Abstract
We performed a meta-analysis to evaluate use of PCR assays for diagnosis of prosthetic joint infection (PJI). The pooled sensitivity and specificity were 0.86 (95% confidence interval [CI], 0.77 to 0.92) and 0.91 (CI, 0.81 to 0.96), respectively. Subgroup analyses showed that use of tissue samples may improve sensitivity, and quantitative PCR and sonication of prostheses fluid may improve specificity. The results showed that PCR is reliable and accurate for detection of PJI.
TEXT
Prosthetic joint infection (PJI) is one of the most common complications of total joint arthroplasty, with an incidence of 1 to 12%, and it always has catastrophic consequences (1, 2). The distinction between PJI and other causes of joint failure, such as aseptic loosening, is frequently difficult and still challenging. Several studies have assessed the diagnostic value of PCR techniques for diagnosing PJI. However, the true diagnostic capabilities of PCR assays remain controversial. Therefore, the aim of our study was to perform a meta-analysis to evaluate the detection validity of PCR in the diagnosis of PJI.
We searched MEDLINE, EMBASE, and OVID for articles that were published between January 1990 and February 2013, using the following medical subject headings (MeSH) or free text words: (i) joint prosthesis, prosthesis infection, septic loosening, aseptic loosening, replacement, or arthroplasty and (ii) PCR. We also manually searched the reference lists of eligible studies and review articles. Our reviewers independently evaluated the selected studies using the following inclusion criteria: (i) the study reported the accuracy of PCR for the diagnosis of joint infection in comparison with visible purulence of joint aspirate or surgical site, presence of a sinus tract (fistula) communicating with the prosthesis, acute inflammation in histopathology sections of periprosthetic tissue, or simultaneously obtained microbiologic cultures from at least two periprosthetic tissue samples (the reference standard); (ii) sufficient data were reported to allow us to calculate the true-positive (TP), false-negative (FN), false-positive (FP), and true-negative (TN) values; (iii) the study reported evaluations of at least 10 patients, from which data could be extracted using our standardized data collection form (X. Qu and Z. Zhai). Discrepancies were resolved by discussion with other investigators and by consulting the original articles (Huiwu Li and K. Dai). We estimated the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the curve (AUC) of summary receiver operating characteristic (ROC) curves to evaluate the capability of PCR assays for diagnosing PJI. We performed meta-regression and subgroup analyses to assess potential heterogeneity, and we constructed Deeks' funnel plot asymmetry test to evaluate potential publication bias. All of the statistical analyses were undertaken using STATA version 11 (StataCorp, College Station, TX).
Our research yielded 2,024 primary studies. Of these, 1,834 were excluded after reviewing the title and abstract, and 190 were excluded after reviewing the full article. A total of 14 articles (3–16) (that included 1,480 patients in total) fulfilled all of the inclusion criteria and were included in the analysis (Table 1; see also Table S1 in the supplemental material). Twelve studies reported patients with FP results. Eight studies used fresh samples, and five used frozen samples. Nine studies detected PJI of multiple joints, two each detected PJI of the hip and knee, and one detected PJI of the shoulder. Eight studies enrolled patients prospectively. Patient enrollments were consecutive in seven studies and were not documented in another seven. We found significant heterogeneity among all test performances.
Table 1.
