Abstract
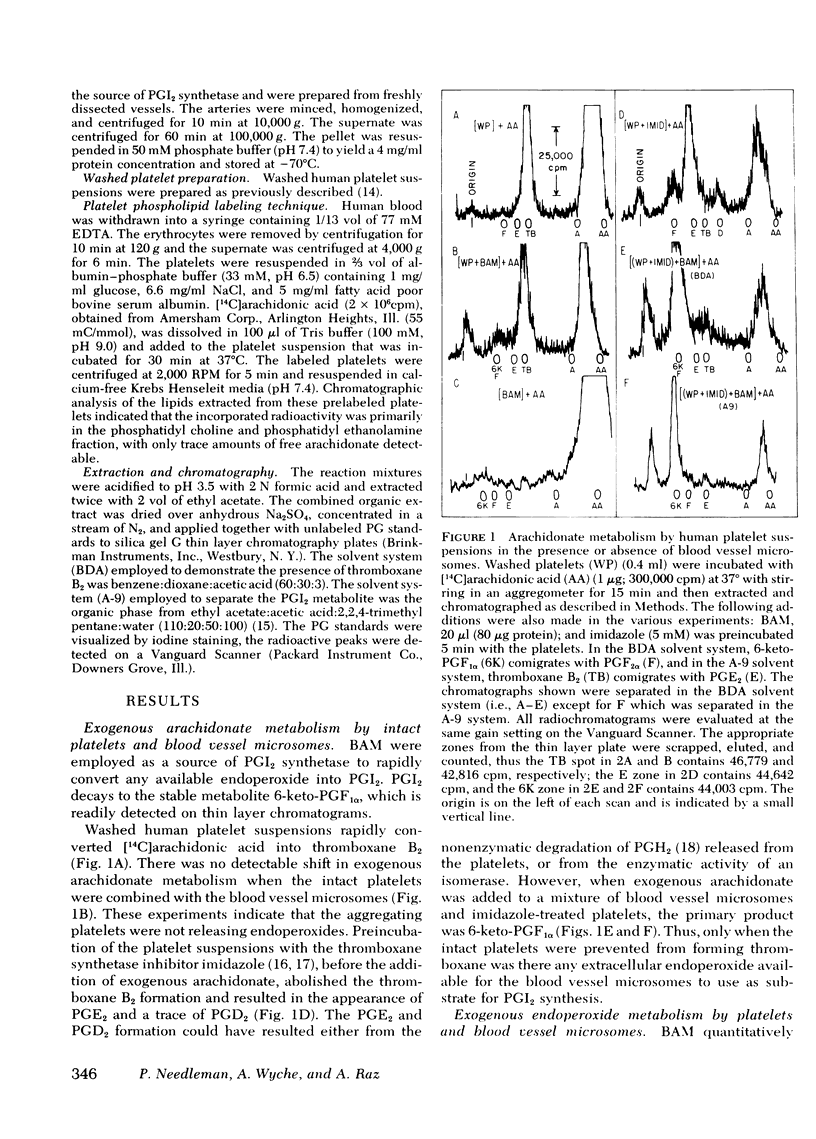
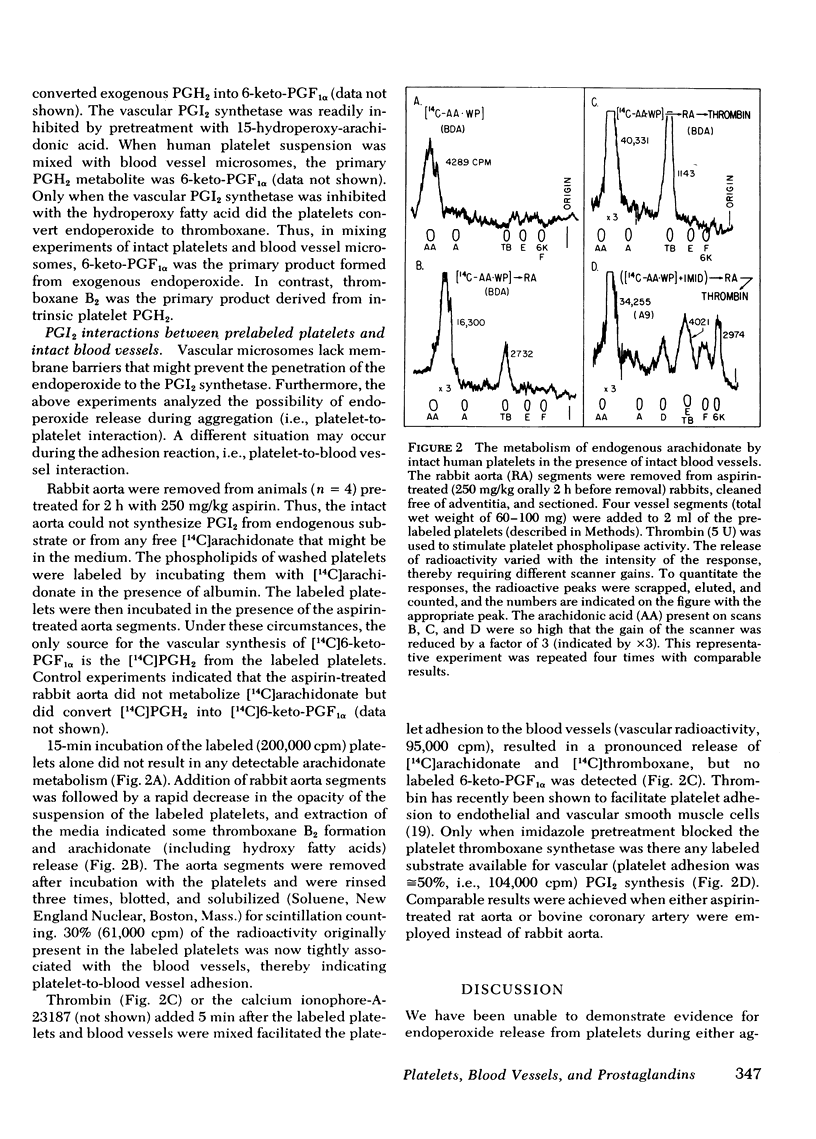
Exogenous arachidonate addition to intact platelets, in the absence or the presence of blood vessel microsomes, results in the production of thromboxane B2 (the stable degradation product of thromboxane A2) only. Prostaglandin (PG) endoperoxides are released from intact platelets only when thromboxane synthetase is inhibited. Thus, addition of exogenous arachidonate to imidazole-pretreated platelets in the presence of bovine aorta microsomes (source of prostacyclin synthetase) results predominantly in the synthesis of 6-keto-PGF1α (the stable degradation product of prostacyclin). Strips of intact aorta were removed from aspirin-treated rabbits, thus the isolated blood vessels were unable to convert endogenous or exogenous arachidonate to prostacyclin. Human platelets, with [14C]arachidonate-labeled phospholipids, adhered to the blood vessel segments and released some thromboxane B2. The subsequent addition of thrombin facilitated the release of endogenous arachidonate and thromboxane, but no labeled 6-keto-PGF1α was detectable. There is therefore no direct chemical evidence of PG-endoperoxide release from human platelets during either aggregation or adhesion, which therefore precludes the possibility that blood vessels use platelet PG-endoperoxide for prostacyclin synthesis. Imidazole inhibited the thromboxane synthetase in the labeled platelets, and thereafter thrombin stimulation resulted in the release of platelet-derived, labeled PG-endoperoxides that were converted to labeled prostacyclin by the vascular prostacyclin synthetase. The latter result suggests a potential antithrombotic therapeutic benefit might be achieved using an effective thromboxane synthetase inhibitor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunting S., Gryglewski R., Moncada S., Vane J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976 Dec;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- Bunting S., Moncada S., Vane J. R. Antithrombotic properties of vascular endothelium. Lancet. 1977 Nov 19;2(8047):1075–1076. doi: 10.1016/s0140-6736(77)91906-7. [DOI] [PubMed] [Google Scholar]
