Abstract
Enterococcus faecalis is a highly stress resistant opportunistic pathogen. The intrinsic ruggedness of this bacterium is supposed to be the basis of its capacity to colonize the hostile environments of hospitals and to cause several kinds of infections. We show in this work that general resistance to very different environmental stresses depends on the ability of E. faecalis to maintain redox balance via lactate dehydrogenase (LDH). Furthermore, LDH-deficient mutants are less successful than the wild type at colonizing host organs in a murine model of systemic infection. Taken together, our results, as well as those previously published for Staphylococcus aureus (A. R. Richardson, S. J. Libby, and F. C. Fang, Science 319:1672–1676, 2008), identify LDH as an attractive drug target. These drugs may have additional applications, as in the fight against glycopeptide antibiotic-resistant bacteria and even cancer.
INTRODUCTION
Enterococcus faecalis is an intestinal resident of humans and many animals and is a major cause of severe nosocomial infections that are difficult to treat due to intrinsic and acquired resistances to major classes of antibiotics. It is a homofermentative lactic acid bacterium that uses mainly the Embden-Meyerhoff-Parnas (glycolysis) pathway for the catabolism of glucose or related carbohydrates (1). The dominant end product of fermentation under these conditions is lactate (2, 3). Reduction of the glycolytic end product pyruvate to lactate by lactate dehydrogenases (LDH) allows the regeneration of the NADH formed during glycolysis to NAD+, which keeps glycolysis going. E. faecalis possesses two cytosolic l-(+)-lactate dehydrogenases encoded by the ldh-1 (EF_0255) and ldh-2 (EF_0641) genes. In the ldh-1 promoter region, a potential cre box was identified 114 bp upstream of the ATG start codon (4). cre boxes are cis-acting sequences targeted by CcpA, the major global transcription regulator of carbon catabolite repression in Firmicutes (5). Northern blot analysis showed that ldh-1 is expressed as a monocistronic transcript. The amount of transcript was 2.2-fold lower in a ccpA mutant than in the wild-type strain, suggesting that CcpA activates ldh-1 transcription (4). Most of the activity is associated with LDH-1, and LDH-2 plays only a minor role (2, 3). Recently, ldh deletion mutants of E. faecalis have been constructed and characterized. Deficiency in either LDH-1, LDH-2, or both activities causes a significant increase in the levels of mixed acid fermentation end products in strain V583 (2, 3, 6). All LDH mutants showed growth rates similar to that of the parental strain (2, 6, 7). This demonstrates that the loss of the characteristic metabolic reaction for lactic acid bacteria does not have an obvious impact on fitness under normal growth conditions in E. faecalis. Similar results have been obtained for Lactococcus lactis (8) and Streptococcus pyogenes (7), where little or no difference in the growth rate between the parent and Δldh mutants has been observed. The growth yields were also comparable in E. faecalis when the strains grew under aerobic conditions, but final cell densities were even higher than those for the wild type when the Δldh-1 mutant and the Δldh-1 Δldh-2 double mutant strain grew under anaerobic conditions (2). These combined results demonstrate the impressive metabolic flexibility of E. faecalis. Transcriptomic and proteomic analysis uncovered these rearrangements, since an important number of metabolic genes and proteins were differentially expressed in the ldh double deletion mutant (6).
E. faecalis is considered a very “hard” bacterium, with the capacity to survive in stressing environments that are lethal for other bacteria (9–11). It is also able to survive for months in a desiccated state on surfaces (12). Nongrowing cells of E. faecalis develop multiple, nonspecific stress resistances that increase their capacity for survival under lethal conditions by several orders of magnitude over that of growing cultures (10). Taken together, the remarkable intrinsic ruggedness and resistance to antibiotics of these bacteria are supposed to be the reason why they so successfully persist and spread within health care settings. However, until now this intrinsic ruggedness has not been explained on a molecular level.
Like Staphylococcus aureus, one of the most successful human pathogens, E. faecalis harbors two lactate dehydrogenase genes. Interestingly, a relationship between lactate metabolism and nitrosative stress has been evidenced in S. aureus. Microarray analysis showed that ldh-1 was among the most highly induced genes when S. aureus cultures were exposed to nitric oxide (NO·) (13). Inactivation of the ldh-1 gene impaired growth, and complete loss of lactate production in the ldh-1 ldh-2 double mutant virtually eliminated growth, during nitrosative stress (14). The latter study also demonstrated that the virulence of the Δldh-1 mutant was decreased in mice, and the Δldh-1 Δldh-2 mutant was virtually avirulent. The combined results for S. aureus led to the conclusion that lactate metabolism in general, and especially the NO·-inducible enzyme LDH-1, is crucial for adaptation to nitrosative stress and for resistance to innate immunity in this important human pathogen.