Characteristics of the 14 reports in our meta-analysis of the diagnosis of PJI based on PCRa
| Study (reference) | Country | No. of patients | Mean age (yrs) | Study design, enrollment | Sample type | Sample condition | Sample site(s) | PCR type | Target gene | Diagnostic criteria of PJI |
|---|---|---|---|---|---|---|---|---|---|---|
| Portillo et al., 2012 (15) | Spain | 86 | 73 | Prospective, consecutive | Sonicated PF | Fresh | Hip, knee, elbow, and shoulder | RT multiplex PCR | NA | IOF, M |
| Marín et al., 2012 (11) | Spain | 122 | 72 | Prospective, NA | BS or SFS | Fresh | Hip, knee, elbow, and shoulder | PCR | 16S rRNA gene | IOF, H |
| Jacovides et al., 2012 (7) | United States | 80 | 67 | Prospective, consecutive | SFS | Frozen | Hip and knee | PCR | 16S rRNA gene | IOF, M |
| Gomez et al., 2012 (6) | United States | 366 | 66 | Retrospective, NA | Sonicated PF | Frozen | Hip and knee | RT-qPCR | 16S rRNA gene | IOF, H |
| Esteban et al., 2012 (4) | Spain | 75 | 66 | NA, consecutive | Sonicated PF | Frozen | Hip and knee | RT-PCR | 16S rRNA gene | M |
| Bergin et al., 2010 (3) | United States | 64 | NA | Prospective, consecutive | SFS | NA | Knee | RT-qPCR | 16S rRNA gene | IOF, H, M |
| Piper et al., 2009 (11) | United States | 134 | 65 | NA, NA | Sonicated PF | Frozen | Shoulder | RT-qPCR | S probes; P 16S rRNA gene | IOF, H |
| Kobayashi et al., 2009 (8) | Japan | 24 | NA | Prospective, consecutive | BS | Fresh | Hip and knee | RT-qPCR | 16S rRNA gene | M |
| Kobayashi et al., 2008 (9) | Japan | 54 | NA | Prospective, consecutive | BS | Fresh | Hip and knee | RT multiplex qPCR | S and P probes | M |
| Gallo et al., 2008 (5) | Czech Republic | 101 | 66 | Prospective, NA | SFS | Fresh | Hip, knee, and elbow | PCR | 16S rRNA gene | IOF, H, M, R |
| Moojen et al., 2007 (12) | Netherlands | 76 | NA | Retrospective, NA | BS | Fresh | Hip | qPCR | 16S rRNA gene | IOF, H, M, R |
| Panousis et al., 2005 (13) | United Kingdom | 91 | 66 | Prospective, consecutive | SFS | Fresh | Hip and knee | Broad-range PCR | 16S rRNA gene | IOF, M |
| Tunney et al., 1999 (16) | United Kingdom | 119 | NA | NA, NA | BS | Fresh | Hip | PCR | 16S rRNA gene | M |
| Mariani et al., 1996 (10) | United States | 50 | NA | NA, NA | SFS | Frozen | Knee | PCR | 16S rRNA gene | M |
Abbreviations: PF, prosthesis fluid; BS, biopsy sample; SFS, synovial fluid sample; RT, real time; qPCR, quantitative PCR; P, Propionibacterium; S, Staphylococcus; H, histological examination; IOF, intraoperative finding; M, microbiological or laboratory examination; R, radiological examination; NA, not available.
