Abstract
This study describes a clinical case of a 71-year-old male with a history of ischemic cardiomyopathy after left ventricular assist device (LVAD) endocarditis caused by methicillin-resistant Staphylococcus epidermidis (MRSE) and a rare linezolid-resistant Streptococcus sanguinis strain (MIC, 32 μg/ml). The patient received courses of several antimicrobial agents, including linezolid for 79 days. The S. sanguinis strain had mutations in the 23S rRNA (T2211C, T2406C, G2576T, C2610T) and an amino acid substitution (N56D) in L22 and exhibited cross-resistance to ribosome-targeting agents.
TEXT
Linezolid has been widely prescribed to treat serious infections caused by multidrug-resistant (MDR) Gram-positive pathogens since its clinical introduction as the first oxazolidinone in 2000 (1). Linezolid is currently approved by the U.S. Food and Drug Administration (FDA) for the treatment of complicated and uncomplicated skin and skin structure infections and nosocomial and community-acquired pneumonia caused by susceptible organisms (2). Linezolid also has an FDA indication for the treatment of vancomycin-resistant Enterococcus faecium (VRE) infections (including bacteremia) (2).
This oxazolidinone alters protein synthesis by interacting with the 50S ribosomal subunit, and recent data suggest that this drug binds to the A site of the peptidyl-transferase center (PTC) of bacterial ribosome, interfering with the positioning of aminoacyl-tRNA and resulting in protein synthesis inhibition (3). Although the prevalence of linezolid resistance among Gram-positive organisms remains low among surveillance clinical isolates (1), the resistance mechanisms detected have been comprised mostly of mutations in domain V of 23S rRNA, but alterations in the ribosomal proteins L3 and L4 have also been associated with decreased susceptibility (3, 4). Moreover, a more recent resistance mechanism, cfr, has been recognized. cfr encodes a methyltransferase that catalyzes the posttranscriptional methylation of nucleotide A2503 in the 23S rRNA, causing decreased susceptibility to phenicol, lincosamide, oxazolidinone, pleuromutilin, and streptogramin A (PhLOPSA) compounds (4).
Overall, staphylococci and enterococci represent the vast majority of linezolid-nonsusceptible clinical pathogens reported (3). Although published reports have described the in vitro selection of laboratory strains of streptococci displaying decreased susceptibility to linezolid due to mutations in the PTC (5, 6), only two clinical cases of linezolid-resistant streptococcal isolates (Streptococcus pneumoniae and Streptococcus oralis) have been described to date (1, 7, 8). Those reports demonstrated that these clinical and laboratory isolates had mutations in the 23S rRNA or amino acid substitutions in L4. Our study reports a clinical case of bacteremia due to a linezolid-resistant Streptococcus sanguinis and methicillin-resistant Staphylococcus epidermidis (MRSE) and investigates in the former the resistance mechanisms associated with ribosome-targeting agents.
Linezolid-resistant S. sanguinis (453-31288R) was recovered from blood cultures from a 71-year-old male who had a history of ischemic cardiomyopathy after placement of a left ventricular assist device (LVAD). The isolate was submitted to a central monitoring laboratory (JMI Laboratories, North Liberty, IA) as part of the 2011 SENTRY Antimicrobial Surveillance Program, according to defined protocols. Species identification was performed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics, Bremen, Germany) and confirmed by 16S rRNA sequencing. Susceptibility testing was performed by broth microdilution methods, according to the Clinical and Laboratory Standards Institute (CLSI) recommendations (M07-A9) (9). Validation of the MIC values was performed by concurrent testing of S. pneumoniae ATCC 49619 (10). MIC interpretations were based on the CLSI M100-S23 document (10) or the FDA package insert (tigecycline) (21).