On the basis of these results, we wondered if there was also a link between lactate metabolism, stress responses, and virulence in E. faecalis. We found that LDH-deficient mutants constructed in two different backgrounds are generally more sensitive than wild-type strains to environmental stresses and exhibit attenuated virulence.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. The E. faecalis strains were cultured on M17 medium (15) supplemented with 0.5% (wt/vol) glucose (GM17 medium) at 37°C. Overnight precultures, grown without agitation in 10 ml GM17 broth, were used to inoculate the cultures for growth analysis under both normal and stress conditions. For the acidic pH stress analysis, M17MOPS medium, which contains 200 mM 3-(N-morpholino)propanesulfonic acid (MOPS) as a buffer instead of the glycerophosphate in M17 medium, was used. For growth analysis, optical densities at 600 nm (OD600) were followed on a BioPhotometer (Eppendorf).
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Bacterial strains | ||
| Enterococcus faecalis | ||
| JH 2-2 | Fusr Rifr; plasmid-free wild-type strain | 27 |
| JH2-2 Δldh mutants | JH2-2 mutant strains with deletions in ldh1 or ldh2 or both genes | 3 |
| JH2-2ldh1R158E | JH2-2 ldh-1 mutant with a replacement of the arginine codon at position 158 with a glutamate codon | This study |
| V583 | VanR | 28 |
| V583 Δldh mutants | V583 mutant strains with deletions in either ldh-1, ldh-2, or both genes | 2 |
| Escherichia coli Top10F′ | F′ [lacIq Tn10 (Tetr)] mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| Plasmid and plasmid-bearing strains | ||
| pMSP3535 | 8.35 kb Eryr nisR nisK PnisA (promoter inducible with nisin) | 18 |
| JH2-2/pMSP3535 | JH2-2 harboring pMSP3535 | This study |
| JH2-2 Δldh-1 Δldh-2/pMSP3535 | ldh double deletion mutant (Δldh-1 Δldh-2) harboring pMSP3535 | This study |
| JH2-2 Δldh-1 Δldh-2/pMSP3535::ldh-1 | Δldh-1 Δldh-2 double mutant harboring recombinant pMSP3535 with the ldh-1 gene under the control of the nisA promoter | This study |
Construction of JH2-2 ldh deletion mutants and the complemented strain.
Plasmids and PCR products were purified using a NucleoSpin plasmid kit and a NucleoSpin Extract II kit, respectively (Macherey-Nagel, Düren, Germany). Other standard techniques were carried out as described by Sambrook et al. (16). The E. faecalis JH2-2 Δldh-1 and Δldh-2 mutants were constructed by deletion of 341-bp and 307-bp internal fragments, respectively. The Δldh-1 Δldh-2 double mutant was constructed by introducing the ldh-2 deletion into the Δldh-1 mutant. JH2-2 mutants were constructed by using the oligonucleotides listed in Table 2 and plasmid pMAD according to the protocol published by La Carbona et al. (17). The construction of the E. faecalis V583 Δldh mutants is described in the article of Jönsson and coworkers (2).
Table 2.
Primers used in the current study
| Primer | Sequence (5′–3′)a |
|---|---|
| Ldh1Sma | GCGCCCGGGCGATCTGCGCGCGCTTCTGG |
| Ldh1Sal | GCGGTCGACGGCTCAACAGAGGTGGGGTAC |
| Ldh1Bam1 | GCGGGATCCCACATGCGAATGTCGCTGGC |
| Ldh1Bam2 | GCGGGATCCCATCATGGCAATCGTCATAAG |
| Ldh1verif1 | CCATGTTACTATCACTTTAGC |
| Ldh1verif2 | GCAGCGTCGCGTACGTTGAAG |
| EF0641For | GCTACGGATCCAATCAAGCCCTTTGATGTCC |
| EF0641Rev | GCTACGGTCGACGCTTTCGCTAAATCGTTTTCC |
| EF0641del1 | GCAAACAAGCGACCTATTATG |
| EF0641del2 | GACAGGATTTGAGGCAATCAC |
| EF0641verif1 | CTTTTTCAGCGGCATTCAGATTC |
| EF0641verif2 | CAAGAAAATGTAAACGTCTGGGC |
| ldh13535Bam | CTCTAGGATCCTCACGAAAAAGAATGTAGAGG |
| ldh13535Sph | CTCTAGCATGCGTGATGAGTAAAGTAAAACAG |
| pMad_ldh_Nco_for | AAGGGGCCATGGTGGCCGCCAGCACTACCTTTTTGC |
| pMad_ldh_Bam_rev | AAGGGGGGATCCAATGTGTGTGGCCCAAGAAGAAATC |
| mut_ldh_for | CTGCTGAATTCCGTCAAGCAATTGCCGAATTAGTTG |
| mut_ldh_rev | TTGACGGAATTCAGCAGAATCTAGTGAAGTTCC |
Underlined sequences correspond to restriction sites, and italicized sequences correspond to reverse complementary regions.
