild-type) spirochetes had decreased in all four coinjected mice from the initial ratio of 1:1 (C12 to A3) to an average ratio of 1:7 (Table 2, experiment 1, and Fig. 7). The change in ratio from 1:1 to 1:7 results in a CI of 0.14 (Table 2, Expt 1; see Table S2 in the supplemental material), suggesting that wild-type spirochetes containing lp28-3 have a selective advantage over spirochetes lacking lp28-3 (C12) during mouse infection.
Table 2.
Competitive index experiments assessing the ratio of lp28-3-deficient spirochetes to wild-type spirochetes in an experimental coinfection modela
| Expt and coinjected mouse | CI result (lp28-3−/wild-type ratio) for: |
||||
|---|---|---|---|---|---|
| Inoculum | Mouse isolates |
Larval tick isolates | |||
| 2 wk | 4 wk | 8 wk | |||
| Expt 1 (C12/A3 ratio)b | |||||
| Mouse 1 | 1:1 | NAc | 1:2.4 | NA | NA |
| Mouse 2 | 1:1 | NA | 1:6.5 | NA | NA |
| Mouse 3 | 1:1 | NA | 0:1d | NA | NA |
| Mouse 4 | 1:1 | NA | 1:8.3 | NA | NA |
| Expt 2 (C33/A3 ratio)e | |||||
| Mouse 1 | 1:1.25 | 1.0:7.7 | 0:1.0d | 1.0:2.7 | 1.0:15.8 |
| Mouse 2 | 1:1.25 | 0:1.0d | NDf | ND | 0:1.0d |
| Mouse 3 | 1:1.25 | 1.0:7.7 | 1.0:10.7 | 0:1.0d | 0:1.0d |
| Mouse 4 | 1:1.25 | 1.0:1.0 | ND | ND | 6.1:1.0 |
| Mouse 5 | 1:1.25 | 1.0:10.6 | 1.0:8.0 | 1.0:5.0 | 1.0:11.5 |
The data used to determine these ratios are provided in Table S2 (CI experiment 1) and Table S3 (CI experiment 2) in the supplemental material.
Ratio of C12 (lp28-3−) to A3 (wild type) determined by enumeration of spirochetes in a Petroff-Hausser chamber prior to preparation of the inoculum. Mice were inoculated with 9 × 103 spirochetes.
NA, not applicable. Samples were not taken at these time points in experiment 1.
All of the tissue isolates screened were positive for the presence of lp28-3 from these samples (for isolate screening data, see Tables S2 and S3 in the supplemental material).
Ratio of C33 (lp28-3−) to A3 (wild type) determined experimentally by PCR screening of colonies for lp28-3 after plating of the inoculum. Mice were inoculated with 1 × 104 spirochetes.
ND, not determined due to contamination of the reisolation culture.
Fig 7.
Competitive index (CI) results. The data are from two CI experiments demonstrating the ratio of C12 to wild-type A3 strain (experiment 1) or C33 to wild-type A3 strain (experiment 2) during mixed infection. Individual mice were inoculated with lp28-3-deficient and wild-type spirochetes at a ratio of 1:1 (closed circles) or 1:1.25 (open circles). Each circle represents the CI calculated from reisolates from an individual mouse at the time point indicated. The ear (E), bladder (B), and tibiotarsal joint (J) tissues were harvested at a 4-week time point in experiment 1 and an 8-week time point in experiment 2, and the colony screening results from those tissues were used to determine the CI. Ear punch (EP) biopsy specimens were taken at 2 and 4 weeks postinoculation in the second experiment to determine spirochete ratios. For tick acquisition, the CI was determined from spirochetes isolated from ticks that fed on individual mice. Colony screening data for determination of the CI are presented in Tables S2 and S3 for CI for experiments 1 and 2, respectively.
