Abstract
An enzyme immunoassay kit that detects serum IgA antibody reacting to glycopeptidolipid core antigen derived from Mycobacterium avium complex (MAC) was not useful for differentiating MAC pulmonary disease (PD) from Mycobacterium abscessus complex PD (MAB-PD). However, this assay could be useful for differentiating MAC- and MAB-PD from pulmonary tuberculosis. (This study has been registered at ClinicalTrials.gov under registration no. NCT00970801.)
TEXT
An enzyme immunoassay (EIA) that detects serum immunoglobulin A (IgA) antibody reacting to the glycopeptidolipid (GPL) core antigen of Mycobacterium avium complex (MAC) was developed (1). This EIA kit was useful for rapid diagnosis of MAC pulmonary disease (MAC-PD) and for differentiating MAC-PD from pulmonary tuberculosis (PTB) (1–5).
MAC, followed by Mycobacterium abscessus complex (MAB), is the most common etiologic organism in PD caused by nontuberculous mycobacteria (NTM-PD) in many countries, including the United States and South Korea (6–9). Because MAB also possesses GPL on its cell surface (10), there is a possibility of false-positive results in patients with MAB-PD. However, this EIA kit has not been evaluated in MAB-PD (1–5). The objective of this study was to evaluate the diagnostic performance of this EIA kit in patients with NTM-PD caused by MAB as well as by MAC.
Serum samples were collected from patients with NTM-PD diagnosed between January 2008 and December 2011 at the Samsung Medical Center (a 1,950-bed referral hospital in Seoul, South Korea). The patients were enrolled in an institutional review board-approved observational cohort study investigating NTM-PD (ClinicalTrials.gov identifier NCT00970801). Informed consent was obtained from all participants. All patients met the diagnostic criteria for NTM-PD according to the guidelines of the American Thoracic Society (11).
The study groups included 40 MAC-PD patients (20 M. avium and 20 M. intracellulare), 40 MAB-PD patients (20 M. abscessus sensu stricto and 20 M. massiliense), 20 culture-confirmed PTB patients, and 20 healthy controls. All patients were newly diagnosed and had not been treated for NTM lung disease at the time of collection of the serum samples.
Serum IgA antibodies against the GPL core antigen were measured using the EIA kit (Tauns Laboratory Inc., Shizuoka, Japan) according to the manufacturer's instructions (2, 3). Results are given as arbitrary U/ml and reported as positive when the IgA titer was more than 0.7 U/ml (2, 3). The titers of each study group are presented as medians and interquartile ranges (IQR) and were compared using the Kruskal-Wallis test with post hoc paired comparisons using the Bonferroni method. We estimated the sensitivity, specificity, and positive predictive value (PPV) and negative predictive value (NPV) for a preset cutoff point (≥0.7 U/ml) and the best cutoff point, which showed the highest Youden index ([sensitivity + specificity] − 1) (12). The discriminative power of the EIA kit was assessed by calculating the area under the receiver operating characteristic curve (AUC). We used STATA ver. 11 (STATA Corp., College Station, TX) for all analyses and considered a 2-sided P of <0.05 to be statistically significant.
The median age was 62 (IQR, 49 to 70) years in the MAC-PD patient group, 56 (IQR, 48 to 68) years in the MAB-PD patient group, 60 (IQR, 53 to 67) years in the PTB patient group, and 56 (IQR, 52 to 61) years in the control groups. The proportions of male patients were 40%, 23%, 75%, and 50% in the MAC-PD, MAB-PD, PTB, and control groups, respectively. The proportions of patients with the nodular bronchiectatic form of MAC- and MAB-PD were 83% and 80%, respectively. None of the subjects were positive for human immunodeficiency virus infection.