The ldh1R158E mutant was generated by site-directed mutagenesis in a 2-step PCR procedure. First, the ldh-1 gene with upstream and downstream sequences was amplified using primer pairs pMad_ldh_Nco_for/Mut_ldh_rev and pMad_ldh_Bam_rev/Mut_ldh_for, respectively (Table 2). The overlapping amplimers were then used as templates for the second step using the pMad_ldh_Nco_for and pMad_ldh_Bam_rev primers, and the PCR product was cloned into the pMAD vector as described previously, generating plasmid pldh1R158E (17). This plasmid was transformed into the JH2-2 Δldh-2 mutant. The replacement of the arginine codon (CGT) with the glutamic acid codon (GAA) was verified by sequencing, and the absence of lactate production in the ldh1R158E mutant was confirmed by high-performance liquid chromatography (HPLC) (data not shown).
For complementation experiments, the ldh-1 gene, including its ribosome binding site (RBS), was amplified with oligonucleotides ldh13535Bam and ldh13535Sph (Table 2) and was cloned into plasmid pMSP3535 (18) under the control of the nisin-inducible promoter of that vector. The plasmid was then transformed into the JH2-2 Δldh-1 Δldh-2 double mutant. Wild-type JH2-2 and the Δldh-1 Δldh-2 double mutant strain, each harboring the empty pMSP3535 plasmid, were used as controls. Where indicated, nisin was added to cultures at an OD600 of 0.2 to a final concentration of 0.5 μg/ml.
Growth of E. faecalis strains under stress conditions.
For H2O2, HOCl, SDS, ethanol, and dimethyl sulfoxide (DMSO) stresses, cells were grown in GM17 medium until they reached an OD600 of 0.2. At this moment, the stressing agent was added. Except for the H2O2 experiments, cultures were grown in 30-ml tubes containing 10 ml of medium and were incubated before and after the addition of the stressing agents without agitation in a 37°C water bath. For H2O2, cultures were grown in 150-ml Erlenmeyer flasks filled with 15 ml of medium and were incubated before and after the addition of the peroxide with agitation at 120 rpm in a 37°C incubator. For the NaCl, glucose, and acid stresses, the cultures were grown in GM17 medium to an OD600 of 0.2. At this moment, cultures were centrifuged (5,000 × g, 4°C, 10 min), and the cells were taken up in GM17 medium containing 8% NaCl or 10% glucose or in M17MOPS medium with 0.25% glucose adjusted with HCl to pH 4.5. In the serum experiments, the cells were cultured in brain heart infusion (BHI) medium containing 40% filter-sterilized serum, in 30-ml tubes with 10 ml of medium, and were incubated without agitation in a 37°C water bath. The final concentrations of the stressing agents were as follows: 2.5 mM H2O2, 0.25% HOCl, 8% ethanol, 0.01% SDS, 10% DMSO, 8% NaCl, and 10% glucose.
Virulence experiments.
To test the virulence of both JH2-2 and V583 ldh deletion mutants, we used an adaptation of a well-established intravenous infection model (19), as described by Michaux et al. previously (20). Briefly, the strains were cultured overnight in BHI broth supplemented with 40% heat-inactivated horse serum; the cultures were centrifuged; and the resulting pellets were resuspended in sterile phosphate-buffered saline (PBS). These bacterial suspensions contained 1 × 109 bacteria/ml. A 100-μl aliquot from each strain suspension was injected into the tail vein of each of five female BALB/c mice (10 weeks old; Harlan Italy Srl, San Pietro al Natisone, Udine, Italy). Seven days after infection, mice were sacrificed, and their organs (kidneys and livers) were removed aseptically, weighed, and homogenized. Serial homogenate dilutions were plated onto Enterococcus selective agar (Fluka Analytical, Switzerland) and were used for the determination of CFU/g organ. CFU counts resulting from three replicated experiments (for a total of 15 mice per infection group) were analyzed by the unpaired t test.
RESULTS AND DISCUSSION
LDH-deficient mutants are generally stress sensitive.
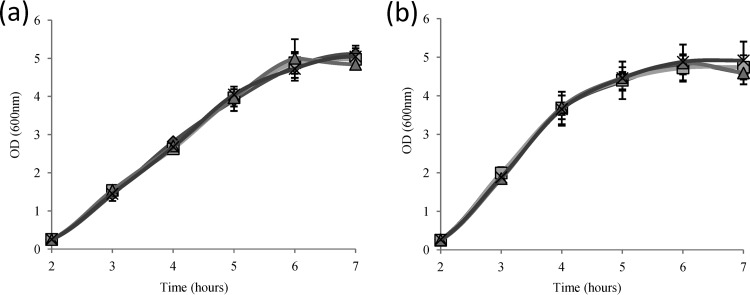
In order to evaluate the metabolic potential of E. faecalis for ethanol production, we recently constructed LDH deletion mutants in strain JH2-2 (3). E. faecalis harbors two separate genes, named ldh-1 and ldh-2, encoding lactate dehydrogenases named LDH-1 and LDH-2. Deletion mutants of each ldh gene, as well as an Δldh-1 Δldh-2 double mutant, were constructed in strain JH2-2. Corresponding LDH deletion mutants of the E. faecalis clinical isolate V583 have also been constructed and characterized recently (2). The results showed that LDH-1 is the main enzyme in lactate production, which we confirmed for strain JH2-2 in our previous study (3). The growth rates of the V583 LDH deletion mutants and of the corresponding wild-type strain were comparable in chemically defined medium (2). This was confirmed and was also found for wild-type V583 and JH2-2 and their ldh deletion mutants in complex GM17 medium (Fig. 1). The final pH values were pH 6 for wild-type JH2-2 and the Δldh-2 strain and pH 6.2 for the isogenic Δldh-1 mutant and the Δldh-1 Δldh-2 double mutant under these experimental conditions.