A similar coinfection experiment was conducted with a separate clone lacking lp28-3 (C33), with comparable results (Table 2, experiment 2). Five mice were inoculated with a mixed culture containing C33 and A3. For the mixed inoculum, the ratio of C33 to A3 was 1:1.25 based on enumeration of viable spirochetes by plating and screening colonies for lp28-3 (Table 2; see Table S3 in the supplemental material). Spirochetes isolated from infected mouse tissues were screened for the presence of lp28-3, and again we found that spirochete populations in mice shifted from an initial ratio of 1:1.25 to a ratio of greater than 1:7 (lp28-3 deficient to wild type) in four of the five mice by 2 weeks postinoculation (Table 2, bottom, and Fig. 7). This trend was maintained at the 4- and 8-week time points, where wild-type bacteria were present in infected mouse tissues at a greater proportion than the lp28-3− strain (Table 2 and Fig. 7). The CIs were determined for the lp28-3-deficient strain at 2, 4, and 8 weeks postinoculation and calculated as 0.16, 0.2, and 0.3, respectively. These results are consistent with our first experiment and taken together suggest that wild-type spirochetes harboring lp28-3 have a selective advantage over lp28-3-deficient spirochetes when coinfecting a mouse.
To further examine the potential selective advantage conferred by lp28-3, we fed larval I. scapularis on the mice coinfected with C33 and A3 to determine the proportion of lp28-3-deficient and wild-type spirochetes acquired by feeding ticks. We found that the majority (>70%) of the spirochetes acquired were wild-type spirochetes harboring lp28-3 (Table 2, experiment 2, and Fig. 7). However, in one coinfected mouse, a few ticks were recovered after feeding that had acquired only lp28-3-deficient spirochetes (Table 2, experiment 2, mouse 4). Taken together, these experiments demonstrate that the lp28-3-deficient spirochetes are at a competitive disadvantage in vivo when coinfecting a mouse in the presence of wild-type B. burgdorferi. The competitive advantage imparted by lp28-3 is further reflected in the proportion of wild-type spirochetes acquired by feeding larval ticks (Table 2 and Fig. 7).
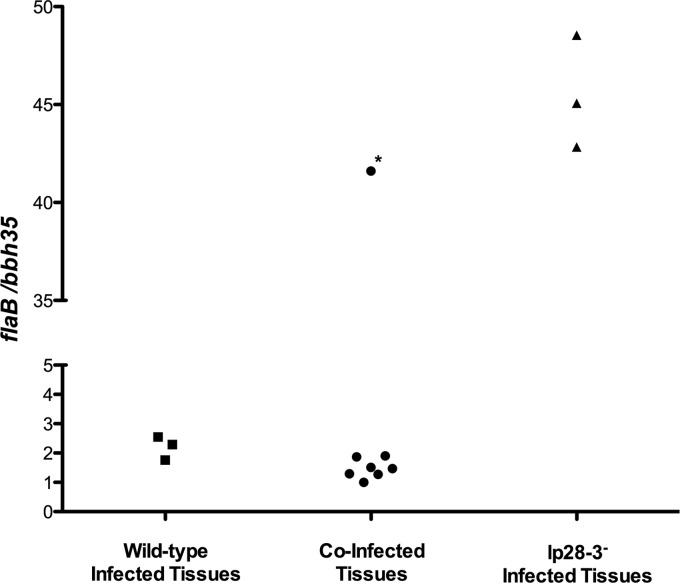
We previously found that the A3, C12, and C33 strains had similar growth rates in vitro and, when mixed, maintained a ratio of ∼1:1 throughout all growth stages (Fig. 4 and data not shown). These data indicate that the ratio of lp28-3-deficient to wild-type spirochetes as determined by screening tissue isolates accurately reflects their status at the point of tissue isolation and did not change during outgrowth from mouse tissues. However, we also used qPCR to directly analyze the ratio of lp28-3-deficient spirochetes to wild-type spirochetes in tissues of coinfected mice harvested concurrent with those used for spirochete outgrowth in the CI experiments. Using gene-specific primer and probe sets for flaB and bbh35, we quantified the number of copies of the chromosome and lp28-3 in DNA extracted from tissues of mice infected with wild-type or lp28-3-deficient spirochetes or coinfected with both (Fig. 8). We found that in wild-type-infected tissues, the flaB/bbh35 ratio exceeded 1:1 and was between 1.7 and 2.5, possibly due to differences in the standard curves used for the individual primer probe sets or actual differences in chromosome versus plasmid copy numbers. The ratio of flaB to bbh35 in tissues from mice infected with lp28-3-deficient spirochetes was >40. For coinfected tissues, the ratio of flaB to bbh35 was similar to that in wild-type-infected tissues: between 1.0 and 1.9 (Fig. 8). These data suggest that at the point of tissue harvest, the spirochetes in these tissues were primarily wild type. A notable exception is mouse 4 from CI experiment 2 (marked with an asterisk in Fig. 8), where the ratio of flaB to bbh35 gene copies is significantly higher, suggesting that lp28-3-deficient spirochetes predominated in that sample. Our data demonstrate that spirochetes harboring lp28-3 predominate at the point of tissue isolation following coinfection and suggest that spirochetes harboring lp28-3 are at a competitive advantage over lp28-3-deficient spirochetes when coinjected into a mouse. These data also demonstrate that the CI experiments determining the ratio of wild-type to lp28-3-deficient spirochetes in outgrowth from tissues are representative of what was present in the infected mouse at the time of tissue isolation.