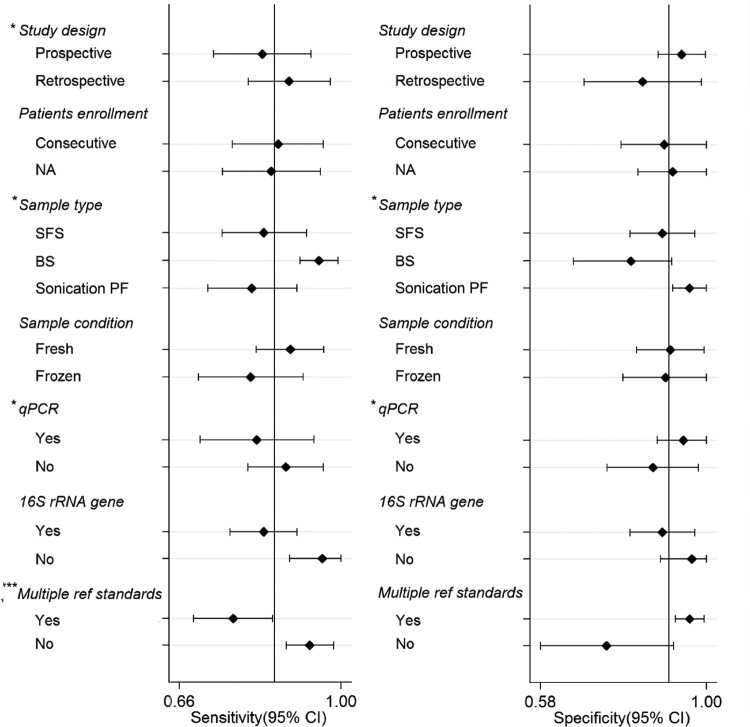
The pooled sensitivity, specificity, PLR, NLR, DOR, and AUC estimates for the detection of PJI using PCR were 0.86 (95% confidence interval [CI], 0.77 to 0.92), 0.91 (CI, 0.81 to 0.96), 9.1 (CI, 4.6 to 18.2), 0.16 (CI, 0.10 to 0.25), 59 (CI, 29 to 118), and 0.94 (CI, 0.91 to 0.95), respectively (Fig. 1). The regression test of asymmetry found no evidence of a small-study effect for PCR (P = 0.64) (see Fig. S1 in the supplemental material). In subgroup analyses, the test performances varied by study design, sample type, sonication of samples, type of PCR, and reference standards (Fig. 2). The sensitivity and specificity of the tissue samples were 0.95 (CI, 0.91 to 0.99) and 0.81 (CI, 0.66 to 0.90), the sensitivity and specificity of the synovial fluid samples were 0.84 (CI, 0.75 to 0.93) and 0.89 (CI, 0.81 to 0.97), and those of the sonicated prostheses fluid samples were 0.81 (CI, 0.71 to 0.91) and 0.96 (CI, 0.92 to 1.00), respectively. Use of multiple reference standards had the lowest sensitivity, at 0.77 (CI, 0.69 to 0.85), and the highest specificity, at 0.96 (CI, 0.92 to 0.99). Compared with nonquantitative PCR, quantitative PCR had a higher specificity of 0.94 (CI, 0.88 to 1.00) (P < 0.05). The sensitivity and specificity of the fresh samples were 0.89 (CI, 0.82 to 0.96) and 0.91 (CI, 0.82 to 0.99), and those of the frozen samples were 0.81 (CI, 0.70 to 0.92) and 0.90 (CI, 0.79 to 1.00), respectively.
Fig 1.
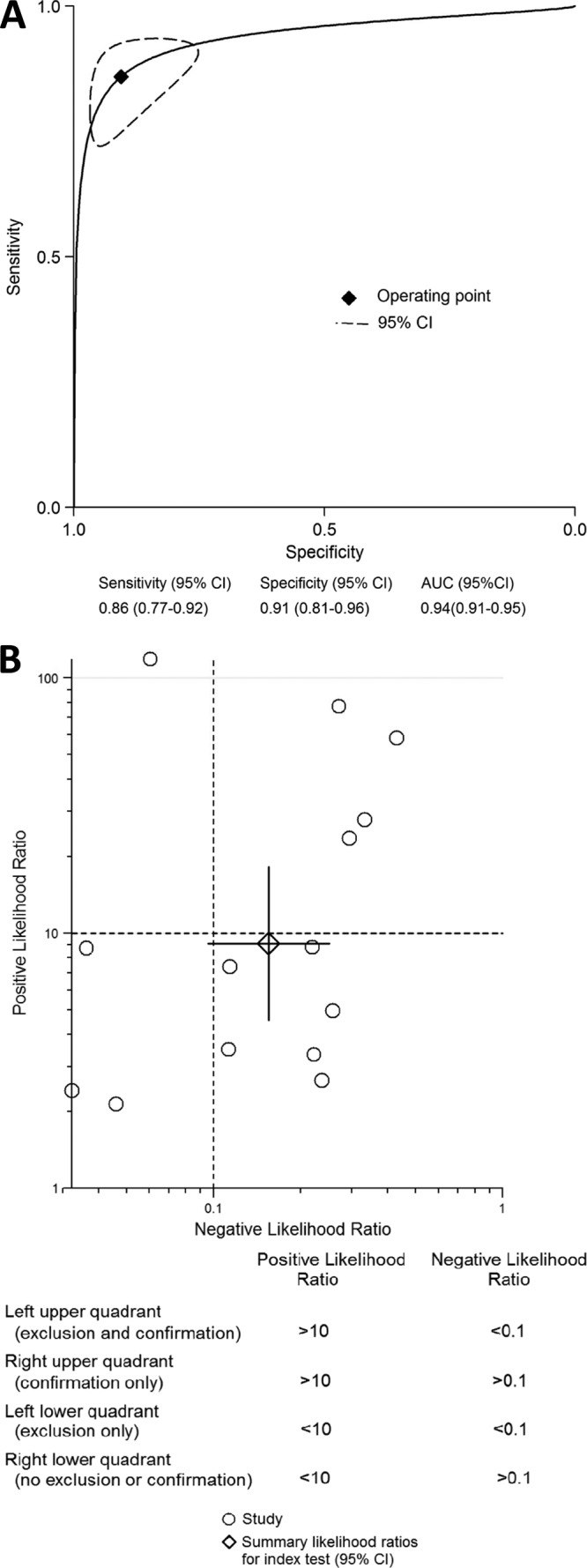
Summary ROC curves (A) and likelihood ratio scattergram (B) for PCR. Curves include a summary operating point for sensitivity and specificity on the curve and a 95% confidence contour ellipsoid. The likelihood ratio profile shows that PCR is a potent tool for ruling out PJI in this patient population.
Fig 2.
Forest plots of subgroup analyses of sensitivity and specificity. BS, biopsy sample; SFS, synovial fluid sample.
Overall, in this meta-analysis we found that PCR has adequate diagnostic value for the detection of PJI. It was estimated that, in current practice, the sensitivity and specificity of PCR are approximately 86% and 91%, respectively.