Fig 1.
Aerobic growth of wild-type E. faecalis JH2-2 (a) and V583 (b) and their isogenic ldh deletion mutants in GM17 medium. Diamonds, wild type; squares, Δldh-1 mutant; triangles, Δldh-2 mutant; multiplication signs, Δldh-1 Δldh-2 mutant.
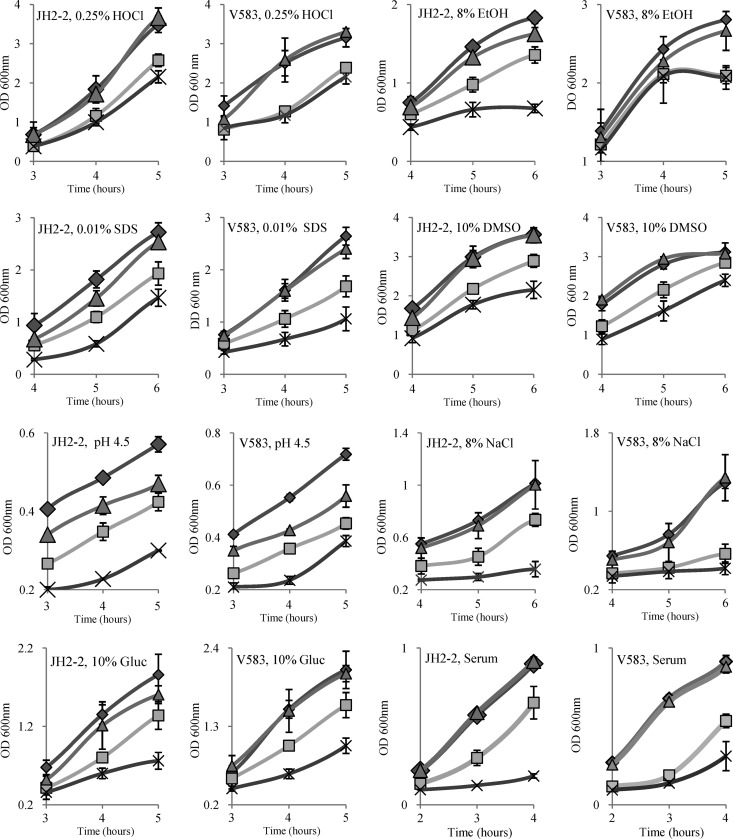
LDH mutants of S. aureus have been shown to be sensitive to nitrosative stress (14). We wondered if this would also be the case for enterococci. To test this possibility, cultures of the JH2-2 wild-type strain and the ldh double deletion mutant were exposed to 1 mM NO· donor S-nitroso-N-acetyl-dl-penicillamine (SNAP). The Δldh-1 Δldh-2 double mutant was more sensitive than the wild type to this treatment. However, this difference in survival was also observed with the control compound NAP (N-acetyl-dl-penicillamine), which does not generate NO. Since the compounds were dissolved in DMSO, the only plausible explanation of the results obtained was that Δldh mutants were sensitive to the solvent and not to the NO·-generating agent. This was tested by exposing the strains to 10% DMSO (Fig. 2). This experiment showed, indeed, that the growth rate of the JH2-2 Δldh-1 mutant and, even more, that of the JH2-2 Δldh-1 Δldh-2 double mutant were lower than that of the parent strain. In contrast, the Δldh-2 mutant grew as well as the wild-type strain (Fig. 2).
Fig 2.
Growth of wild-type JH2-2 and V583 cells (filled diamonds) and their isogenic ldh-deficient mutants (shaded triangles, Δldh-2 mutant; shaded squares, Δldh-1 mutant; multiplication signs, Δldh-1 Δldh-2 mutant) in different stressing environments. Only relevant parts of the growth curves are shown for each treatment. EtOH, ethanol; Gluc, glucose. See Fig. S2 in the supplemental material for full growth curves.