Fig 8.
Determination of the relative proportion of wild-type and lp28-3-deficient spirochetes in tissues of infected mice by qPCR. Each symbol represents a different mouse, and the symbol marked with an asterisk corresponds to mouse 4 in CI experiment 2, in which primarily lp28-3-deficient spirochetes were present and acquired by feeding ticks.
DISCUSSION
Although lp28-3 does not provide any genes that are essential for B. burgdorferi to establish and maintain infection, we have now demonstrated that the linear plasmid lp28-3 confers a competitive advantage to the spirochetes that retain it. Specifically, in an experimental coinfection, strains lacking lp28-3 are attenuated relative to wild-type spirochetes in their ability to compete in a mammalian environment. Furthermore, the selective advantage of wild-type spirochetes harboring lp28-3 is reflected in the composition of spirochetes acquired by larval ticks feeding on coinfected mice. Taken together, these data indicate that genes on lp28-3 contribute to the overall fitness of B. burgdorferi in its natural infectious cycle.
Several lines of evidence suggested a role for genes carried by lp28-3 in B. burgdorferi survival in vivo. By both genomic sequencing and genomic array hybridization, the gene composition and sequence of lp28-3 are highly conserved, except for the very distal ends (22, 54). Additionally, lp28-3 is present in most genomic isolates and persists after serial passage in culture (21, 22, 35, 50, 55). With the data presented here, it is becoming clear that genes carried by lp28-3 have been conserved to fulfill functionally important roles in the survival of B. burgdorferi and that the competitive advantage conferred to spirochetes harboring lp28-3 could explain why the majority of isolates retain this plasmid.
Initially, the B. burgdorferi strains lacking lp28-3 did not yield any obvious in vivo or in vitro phenotype. Surprisingly, using two independent clones, we found that lp28-3 does not contain any genes strictly required to complete any stage of an experimental mouse-tick-mouse infectious cycle. However, determination of the CI in the mouse coinfection experiments, which directly compare lp28-3-deficient spirochetes to wild-type spirochetes, revealed that lp28-3 makes a distinct contribution to the fitness of B. burgdorferi in vivo. These data were also reinforced by qPCR data demonstrating that at the point of tissue isolation, the wild-type strain predominated over the lp28-3-deficient strains. This type of analysis has been similarly effective in determining the contribution of biosynthetic enzymes and iron-regulated virulence factors to the in vivo fitness of Vibrio cholerae, again, for which no obvious phenotype was apparent for mutants alone (41, 43).
Our data demonstrate the utility of using a coinfection model to analyze B. burgdorferi mutants for which there is no obvious phenotype when assayed individually. Testing B. burgdorferi mutants for their competence in an experimental infectious cycle, while also determining their CI, offers a powerful assessment of the contribution of the gene or genes to B. burgdorferi pathogenesis. This strategy facilitates determination of the strict requirement for a genetic element during infection, while also providing a quantitative assessment of the contribution to the fitness of Borrelia in vivo. This approach could be especially useful for studying B. burgdorferi variants in which seemingly important genetic elements, such as lp38, ospD, and others, have been shown to be dispensable in an experimental infectious cycle (35, 36, 40). Highlighting the usefulness of such an approach, it has been recently demonstrated that BBA03, another conserved but nonessential lipoprotein, contributes to the success of transmission of B. burgdorferi by ticks in the context of a mixed infection (56).