The presence of cfr and mutations in the 23S rRNA and ribosomal proteins (L3, L4, and L22) was investigated by PCR and sequencing. Primers utilized were as follows: L3-F, ATGACCAAAGGAATCTTAGGG; L3-R, TTAGCACCTGGTACGTTACC; L4-F, AGCGATGCAGTATTTGGTATCG; L4-R, AAATTCATTATGCAAGAACC; L22-F, GAACTCAGCTGTAGCTAACGC; L22-R, TTCTGCAACAGCTACAGTGATG. The PCR conditions were as follows: initial denaturation at 95°C (5 min); 35 cycles of 94°C (30 s), 55°C (30 s), and 72°C (1 min); and final extension at 72°C (5 min). Amplicons were sequenced on both strands. 23S rRNA and ribosomal proteins obtained were compared to those from wild-type S. sanguinis ATCC 10556 using the Lasergene software package (DNAStar, Madison, WI). This isolate was also screened for vga(ABCD), lsa(ABC), and tetracycline [tet(KLMO)] genes by PCR (11). The S. sanguinis strain was subjected to serial daily passage on drug-free blood agar plates for 60 days, followed by susceptibility testing. Growth curves were performed in duplicate for the index and wild-type ATCC 10556 strains, as previously described (12).
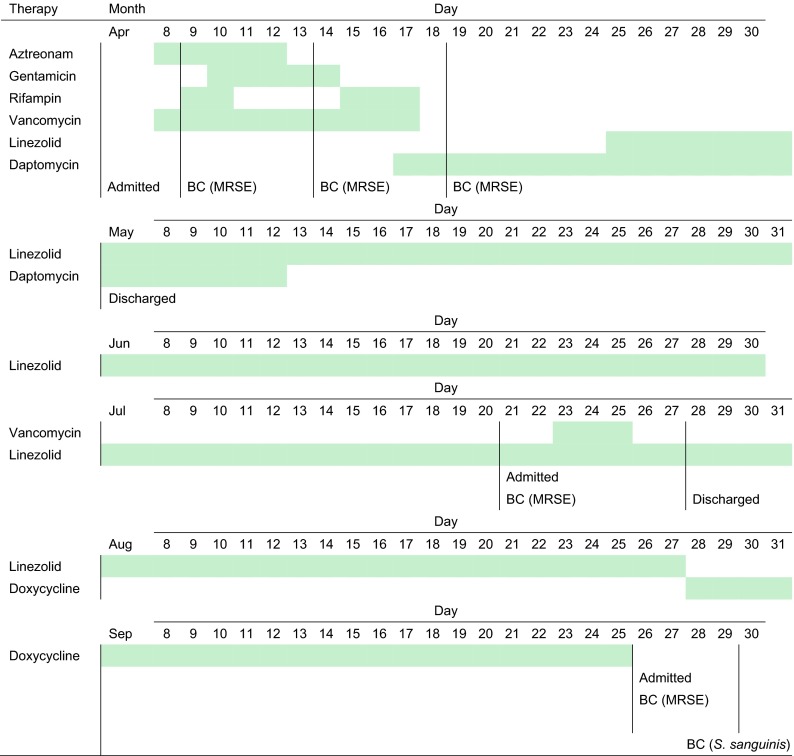
In April 2011, a 71-year-old male patient was diagnosed with LVAD endocarditis caused by MRSE, and aztreonam (5-day therapy), gentamicin (5 days), rifampin (5 days), and vancomycin (10 days) were prescribed, followed by daptomycin (25 days) and linezolid (8 days) (April to May 2011) (Fig. 1). The patient was discharged and continued receiving linezolid (79 days). He was readmitted in July 2011 due to persistent MRSE bacteremia, and vancomycin therapy (3 days) was introduced, while linezolid (3 days) remained. The patient was discharged on linezolid and then switched to doxycycline. The patient was readmitted in September 2011, and blood cultures yielded MRSE, followed by linezolid-resistant S. sanguinis (MIC, 32 μg/ml) (Fig. 1 and Table 1). S. sanguinis was cleared, but the MRSE infection persisted, with another positive blood culture in December 2011, despite several (five) changes of peripherally inserted central venous catheters (PICCs) between April and December 2011. In March and May 2012, Pseudomonas aeruginosa was recovered from blood cultures, which cleared later. The patient was discharged but expired in May from further complications.
Fig 1.
Summary of patient hospitalization history prior to the recovery of linezolid-resistant S. sanguinis, antimicrobial agents prescribed (green dates/lines), and culture dates of organisms recovered from blood cultures (BC). MRSE, methicillin-resistant S. epidermidis.