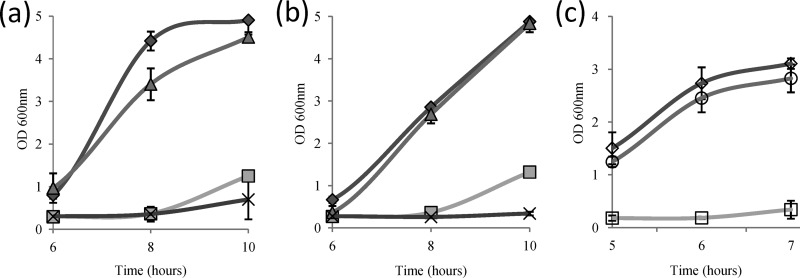
Since an effect of reactive nitrogen species (RNS) on the physiology of the bacterium might have been masked by the stress imposed by the solvent DMSO, we subsequently tested the resistance of the various ldh deletion mutants of both strains (JH2-2 and V583) to reactive oxygen species (ROS). The growth kinetics of wild-type JH2-2 and V583 and their corresponding ldh deletion mutant strains were monitored in the presence of 2.5 mM H2O2 (Fig. 3a and b). This treatment provoked an extended growth lag of several hours for all strains. In both backgrounds, Ldh-2-deficient mutants showed little (JH2-2 Δldh-2) or no (V583 Δldh-2) difference in growth recovery from the corresponding parent strains. In contrast, the JH2-2 and V583 Δldh-1 single mutants and Δldh-1 Δldh-2 double mutants were greatly affected by this treatment (Fig. 3a and b). The Δldh-1 mutants escaped from growth inhibition earlier (significant growth after 8 h of incubation) than the corresponding double mutants. This difference was more pronounced in the V583 background, where the Δldh-1 Δldh-2 mutant demonstrated no growth even at the end of the experiment. Bringing back the wild-type ldh-1 gene on a plasmid under the control of a nisin-inducible promoter into the JH2-2 Δldh-1 Δldh-2 double mutant restored growth to near-wild-type levels (Fig. 3c), demonstrating that LDH deficiency is responsible for the observed sensitivity to the oxidant.
Fig 3.
Growth of wild-type E. faecalis, ldh deletion mutants, and a complemented strain in the presence of 2.5 mM H2O2. (a) Wild-type JH2-2 (filled diamonds) and isogenic ldh deletion mutants (shaded triangles, Δldh-2 mutant; shaded squares, Δldh-1 mutant; multiplication signs, Δldh-1 Δldh-2 mutant). (b) Wild-type V583 (filled diamonds) and isogenic ldh deletion mutants (shaded triangles, Δldh-2 mutant; shaded squares, Δldh-1 mutant; multiplication signs, Δldh-1 Δldh-2 mutant). (c) Wild-type JH2-2 with plasmid pMSP3535 (open diamond), JH2-2 Δldh-1 Δldh-2/pMSP3535 (open squares), and the complemented strain JH2-2 Δldh-1 Δldh-2/pMSP3535::ldh1 (open circles). Nisin was added at an OD600 of 0.2 to a final concentration of 0.5 μg/ml. Only relevant parts of the growth curves are shown, but full growth curves are presented in Fig. S1 in the supplemental material.
Since we observed that Δldh mutants were more susceptible to such different treatments as a solvent and an oxidant, we wondered if a deficiency in lactate production might have a more general negative effect on stress resistance. Therefore, we tested the growth of the parent strains and the Δldh mutants in the presence of various stressing agents. As can be seen from Fig. 2, the growth of the JH2-2 and V583 Δldh-1 mutants and, especially, the Δldh-1 Δldh-2 double mutants was always more affected in the presence of ethanol (8%), the strong oxidant hypochlorite (0.25%), or the detergent SDS (0.01%), at an acidic pH (pH 4.5), or under conditions of high osmolarity (8% NaCl or 10% glucose) than that of their parent strains (see also Fig. S2 in the supplemental material). Except for ethanol, which had greater effects in the JH2-2 background, the effects of the stresses on the growth of the wild-type and mutant strains were very comparable for the JH2-2 and V583 backgrounds. The growth performance of the Δldh-2 mutants under most stress conditions was similar to that of the corresponding wild-type strains, with the exception of growth at pH 4.5. Under these conditions, the Δldh-2 mutant was slightly but reproducibly more susceptible than the parent strain (Fig. 2).
The combined results seem to indicate that the characteristic intrinsic ruggedness of E. faecalis under environmental stresses is dependent on the metabolic capacity to reduce pyruvate to lactate by lactate dehydrogenase. However, the possibility that the growth deficiency of ldh deletion mutants in stressing environments is related not to the activity of the LDH proteins but simply to their physical disappearance could not be excluded. We therefore constructed another ldh double mutant by introducing an ldh-1 allele with point mutations in the substrate binding domain (http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?uid=96482), leading to the replacement of the critical arginine at position 158 of the enzyme with glutamate, into the ldh-2 mutant (see Materials and Methods for more details). Arginines at this position in LDH interact with the carboxyl group of pyruvate. The replacement of this residue should therefore lead to inactivation of the enzyme, and HPLC analysis of the Δldh1R158E/ldh-2 mutant confirmed the absence of lactate production (data not shown). This new mutant showed a fitness deficiency with regard to growth in stressing environments comparable to that of the Δldh-1 Δldh-2 double mutants, supporting our initial hypothesis that a deficiency in LDH activity is responsible for the elevated stress sensibility (data not shown).