Because of the absence of highly accurate diagnostic methods, the gold standard for diagnosis of PJI is still controversial among clinicians (17). Intraoperative tissue culture has historically been used as the gold standard in most hospitals, although several other tests are available (17). However, the results of culture do not have optimal sensitivity or specificity and are sometimes difficult to interpret, especially when few samples are analyzed (11). The sensitivity of culture ranges from 0.7 to 0.9, and the specificity ranges from 0.75 to 0.95 (3, 11, 17–20). In recent years, PCR methods for the diagnosis of PJI have been investigated and have received much attention. Compared to intraoperative tissue culture, PCR theoretically has higher sensitivity, a faster turnaround time, and is not as affected by treatment (21). Guidelines for PJI by the American Academy of Orthopaedic Surgeons and the Infectious Diseases Society of America recommend further “high evidence”-based studies to assess the diagnostic value of PCR (22, 23).
Our results showed that PCR is another diagnostic method that has an equivalent or better diagnostic value to that of intraoperative tissue culture and may add important insight into the diagnosis of PJI. However, the main problem in the diagnosis of PJI is recovery of bacteria from the samples. Whether relying on intraoperative tissue culture or PCR, the bacterial recovery from the samples is always one of the most important aspects in the diagnosis of PJI. In this meta-analysis, there were three types of samples for PCR: tissue samples, synovial fluid samples, and sonicated prostheses fluid samples. Our subgroup analyses showed that use of tissue samples may improve sensitivity and that sonication of prostheses fluid samples may improve specificity. However, none of the sampling methods can satisfy both increased sensitivity and increased specificity concurrently. Perhaps vortexing of tissue samples by using sonicated prostheses fluid may offer an additional insight into the improvement of sensitivity and specificity concurrently in the diagnosis of PJI.