- Czervionke R. L., Hoak J. C., Fry G. L. Effect of aspirin on thrombin-induced adherence of platelets to cultured cells from the blood vessel wall. J Clin Invest. 1978 Oct;62(4):847–856. doi: 10.1172/JCI109197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusting G. J., Moncada S., Vane J. R. Prostacyclin (PGX) is the endogenous metabolite responsible for relaxation of coronary arteries induced by arachindonic acid. Prostaglandins. 1977 Jan;13(1):3–15. doi: 10.1016/0090-6980(77)90037-5. [DOI] [PubMed] [Google Scholar]
- Funk M. O., Isacc R., Porter N. A. Preparation and purification of lipid hydroperoxides from arachidonic and gamma-linolenic acids. Lipids. 1976 Feb;11(2):113–117. doi: 10.1007/BF02532660. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Sun F. F., Miller O. V., Johnson R. A. Prostaglandins H1 and H2. Convenient biochemical synthesis and isolation. Further biological and spectroscopic characterization. Prostaglandins. 1977 Jun;13(6):1043–1053. doi: 10.1016/0090-6980(77)90132-0. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Fredholm B. B. Isomerization of prostaglandin H2 into prostaglandin D2 in the presence of serum albumin. Biochim Biophys Acta. 1976 Apr 22;431(1):189–183. doi: 10.1016/0005-2760(76)90273-3. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandins in human seminal plasma. Prostaglandins and related factors 46. J Biol Chem. 1966 Jan 25;241(2):257–263. [PubMed] [Google Scholar]
- Kelton J. G., Hirsh J., Carter C. J., Buchanan M. R. Thrombogenic effect of high-dose aspirin in rabbits. Relationship to inhibition of vessel wall synthesis of prostaglandin I2-like activity. J Clin Invest. 1978 Oct;62(4):892–895. doi: 10.1172/JCI109203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni P. S., Roberts R., Needleman P. Paradoxical endogenous synthesis of a coronary dilating substance from arachidonate. Prostaglandins. 1976 Sep;12(3):337–353. doi: 10.1016/0090-6980(76)90015-0. [DOI] [PubMed] [Google Scholar]