What might be the molecular explanations for the observed multiple stress sensitivities of LDH-deficient mutants? Based on data in the literature, at least two hypotheses, which are not mutually exclusive, could be proposed. In the first, the functioning of the alternative pathways for redox homeostasis in the LDH-deficient strains may be generally more sensitive to perturbations of cell physiology in stressing environments than the maintenance of redox homeostasis via LDH. Some results obtained with S. aureus and E. faecalis point in this direction. Indeed, the activities at the beginning of these alternative pathways, i.e., pyruvate dehydrogenase (PDH) and pyruvate formate lyase (PFL), are strongly inhibited by NO· in extracts of S. aureus cells, whereas LDH remains nearly 100% active under these conditions (14). Furthermore, the PFL activity has also been shown to be sensitive to an acidic pH in E. faecalis (21). Interestingly, expression of the operons encoding PDH and PFL is induced in the V583 Δldh-1 Δldh-2 double mutant (6). Both PDH and PFL compete with LDH for the substrate pyruvate. PDH generates acetyl coenzyme A (acetyl-CoA) in the following oxidative decarboxylation reaction: pyruvate + NAD+ + CoASH → acetyl-CoA + NADH + CO2 (where CoASH is coenzyme A). PFL converts pyruvate to acetyl-CoA and formate in the following reaction: pyruvate + CoASH → acetyl-CoA + formate. The redox balance can then be achieved by reducing acetyl-CoA to ethanol, which allows the oxidation of 2 NADH molecules to NAD+.
The second explanation could be that lactate formation may trigger a process that induces a general stress resistance response. This hypothesis seems to be supported by results from cancer research. Most solid tumors are characterized by increased glucose uptake and lactate formation (22). Studies on experimental tumors have shown that lactate concentrations are positively correlated with radioresistance (23), and a high intratumor lactate concentration might also be responsible for the chemoresistance of some tumors (24).
In any case, our results clearly demonstrate that the fitness of LDH-deficient strains is significantly reduced in stressing environments. We wondered if this LDH deficiency also influences the virulence of the mutants.
LDH-deficient mutants exhibit attenuated virulence.
Pathogens invading a host are exposed to numerous stresses, and stress resistance has been correlated with virulence (25). To analyze if LDH deficiency reduces the resistance of E. faecalis in vivo, we first tested the growth of the ldh deletion mutants and of the parent strains, JH2-2 and V583, in BHI medium containing 40% horse serum (Fig. 2). LDH-2 deficiency had no effect on growth relative to the growth of controls under these conditions. In contrast, the growth of the Δldh-1 mutants was significantly reduced, and growth restriction was even more pronounced in the Δldh-1 Δldh-2 double mutants. Indeed, whereas the wild-type strains reached an OD600 of ca. 0.63 after 3 h of growth, the strain deficient in both LDH-1 and LDH-2 showed no significant growth until this time point. Inhibition of the growth of the ldh double deletion mutant was also observed by using serum pretreated at 56°C for 30 min, a treatment generally used to inactivate the complement system. However, the growth of the Δldh-1 Δldh-2 double mutant was comparable to that of the wild-type strain in BHI medium containing 40% serum treated at 65°C for 30 min, demonstrating that proteins more heat stable than the complement system are involved in this inhibition of the growth of LDH-deficient strains.
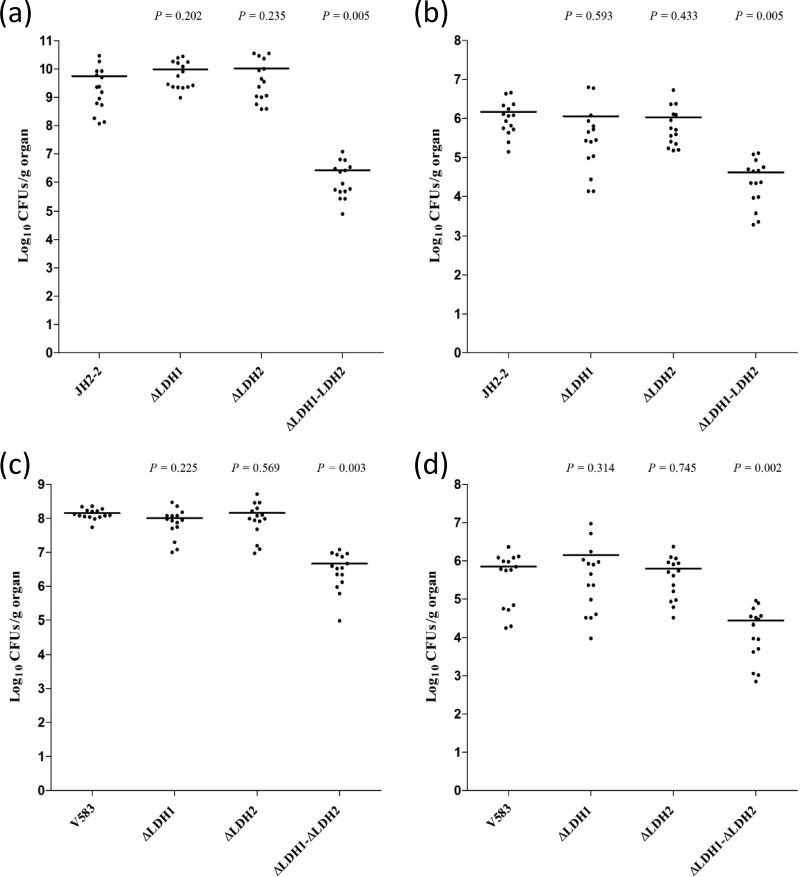