Table 1.
Antimicrobial susceptibility profile and molecular findings for the linezolid-resistant S. sanguinis strain (453-31288R) investigated in this study
| Parameter | Value/result |
|
|---|---|---|
| 453-31288R | 453-31288R60b | |
| Antimicrobial agent MIC (μg/ml) [susceptibility category]a | ||
| Linezolid | 32 [R] | 64 [R] |
| Chloramphenicol | 32 [R] | 32 [R] |
| Clindamycin | 2 [R] | 1 [R] |
| Virginiamycin | 2 | 2 |
| Quinupristin-dalfopristin | 4 [R] | 4 [R] |
| Retapamulin | 2 | 2 |
| Tiamulin | 32 | 32 |
| Tigecycline | ≤0.03 [S] | ≤0.03 [S] |
| Tetracycline | >16 [R] | >16 [R] |
| Doxycycline | 8 | |
| Vancomycin | 0.5 [S] | |
| Levofloxacin | 0.5 [S] | |
| Erythromycin | ≤0.12 [S] | |
| Penicillin | ≤0.06 [S] | |
| Molecular finding | ||
| cfr | Negative | |
| 23S rRNA | T2211C, T2406C, G2576T, and C2610T | |
| L3 | Wild type | |
| L4 | Wild type | |
| L22 | N56D | |
MIC interpretive criteria as published by CLSI M100-S23 (10), when available. The tigecycline susceptible breakpoint for Streptococcus spp. other than S. pneumoniae (≤0.25 μg/ml) approved by the Food and Drug Administration was applied (21). S, susceptible; I, intermediate; R, resistant.
MIC results and interpretations obtained for some antimicrobial agents tested against the S. sanguinis strain 31288R60 after 60 subcultures on drug-free blood agar plates.
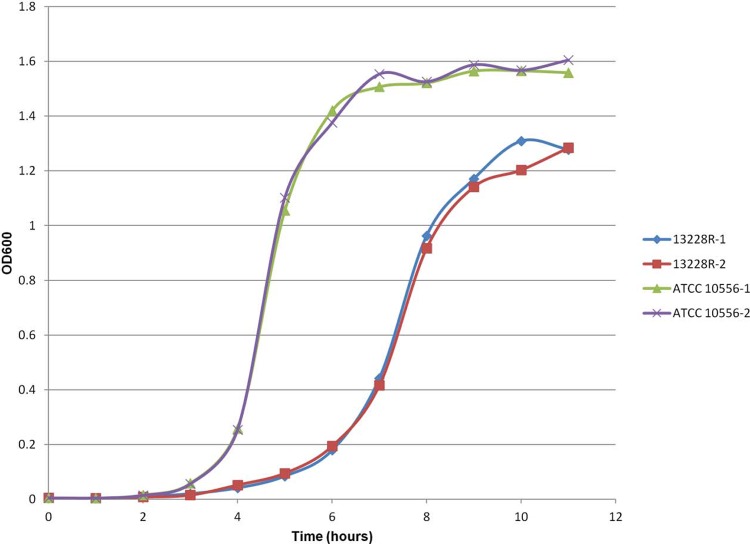
Linezolid-resistant S. sanguinis also exhibited elevated MIC results for chloramphenicol, clindamycin, tiamulin, tetracycline, doxycycline, and quinupristin-dalfopristin (Table 1). When tested against other ribosome-targeting agents, the S. sanguinis strain showed susceptible and/or low MIC values for erythromycin and tigecycline. Mutations were observed within the 23S rRNA at positions T2211, T2406, G2576, and C2610 (Escherichia coli numbering of rRNA), and there was an N56D amino acid substitution in L22. Wild-type amino acid sequences were observed for L3 and L4 (Table 1). The S. sanguinis strain was PCR negative for cfr, vga, and lsa genes and positive for tet(M). After 60 days of passage in nonselective medium, passage-derived S. sanguinis strains demonstrated antimicrobial susceptibility profiles equivalent to that of the parent strain (Table 1). The S. sanguinis mutant strain demonstrated a lag phase longer than the control strain, and both growth curves had distinct log and stationary phases (Fig. 2). In addition, 31228R showed a generation time of approximately 30 min, which was 2-fold longer than that of ATCC 10556 (i.e., ∼15 min).