Moreover, the number of samples taken for PCR may impact the diagnostic sensitivity and specificity of PCR (11). Marín et al. showed that when only considering the number of positive samples, a PCR-positive result in one sample had good specificity and a positive predictive value for PJI (specificity, 0.96; positive predictive value, 0.92). The best combination of results for PCR was observed when 5 samples were studied and the same microorganism was detected in 2 of them (sensitivity, 0.94; specificity, 1.00) (11). In addition, in our meta-analysis, there were 80 false-negative results from 12 studies. Most of the included studies explained that the false-negative resulted from the patient receiving antibiotics previous to sampling (3, 5–8, 11–15).
Compared to intraoperative tissue culture, PCR is expensive and involves complex techniques. To assess the value of PCR, cost-effectiveness studies should be conducted. Furthermore, we must highlight that PCR can serve as a valuable additional tool for diagnosing PJI, but it cannot replace intraoperative tissue culture, since the antibiotic susceptibility testing included in the tissue culture method is highly important for adequate treatment.
Our study had some limitations. First, there was no established gold standard, which is a universal drawback to all studies assessing PCR procedures for diagnostic accuracy in the detection of PJI. In this meta-analysis, the reference standards of the included studies varied. We performed subgroup analysis and examined reference standards as possible sources of heterogeneity. Second, not all studies explicitly stated whether they were performed in a prospective manner. Subgroup analysis showed that a prospective study design as a covariate in the bivariate statistical model may have significantly influenced the sensitivity. Third, the summary results of this meta-analysis had high statistical heterogeneity. The heterogeneity had multiple sources, including study design, sample type, sonication of samples, type of PCR, and reference standards, which may have led to an overestimation of the true diagnostic performance.
In summary, this meta-analysis of diagnostic accuracy demonstrated that PCR has an adequate diagnostic value for the detection of PJI, with a sensitivity of 86% and specificity of 91%, which is acceptable for clinical practice. Future studies should assess the cost-effectiveness of this test.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Fund for Key National Basic Research Program of China (grant number 2012CB619101), Major Basic Research of Science and Technology Commission of Shanghai Municipality (grant number 11DJ1400303), and Key Disciplines of Shanghai Municipal Education Commission (grant number J50206).
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 5 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00657-13.
REFERENCES
- 1. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. 2004. Reasons for revision hip surgery: a retrospective review. Clin. Orthop. Relat. Res. 429:188–192 [DOI] [PubMed] [Google Scholar]
- 2. Saleh KJ, Rand JA, McQueen DA. 2003. Current status of revision total knee arthroplasty: how do we assess results? J. Bone Joint Surg. Am. 85-A(Suppl 1):S18–S20 [DOI] [PubMed] [Google Scholar]
- 3. Bergin PF, Doppelt JD, Hamilton WG, Mirick GE, Jones AE, Sritulanondha S, Helm JM, Tuan RS. 2010. Detection of periprosthetic infections with use of ribosomal RNA-based polymerase chain reaction. J. Bone Joint Surg. Am. 92:654–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Esteban J, Alonso-Rodriguez N, del-Prado G, Ortiz-Perez A, Molina-Manso D, Cordero-Ampuero J, Sandoval E, Fernandez-Roblas R, Gomez-Barrena E. 2012. PCR-hybridization after sonication improves diagnosis of implant-related infection. Acta Orthop. 83:299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gallo J, Kolar M, Dendis M, Loveckova Y, Sauer P, Zapletalova J, Koukalova D. 2008. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 31:97–104 [PubMed] [Google Scholar]