Then we assessed whether the single Δldh-1 or Δldh-2 mutation or the double Δldh-1 Δldh-2 mutation could affect the virulence of JH2-2 and V583 by comparing the mutant and wild-type strains in a murine model of systemic infection. Seven days after intravenous bacterial challenge, groups of mice were sacrificed, and their kidneys and livers were removed for the enumeration of viable bacteria. As shown in Fig. 4, the counts (in CFU per gram of tissue) of both JH2-2 (Fig. 4a and b) and V583 (Fig. 4c and d) in the infected organs did not differ significantly between the single mutants and the parent strains. Conversely, the numbers of cells of the Δldh-1 Δldh-2 double mutants recovered were significantly lower in both the kidneys (P, 0.005 for JH2-2 and 0.003 for V583) and the liver (P, 0.005 for JH2-2 and 0.002 for V583) than those of the wild-type strains in both the JH2-2 and V583 backgrounds (Fig. 4). These findings indicate that functionally active Δldh-1 and Δldh-2 genes contribute together to enterococcal persistence within mouse organs, since only the simultaneous inactivation of both genes greatly reduces the ability of E. faecalis to colonize and infect the host. From these results, it can also be deduced that the residual activity of LDH-2 in the Δldh-1 single mutant seems to be sufficient for colonization of the organs. Of note also, strain JH2-2 accumulated to very high numbers in the kidneys compared to strain V583, but the corresponding double mutant did not grow to numbers higher than those of the V583 homologous mutant.
Fig 4.
Effect of ldh deficiency on enterococcal burdens within mouse organs. Groups of BALB/c mice (five per group) were infected intravenously with the wild-type strain JH2-2 or V583 or with the Δldh-1, Δldh-2, or Δldh-1 Δldh-2 isogenic mutant. After 7 days of infection, the mice were sacrificed and necropsied. Organs were homogenized, and bacterial cells on agar plates were counted. Each infection experiment was repeated three times. (a and c) Log10 CFU count per gram of kidney pairs; (b and d) log10 CFU count per gram of liver. Horizontal bars represent geometric means. P values of <0.05 were considered to be significant.
In conclusion, the results presented in this report establish for the first time that the intrinsic ruggedness of E. faecalis, supposed to be important for its persistence in hospital settings and its capacity to cause infections, can be weakened by disabling its capacity to maintain redox balance via the LDH reaction. Furthermore, we showed that this also decreases fitness during infection, arguing that LDH may be an attractive drug target. Such a drug would have additional applications. Glycopeptide antibiotics such as vancomycin inhibit peptidoglycan synthesis by binding to the C-terminal d-alanyl-d-alanine of pentapeptide precursors, preventing transglycosylation and transpeptidation in cell wall assembly. Vancomycin resistance is based on the synthesis of modified peptidoglycan precursors ending in d-alanyl-d-lactate, to which glycopeptides exhibit low binding affinities. d-Lactate is synthesized by VanH dehydrogenase, a d-LDH converting pyruvate into d-lactate (26). Inactivation of this enzyme would counteract glycopeptide resistance. Finally, drugs inactivating LDH might also be useful for increasing the sensitivity of tumors to radiation and/or chemotherapy (23, 24).
Supplementary Material
ACKNOWLEDGMENTS
We thank Isabelle Rincé for excellent technical assistance.
N.F.R. was the recipient of a doctoral fellowship from the Higher Education Commission of Pakistan. The work of I.N. was supported by the SysMO-LAB project, which is financed by the Research Council of Norway.
Footnotes
Published ahead of print 6 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01299-12.