Fig 2.
Growth pattern experiments performed for S. sanguinis 453-31288R and control strain ATCC 10556. The optical density at 600 nm (OD600) values of two cultures for each strain are shown for each time point.
Among the 23S rRNA mutations observed, G2576T and C2610T are within the PTC and are known to affect the linezolid MIC values and to cause cross-resistance to other ribosome-targeting agents, such as clindamycin, chloramphenicol, streptogramin A, and pleuromutilins (13–16). However, strain 31288R was susceptible to erythromycin, which corroborates the fact that this drug interacts with the 50S rRNA exit tunnel wall, and binding is mostly affected by mutations at 23S rRNA residues 2058 and 2059 (17). Moreover, amino acid substitutions in L22 can result in macrolide resistance, but the ribosomal protein alteration observed here seems to have little effect on erythromycin (18).
It is well known that mutations in the 23S rRNA may affect protein synthesis and confer lethal phenotypes; otherwise, they may confer a high bacterial fitness cost (16). Although the resistance phenotype displayed by strain 31228R seemed to be stable based on the passaging experiment, the ribosomal mutations and/or the L22 amino acid alteration seemed to adversely affect the growth rate (fitness) of S. sanguinis. However, it is important to mention the limitations of the growth rate experiments, since strains with different backgrounds were utilized and the results observed may have been originated due to factors other than the ribosomal modifications detected. In summary, further experiments are necessary to understand the impact of each 23S rRNA and the L22 amino acid substitution on the susceptibility profile and fitness cost. In addition, any modifications observed in the 23S rRNA may also act as compensatory mutations, as well as the L22 amino acid substitution, since alterations in this protein have not been associated with linezolid resistance due to a more distal position from the PTC (6, 19, 20). Lastly, the combination of ribosomal modifications observed in this strain appears to affect binding of several ribosome-targeting agents and produce a resistance profile similar to that of Cfr (PhLOPSA) (4).
ACKNOWLEDGMENTS
We thank the following staff members at JMI Laboratories (North Liberty, IA) for technical support and manuscript assistance: S. Benning, M. Castanheira, S. Farrell, and A. Costello.
The S. sanguinis strain was obtained through the LEADER Program, which is sponsored by educational/research grants from Pfizer Inc., Specialty Business Unit (Collegeville, PA).
JMI Laboratories, Inc. (R.E.M., L.M.D., J.E.R., and R.N.J.), has received research and educational grants from 2009 to 2012 from American Proficiency Institute (API), Anacor, Astellas, AstraZeneca, Bayer, Cempra, Cerexa, Contrafect, Cubist, Daiichi, Dipexium, Enanta, Furiex, GlaxoSmithKline, Johnson & Johnson (Ortho McNeil), LegoChem Biosciences Inc., Meiji Seika Kaisha, Merck, Nabriva, Novartis, Pfizer (Wyeth), Rempex, Rib-X Pharmaceuticals, Seachaid, Shionogi, The Medicines Co., Theravance, ThermoFisher, and some other corporations. Some JMI employees are advisors/consultants for Astellas, Cubist, Pfizer, Cempra, Cerexa-Forest, Johnson & Johnson, and Theravance.
In regard to speakers bureaus and stock options, there are no conflicts to declare. J.K. and D.S.M. have nothing to declare.