Our data suggest that although many B. burgdorferi genes are not strictly required for completion of an experimental mouse-tick-mouse infectious cycle, they may make substantial contributions in the competitive environment of a mixed infection that is normally encountered in a natural infectious cycle. Here we show that spirochetes lacking the conserved linear plasmid lp28-3 can only suboptimally fulfill an experimental mouse-tick infectious cycle when wild-type spirochetes are simultaneously present. Currently, we do not know what gene or genes are responsible for this phenotype. Most lp28-3 genes are hypothetical or conserved hypothetical genes with little or no homology to genes outside the genus Borrelia (14, 22). However, lp28-3 does encode CRASP2, a factor-H binding protein, and multiple hypothetical lipoproteins that might mediate interactions within the host environment and contribute to the in vivo fitness of B. burgdorferi (14, 26). Interestingly, the hypothetical lipoprotein BBH13 displays a gene expression profile indicative of a potential role in the mammalian host (29). Although we did not observe a severe decrease in the infectivity of our lp28-3-deficient strains in laboratory mice, it is possible that lp28-3-borne genes play a more important role in other mammalian hosts.
With the recent development of a signature-tagged transposon mutagenesis (STM) library, in which individually tagged mutant strains have been pooled and used for coinfections, we now have another tool to analyze the contribution of individual genes to the fitness of B. burgdorferi in vivo (57). Not surprisingly, some transposon mutants of lp28-3-carried genes, such as bbh06 (CRASP2), bbh18, and bbh20, were reisolated at a very low frequency, or not at all, when analyzed in a pool of 11 mutants (57). Intriguingly, bbh20 is annotated as a pseudogene, making its contribution to virulence unclear but possibly suggestive of important unannotated genes or small RNAs in that region. However, it is important to point out, as seen here with the loss of lp28-3, that the inability to reisolate a certain clone from coinfected mice does not necessarily indicate that those genes are essential for infection. With the availability of the STM library, analysis of individual mutants during coinfections using a 1:1 mutant/wild-type ratio could help elucidate which specific lp28-3 genes are playing a role in the infectious cycle and the role of these genes in the pathogenesis of B. burgdorferi.
Supplementary Material
ACKNOWLEDGMENTS
We thank all members of the Rosa laboratory, Ted Hackstadt, and Laszlo Kari for insightful review of the manuscript, as well as Ryan Rego for providing the mouse isolate used to identify C33 in this study. We are also grateful to Anita Mora and Heather Murphy for help with figures and graphics and Dan Sturdevant for designing the primer-probe sets used in this study.
This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Published ahead of print 10 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00219-13.
REFERENCES
- 1. Steere AC, Coburn J, Glickstein L. 2004. The emergence of Lyme disease. J. Clin. Invest. 113:1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steere AC. 1994. Lyme disease: a growing threat to urban populations. Proc. Natl. Acad. Sci. U. S. A. 91:2378–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317–1319 [DOI] [PubMed] [Google Scholar]
- 4. Lane RS, Piesman J, Burgdorfer W. 1991. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 36:587–609 [DOI] [PubMed] [Google Scholar]
- 5. Lane RS. 1994. Competence of ticks as vectors of microbial agents with an emphasis on Borrelia burgdorferi, p 45–67 In Sonenshine DE, Mather TN. (ed), Ecological dynamics of tick-borne zoonoses. Oxford University Press, New York, NY [Google Scholar]
- 6. Lane RS, Berger DM, Casher LE, Burgdorfer W. 1994. Experimental infection of Columbian black-tailed deer with the Lyme disease spirochete. J. Wildl. Dis. 30:20–28 [DOI] [PubMed] [Google Scholar]
- 7. Peavey CA, Lane RS. 1995. Transmission of Borrelia burgdorferi by Ixodes pacificus nymphs and reservoir competence of deer mice (Peromyscus maniculatus) infected by tick-bite. J. Parasitol. 81:175–178 [PubMed] [Google Scholar]
- 8. Maupin GO, Gage KL, Piesman J, Montenieri J, Sviat SL, VanderZanden L, Happ CM, Dolan M, Johnson BJB. 1994. Discovery of an enzootic cycle of Borrelia burgdorferi in Neotoma mexicana and Ixodes spinipalpis from Northern Colorado, an area where Lyme disease is nonendemic. J. Infect. Dis. 170:36–43 [DOI] [PubMed] [Google Scholar]