- 6. Gomez, Cazanave C, Cunningham SA, Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, Hanssen AD, Karau MJ, Schmidt SM, Osmon DR, Berbari EF, Mandrekar J, Patel R. 2012. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J. Clin. Microbiol. 50:3501–3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. 2012. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J. Bone Joint Surg. Am. 94:2247–2254 [DOI] [PubMed] [Google Scholar]
- 8. Kobayashi N, Inaba Y, Choe H, Aoki C, Ike H, Ishida T, Iwamoto N, Yukizawa Y, Saito T. 2009. Simultaneous intraoperative detection of methicillin-resistant Staphylococcus and pan-bacterial infection during revision surgery: use of simple DNA release by ultrasonication and real-time polymerase chain reaction. J. Bone Joint Surg. Am. 91:2896–2902 [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi N, Procop GW, Krebs V, Kobayashi H, Bauer TW. 2008. Molecular identification of bacteria from aseptically loose implants. Clin. Orthop. Relat. Res. 466:1716–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mariani BD, Martin DS, Levine MJ, Booth RE, Jr., Tuan RS. 1996. The Coventry Award. Polymerase chain reaction detection of bacterial infection in total knee arthroplasty. Clin. Orthop. Relat. Res. 311:11–22 [DOI] [PubMed] [Google Scholar]
- 11. Marin M, Garcia-Lechuz JM, Alonso P, Villanueva M, Alcala L, Gimeno M, Cercenado E, Sanchez-Somolinos M, Radice C, Bouza E. 2012. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J. Clin. Microbiol. 50:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moojen DJ, Spijkers SN, Schot CS, Nijhof MW, Vogely HC, Fleer A, Verbout AJ, Castelein RM, Dhert WJ, Schouls LM. 2007. Identification of orthopaedic infections using broad-range polymerase chain reaction and reverse line blot hybridization. J. Bone Joint Surg. Am. 89:1298–1305 [DOI] [PubMed] [Google Scholar]
- 13. Panousis K, Grigoris P, Butcher I, Rana B, Reilly JH, Hamblen DL. 2005. Poor predictive value of broad-range PCR for the detection of arthroplasty infection in 92 cases. Acta Orthop. 76:341–346 [PubMed] [Google Scholar]
- 14. Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. 2009. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J. Clin. Microbiol. 47:1878–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Portillo ME, Salvado M, Sorli L, Alier A, Martinez S, Trampuz A, Gomez J, Puig L, Horcajada JP. 2012. Multiplex PCR of sonication fluid accurately differentiates between prosthetic joint infection and aseptic failure. J. Infect. 65:541–548 [DOI] [PubMed] [Google Scholar]
- 16. Tunney MM, Patrick S, Curran MD, Ramage G, Hanna D, Nixon JR, Gorman SP, Davis RI, Anderson N. 1999. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J. Clin. Microbiol. 37:3281–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. 2012. Management of periprosthetic joint infection: the current knowledge. AAOS exhibit selection. J. Bone Joint Surg. Am. 94:e104. [DOI] [PubMed] [Google Scholar]
- 18. Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. 2010. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J. Clin. Microbiol. 48:1208–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feldman DS, Lonner JH, Desai P, Zuckerman JD. 1995. The role of intraoperative frozen sections in revision total joint arthroplasty. J. Bone Joint Surg. Am. 77:1807–1813 [DOI] [PubMed] [Google Scholar]
- 20. Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. 2008. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J. Bone Joint Surg. Am. 90:1869–1875 [DOI] [PubMed] [Google Scholar]
- 21. Cazanave C, Greenwood-Quaintance KE, Hanssen AD, Karau MJ, Schmidt SM, Gomez Urena EO, Mandrekar JN, Osmon DR, Lough LE, Pritt BS, Steckelberg JM, Patel R. 8 May 2013. Rapid molecular microbiologic diagnosis of prosthetic joint infection. J. Clin. Microbiol. 10.1128/JCM.00335-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the infectious diseases society of america. Clin. Infect. Dis. 56:e1–e25 [DOI] [PubMed] [Google Scholar]
- 23. Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. 2013. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 56:1–10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.