REFERENCES
- 1. Brown AT, Wittenberger CL. 1971. Mechanism for regulating the distribution of glucose carbon between the Embden-Meyerhof and hexose-monophosphate pathways in Streptococcus faecalis. J. Bacteriol. 106:456–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jönsson M, Saleihan Z, Nes IF, Holo H. 2009. Construction and characterization of three lactate dehydrogenase-negative Enterococcus faecalis V583 mutants. Appl. Environ. Microbiol. 75:4901–4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rana NF, Gente S, Rincé A, Auffray Y, Laplace JM. 2012. Genetic modifications and introduction of heterologous pdc genes in Enterococcus faecalis for its use in production of bioethanol. Biotechnol. Lett. 34:1651–1657 [DOI] [PubMed] [Google Scholar]
- 4. Leboeuf C, Leblanc L, Auffray Y, Hartke A. 2000. Characterization of the ccpA gene of Enterococcus faecalis: identification of starvation-inducible proteins regulated by CcpA. J. Bacteriol. 182:5799–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deutscher J. 2008. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 11:87–93 [DOI] [PubMed] [Google Scholar]
- 6. Mehmeti I, Jönsson M, Fergestad EM, Mathiesen G, Nes IF, Holo H. 2011. Transcriptome, proteome, and metabolite analyses of a lactate dehydrogenase-negative mutant of Enterococcus faecalis V583. Appl. Environ. Microbiol. 77:2406–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fiedler T, Bekker M, Jonsson M, Mehmeti I, Pritzschke A, Siemens N, Nes I, Hugenholtz J, Kreikemeyer B. 2011. Characterization of three lactic acid bacteria and their isogenic ldh deletion mutants shows optimization for YATP (cell mass produced per mole of ATP) at their physiological pHs. Appl. Environ. Microbiol. 77:612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bongers RS, Hoefnagel MHN, Starrenburg MJC, Siemerink MAJ, Arends JGA, Hugenholtz J, Kleerebezem M. 2003. IS981-mediated adaptive evolution recovers lactate production by ldhB transcription activation in a lactate dehydrogenase-deficient strain of Lactococcus lactis. J. Bacteriol. 185:4499–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sherman JM. 1938. The enterococci and related streptococci. J. Bacteriol. 35:81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giard J-C, Rincé A, Benachour A, Hartke A, Laplace JM, Le Breton Y, Pichereau V, Verneuil N, Auffray Y. 2003. The response to environmental stresses in Enterococcus faecalis. Recent Res. Dev. Microbiol. 7:325–339 [Google Scholar]
- 11. Rincé A, Le Breton Y, Verneuil N, Giard Hartke J-CA, Auffray Y. 2003. Physiological and molecular aspects of bile salt response in Enterococcus faecalis. Int. J. Food Microbiol. 88:207–213 [DOI] [PubMed] [Google Scholar]
- 12. Kramer A, Schwebke I, Kampf G. 2006. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 6:130. 10.1186/1471-2334-6-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richardson AR, Dunman PM, Fang FC. 2006. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol. Microbiol. 61:927–939 [DOI] [PubMed] [Google Scholar]
- 14. Richardson AR, Libby SJ, Fang FC. 2008. A nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunity. Science 319:1672–1676 [DOI] [PubMed] [Google Scholar]
- 15. Terzaghi BE, Sandine WE. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning; a laboratory manual, 2nd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 17. La Carbona S, Sauvageot N, Giard J-C, Benachour A, Posteraro B, Auffray Y, Sanguinetti M, Hartke A. 2007. Comparative study of the physiological roles of three peroxidases (NADH peroxidase, alkyl hydroperoxide reductase and thiol peroxidase) in oxidative stress response, survival inside macrophages and virulence of Enterococcus faecalis. Mol. Microbiol. 66:1148–1163 [DOI] [PubMed] [Google Scholar]
- 18. Bryan EM, Bae T, Kleerebezem M, Dunny GM. 2000. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid 44:183–190 [DOI] [PubMed] [Google Scholar]
- 19. Gentry-Weeks C, Estay M, Loui C, Baker D. 2003. Intravenous mouse infection model for studying the pathology of Enterococcus faecalis infections. Infect. Immun. 71:1434–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michaux C, Sanguinetti M, Reffuveille F, Auffray Y, Posteraro B, Gilmore MS, Hartke A, Giard J-C. 2011. SlyA is a transcriptional regulator involved in the virulence of Enterococcus faecalis. Infect. Immun. 79:2638–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Snoep JL, van Bommel M, Lubbers F, Teixeira de Mattos MJ, Neijssel OM. 1993. The role of lipoic acid in product formation by Enterococcus faecalis NCTC 775 and reconstitution in vivo and in vitro of the pyruvate dehydrogenase complex. J. Gen. Microbiol. 139(Part 6):1325–1329 [DOI] [PubMed] [Google Scholar]
- 22. DeBerardinis RJ. 2008. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet. Med. 10:767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sattler UGA, Meyer SS, Quennet V, Hoerner C, Knoerzer H, Fabian C, Yaromina A, Zips D, Walenta S, Baumann M, Mueller-Klieser W. 2010. Glycolytic metabolism and tumour response to fractionated irradiation. Radiother. Oncol. 94:102–109 [DOI] [PubMed] [Google Scholar]
- 24. Hirschhaeuser F, Sattler UGA, Mueller-Klieser W. 2011. Lactate: a metabolic key player in cancer. Cancer Res. 71:6921–6925 [DOI] [PubMed] [Google Scholar]
- 25. Riboulet E, Verneuil N, La Carbona S, Sauvageot N, Auffray Y, Hartke A, Giard J-C. 2007. Relationships between oxidative stress response and virulence in Enterococcus faecalis. J. Mol. Microbiol. Biotechnol. 13:140–146 [DOI] [PubMed] [Google Scholar]
- 26. Leclercq R, Courvalin P. 1997. Resistance to glycopeptides in enterococci. Clin. Infect. Dis. 24:545–554 [DOI] [PubMed] [Google Scholar]
- 27. Yagi Y, Clewell DB. 1980. Recombination-deficient mutant of Streptococcus faecalis. J. Bacteriol. 143:966–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahm DF, Kissinger J, Gilmore MS, Murray PR, Mulder R, Solliday J, Clarke B. 1989. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 33:1588–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.