- 9. Barbour AG. 1988. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J. Clin. Microbiol. 26:475–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casjens S, DeLange M, Ley HL, III, Rosa P, Huang WM. 1995. Linear chromosomes of Lyme disease agent spirochetes: genetic diversity and conservation of gene order. J. Bacteriol. 177:2769–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, Haft D, Hickey E, Gwinn M, White O, Fraser C. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490–516 [DOI] [PubMed] [Google Scholar]
- 12. Barbour AG, Carter CJ, Bundoc V, Hinnebusch J. 1996. The nucleotide sequence of a linear plasmid of Borrelia burgdorferi reveals similarities to those of circular plasmids of other prokaryotes. J. Bacteriol. 178:6635–6639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saint Girons I, Old IG, Davidson BE. 1994. Molecular biology of the Borrelia, bacteria with linear replicons. Microbiology 140:1803–1816 [DOI] [PubMed] [Google Scholar]
- 14. Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidmann J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586 [DOI] [PubMed] [Google Scholar]
- 15. Hinnebusch J, Tilly K. 1993. Linear plasmids and chromosomes in bacteria. Mol. Microbiol. 10:917–922 [DOI] [PubMed] [Google Scholar]
- 16. Ferdows MS, Barbour AG. 1989. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc. Natl. Acad. Sci. U. S. A. 86:5969–5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10:87–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samuels DS. 2011. Gene regulation in Borrelia burgdorferi. Annu. Rev. Microbiol. 65:479–499 [DOI] [PubMed] [Google Scholar]
- 19. Parveen N, Cornell KA. 2011. Methylthioadenosine/S-adenosylhomocysteine nucleosidase, a critical enzyme for bacterial metabolism. Mol. Microbiol. 79:7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tilly K, Rosa PA, Stewart PE. 2008. Biology of infection with Borrelia burgdorferi. Infect. Dis. Clin. North Am. 22:217–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stewart PE, Byram R, Grimm D, Tilly K, Rosa PA. 2005. The plasmids of Borrelia burgdorferi: essential genetic elements of a pathogen. Plasmid 53:1–13 [DOI] [PubMed] [Google Scholar]
- 22. Casjens SR, Mongodin EF, Qiu WG, Luft BJ, Schutzer SE, Gilcrease EB, Huang WM, Vujadinovic M, Aron JK, Vargas LC, Freeman S, Radune D, Weidman JF, Dimitrov GI, Khouri HM, Sosa JE, Halpin RA, Dunn JJ, Fraser CM. 2012. Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One 7:e33280. 10.1371/journal.pone.0033280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haupt K, Kraiczy P, Wallich R, Brade V, Skerka C, Zipfel PF. 2007. Binding of human factor H-related protein 1 to serum-resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J. Infect. Dis. 196:124–133 [DOI] [PubMed] [Google Scholar]
- 24. Kraiczy P, Skerka C, Brade V, Zipfel PF. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800–7809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kraiczy P, Skerka C, Kirschfink M, Brade V, Zipfel PF. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 31:1674–1684 [DOI] [PubMed] [Google Scholar]
- 26. Coleman AS, Yang X, Kumar M, Zhang X, Promnares K, Shroder D, Kenedy MR, Anderson JF, Akins DR, Pal U. 2008. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS One 3:3010e. 10.1371/journal.pone.0003010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rogers EA, Abdunnur SV, McDowell JV, Marconi RT. 2009. Comparative analysis of the properties and ligand binding characteristics of CspZ, a factor H binding protein, derived from Borrelia burgdorferi isolates of human origin. Infect. Immun. 77:4396–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rogers EA, Marconi RT. 2007. Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect. Immun. 75:5272–5281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Revel AT, Talaat AM, Norgard MV. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. U. S. A. 99:1562–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brooks CS, Hefty PS, Jolliff SE, Akins DR. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tokarz R, Anderton JM, Katona LI, Benach JL. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ojaimi C, Brooks C, Casjens S, Rosa P, Elias A, Barbour AG, Jasinskas A, Benach J, Katona L, Radolf J, Caimano M, Skare J, Swingle K, Akins D, Schwartz I. 2003. Profiling temperature-induced changes in Borrelia burgdorferi gene expression using whole genome arrays. Infect. Immun. 71:1689–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bono JL, Elias AF, Kupko JJ, III, Stevenson B, Tilly K, Rosa P. 2000. Efficient targeted mutagenesis in Borrelia burgdorferi. J. Bacteriol. 182:2445–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K, Bono JL, Akins DR, Radolf JD, Schwan TG, Rosa P. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Purser JE, Norris SJ. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 97:13865–13870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dulebohn DP, Bestor A, Rego RO, Stewart PE, Rosa PA. 2011. Borrelia burgdorferi linear plasmid 38 is dispensable for completion of the mouse-tick infectious cycle. Infect. Immun. 79:3510–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Q, Fischer JR, Benoit VM, Dufour NP, Youderian P, Leong JM. 2008. In vitro CpG methylation increases the transformation efficiency of Borrelia burgdorferi strains harboring the endogenous linear plasmid lp56. J. Bacteriol. 190:7885–7891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rego RO, Bestor A, Rosa PA. 2011. Defining the plasmid-encoded restriction-modification systems of the Lyme disease spirochete Borrelia burgdorferi. J. Bacteriol. 193:1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Samuels DS, Mach KE, Garon CF. 1994. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J. Bacteriol. 176:6045–6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stewart PE, Bestor A, Cullen JN, Rosa PA. 2008. Tightly regulated surface protein of Borrelia burgdorferi is not essential to the mouse-tick infectious cycle. Infect. Immun. 76:1970–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Almagro-Moreno S, Boyd EF. 2009. Sialic acid catabolism confers a competitive advantage to pathogenic Vibrio cholerae in the mouse intestine. Infect. Immun. 77:3807–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Freter R, O'Brien PC, Macsai MS. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect. Immun. 34:234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldberg MB, DiRita VJ, Calderwood SB. 1990. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect. Immun. 58:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jewett MW, Lawrence K, Bestor AC, Tilly K, Grimm D, Shaw P, VanRaden M, Gherardini F, Rosa PA. 2007. The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Mol. Microbiol. 64:1358–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 46. Stewart PE, Thalken R, Bono JL, Rosa P. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714–721 [DOI] [PubMed] [Google Scholar]
- 47. Grimm D, Eggers CH, Caimano MJ, Tilly K, Stewart PE, Elias AF, Radolf JD, Rosa PA. 2004. Experimental assessment of the roles of linear plasmids lp25 and lp28-1 of Borrelia burgdorferi throughout the infectious cycle. Infect. Immun. 72:5938–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stewart PE, Chaconas G, Rosa P. 2003. Conservation of plasmid maintenance functions between linear and circular plasmids in Borrelia burgdorferi. J. Bacteriol. 185:3202–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Elias AF, Bono JL, Kupko JJ, Stewart PE, Krum JG, Rosa PA. 2003. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi. J. Mol. Microbiol. Biotechnol. 6:29–40 [DOI] [PubMed] [Google Scholar]
- 50. Grimm D, Elias AF, Tilly K, Rosa PA. 2003. Plasmid stability during in vitro propagation of Borrelia burgdorferi assessed at a clonal level. Infect. Immun. 71:3138–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schwan TG, Burgdorfer W, Garon CF. 1988. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect. Immun. 56:1831–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Busch U, Will G, Hizo-Teufel C, Wilske B, Preac-Mursic V. 1997. Long term in vitro cultivation of Borrelia burgdorferi sensu lato strains: influence on plasmid patterns, genome stability and expression of proteins. Res. Microbiol. 148:109–118 [DOI] [PubMed] [Google Scholar]
- 53. Almagro-Moreno S, Boyd EF. 2010. Bacterial catabolism of nonulosonic (sialic) acid and fitness in the gut. Gut Microbes 1:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Terekhova D, Iyer R, Wormser GP, Schwartz I. 2006. Comparative genome hybridization reveals substantial variation among clinical isolates of Borrelia burgdorferi sensu stricto with different pathogenic properties. J. Bacteriol. 188:6124–6134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Iyer R, Kalu O, Purser J, Norris S, Stevenson B, Schwartz I. 2003. Linear and circular plasmid content in Borrelia burgdorferi clinical isolates. Infect. Immun. 71:3699–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bestor A, Rego RO, Tilly K, Rosa PA. 2012. Competitive advantage of Borrelia burgdorferi with outer surface protein BBA03 during tick-mediated infection of the mammalian host. Infect. Immun. 80:3501–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin T, Gao L, Zhang C, Odeh E, Jacobs MB, Coutte L, Chaconas G, Philipp MT, Norris SJ. 2012. Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS One 7:e47532. 10.1371/journal.pone.0047532 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.