Footnotes
Published ahead of print 22 May 2013
REFERENCES
- 1. Flamm RK, Mendes RE, Ross JE, Sader HS, Jones RN. 2013. Linezolid surveillance results for the United States: LEADER Surveillance Program 2011. Antimicrob. Agents Chemother. 57:1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pfizer 2010. Zyvox package insert. Pfizer Inc., New York, NY: http://www.pfizer.com/products/rx/rx_product_zyvox.jsp [Google Scholar]
- 3. Shaw KJ, Barbachyn MR. 2011. The oxazolidinones: past, present, and future. Ann. N. Y. Acad. Sci. 1241:48–70 [DOI] [PubMed] [Google Scholar]
- 4. Diaz L, Kiratisin P, Mendes R, Panesso D, Singh KV, Arias CA. 2012. Transferable plasmid-mediated resistance to linezolid due to cfr in a human clinical isolate of Enterococcus faecalis. Antimicrob. Agents Chemother. 56:3917–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bozdogan B, Appelbaum PC. 2004. Oxazolidinones: activity, mode of action, and mechanism of resistance. Int. J. Antimicrob. Agents 23:113–119 [DOI] [PubMed] [Google Scholar]
- 6. Long KS, Vester B. 2012. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob. Agents Chemother. 56:603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolter N, Smith AM, Farrell DJ, Schaffner W, Moore M, Whitney CG, Jorgensen JH, Klugman KP. 2005. Novel mechanism of resistance to oxazolidinones, macrolides, and chloramphenicol in ribosomal protein L4 of the pneumococcus. Antimicrob. Agents Chemother. 49:3554–3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mutnick AH, Enne V, Jones RN. 2003. Linezolid resistance since 2001: SENTRY Antimicrobial Surveillance Program. Ann. Pharmacother. 37:769–774 [DOI] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—ninth edition. CLSI document M07-A9. CLSI, Wayne, PA [Google Scholar]
- 10. Clinical and Laboratory Standards Institute 2013. Performance standards for antimicrobial susceptibility testing: 23rd informational supplement. CLSI document M100-S23. CLSI, Wayne, PA [Google Scholar]
- 11. Mendes RE, Deshpande LM, Farrell DJ, Spanu T, Fadda G, Jones RN. 2010. Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J. Antimicrob. Chemother. 65:2329–2335 [DOI] [PubMed] [Google Scholar]
- 12. Locke JB, Rahawi S, Lamarre J, Mankin AS, Shaw KJ. 2012. Genetic environment and stability of cfr in methicillin-resistant Staphylococcus aureus CM05. Antimicrob. Agents Chemother. 56:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meka VG, Gold HS. 2004. Antimicrobial resistance to linezolid. Clin. Infect. Dis. 39:1010–1015 [DOI] [PubMed] [Google Scholar]
- 14. Miller K, Dunsmore CJ, Fishwick CW, Chopra I. 2008. Linezolid and tiamulin cross-resistance in Staphylococcus aureus mediated by point mutations in the peptidyl transferase center. Antimicrob. Agents Chemother. 52:1737–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCoy LS, Xie Y, Tor Y. 2011. Antibiotics that target protein synthesis. Wiley Interdiscip. Rev. RNA 2:209–232 [DOI] [PubMed] [Google Scholar]
- 16. Polacek N, Mankin AS. 2005. The ribosomal peptidyl transferase center: structure, function, evolution, inhibition. Crit. Rev. Biochem. Mol. Biol. 40:285–311 [DOI] [PubMed] [Google Scholar]
- 17. Kannan K, Mankin AS. 2011. Macrolide antibiotics in the ribosome exit tunnel: species-specific binding and action. Ann. N. Y. Acad. Sci. 1241:33–47 [DOI] [PubMed] [Google Scholar]
- 18. Farrell DJ, Morrissey I, Bakker S, Buckridge S, Felmingham D. 2004. In vitro activities of telithromycin, linezolid, and quinupristin-dalfopristin against Streptococcus pneumoniae with macrolide resistance due to ribosomal mutations. Antimicrob. Agents Chemother. 48:3169–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Long KS, Poehlsgaard J, Hansen LH, Hobbie SN, Bottger EC, Vester B. 2009. Single 23S rRNA mutations at the ribosomal peptidyl transferase centre confer resistance to valnemulin and other antibiotics in Mycobacterium smegmatis by perturbation of the drug binding pocket. Mol. Microbiol. 71:1218–1227 [DOI] [PubMed] [Google Scholar]
- 20. Billal DS, Feng J, Leprohon P, Legare D, Ouellette M. 2011. Whole genome analysis of linezolid resistance in Streptococcus pneumoniae reveals resistance and compensatory mutations. BMC Genomics 12:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wyeth Pharmaceuticals Inc 2005. Tygacil package insert. Wyeth Pharmaceuticals Inc., Philadelphia, PA [Google Scholar]