- Minkes M., Stanford N., Chi M. M., Roth G. J., Raz A., Needleman P., Majerus P. W. Cyclic adenosine 3',5'-monophosphate inhibits the availability of arachidonate to prostaglandin synthetase in human platelet suspensions. J Clin Invest. 1977 Mar;59(3):449–454. doi: 10.1172/JCI108659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Bunting S., Mullane K., Thorogood P., Vane J. R., Raz A., Needleman P. Imidazole: a selective inhibitor of thromboxane synthetase. Prostaglandins. 1977 Apr;13(4):611–618. doi: 10.1016/0090-6980(77)90232-5. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R. J., Bunting S., Vane J. R. A lipid peroxide inhibits the enzyme in blood vessel microsomes that generates from prostaglandin endoperoxides the substance (prostaglandin X) which prevents platelet aggregation. Prostaglandins. 1976 Nov;12(5):715–737. doi: 10.1016/0090-6980(76)90048-4. [DOI] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A., Vane J. R. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977 Jan 1;1(8001):18–20. doi: 10.1016/s0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Unstable metabolites of arachidonic acid and their role in haemostasis and thrombosis. Br Med Bull. 1978 May;34(2):129–135. doi: 10.1093/oxfordjournals.bmb.a071482. [DOI] [PubMed] [Google Scholar]
- Needleman P., Bronson S. D., Wyche A., Sivakoff M., Nicolaou K. C. Cardiac and renal prostaglandin I2. Biosynthesis and biological effects in isolated perfused rabbit tissues. J Clin Invest. 1978 Mar;61(3):839–849. doi: 10.1172/JCI108998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Minkes M., Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976 Jul 9;193(4248):163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- Needleman P., Raz A., Ferrendelli J. A., Minkes M. Application of imidazole as a selective inhibitor thromboxane synthetase in human platelets. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1716–1720. doi: 10.1073/pnas.74.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Isakson P. C., Minkes M. S., Needleman P. Characterization of a novel metabolic pathway of arachidonate in coronary arteries which generates a potent endogenous coronary vasodilator. J Biol Chem. 1977 Feb 10;252(3):1123–1126. [PubMed] [Google Scholar]
- Tansik R. L., Namm D. H., White H. L. Synthesis of prostaglandin 6-keto F1alpha by cultured aortic smooth muscle cells and stimulation of its formation in a coupled system with platelet lysates. Prostaglandins. 1978 Mar;15(3):399–408. doi: 10.1016/0090-6980(78)90123-5. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]