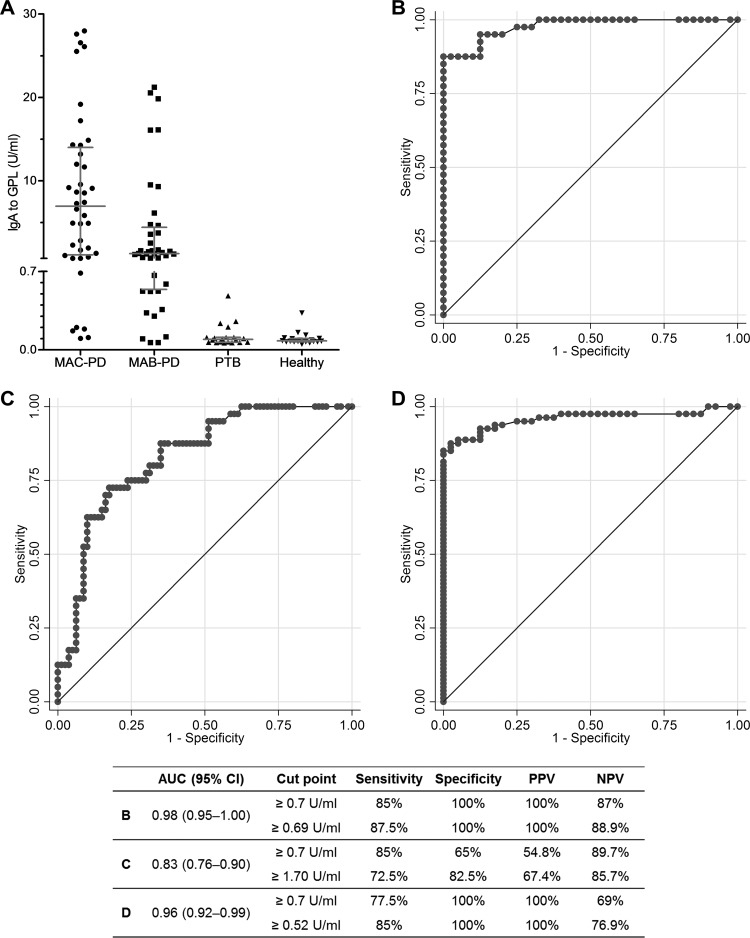
Figure 1A shows a scattergram of IgA antibody titers plotted against the GPL core antigen in each group. Significantly higher levels were detected in the MAC-PD group (median, 6.96; IQR, 1.12 to 14.00 U/ml) than in the other groups (P = 0.030 for MAB-PD, P < 0.001 for PTB, and P < 0.001 for the control group). However, the MAB-PD group (median, 1.28; IQR, 0.54 to 4.43 U/ml) also had a higher titer than the PTB group (median, 0.09; IQR, 0.07 to 0.11 U/ml; P < 0.001) and controls (median, 0.08; IQR, 0.07 to 0.10 U/ml; P < 0.001). The positivity rates for the EIA were 85%, 70%, 0%, and 0% in the MAC-PD, MAB-PD, PTB, and control groups, respectively.
Fig 1.
Comparison of the IgA antibody response to glycopeptidolipid (GPL) core antigen and the sensitivity of the enzyme immunoassay. (A) Scattergram of IgA antibody titers plotted against GPL core antigen from 40 patients with Mycobacterium avium complex pulmonary disease (MAC-PD), 40 patients with M. abscessus complex pulmonary disease (MAB-PD), 20 patients with pulmonary tuberculosis (PTB), and 20 healthy controls. (B) Receiver operating characteristic (ROC) curve for detection of MAC-PD in the study subjects, excluding MAB-PD (area under the curve [AUC], 0.98; 95% confidence interval [CI], 0.95 to 1.00). (C) ROC curve for detection of MAC-PD among all study subjects (AUC, 0.83; 95% CI, 0.76 to 0.90). (D) ROC curve for detection of nontuberculous mycobacterial lung disease (both MAC- and MAB-PD) among all study subjects (AUC, 0.96; 95% CI, 0.92 to 0.99). PPV, positive predictive value; NPV, negative predictive value.
In the study subjects, excluding MAB-PD, the discriminatory power for detection of MAC-PD was high (AUC, 0.98; 95% confidence interval [CI], 0.95 to 1.00), with 85% sensitivity, 100% specificity, and 100% PPV at a preset cutoff of 0.7 U/ml, which were similar to values seen at the best cutoff point (≥0.69 U/ml) (Fig. 1B). Among all study subjects, including those with MAB-PD, however, the discriminatory power and the PPV for detection of MAC-PD were weakened to an AUC of 0.83 (95% CI, 0.76 to 0.90), with 85% sensitivity, 65% specificity, and 54.8% PPV at a preset cutoff value and 72.5% sensitivity, 82.5% specificity, and 67.4% PPV at the best cutoff point (≥1.70 U/ml) (Fig. 1C). In terms of distinguishing NTM lung disease (both MAC- and MAB-PD) from others (PTB and control groups), the AUC was 0.96 (95% CI, 0.92 to 0.99), with 77.5% sensitivity, 100% specificity, and 100% PPV at a preset cutoff value and 85% sensitivity, 100% specificity, and 100% PPV at the best cutoff point (≥0.52 U/ml) (Fig. 1D).
NTM causes a chronic, slowly progressive pulmonary infection that resembles PTB. In clinical practice, it is difficult to distinguish NTM-PD from PTB, because the symptoms and radiographic findings are often similar. This leads to an incorrect diagnosis or unnecessary treatment in many patients with NTM-PD (13). Therefore, serodiagnosis of NTM-PD could be useful for rapid differentiation of NTM-PD from PTB. Our data revealed that this EIA kit could be useful for differential serodiagnosis of MAC-PD and PTB, which is consistent with previous studies (1–5).
We found that the level of serum IgA antibody to the GPL core antigen was higher in both MAB- and MAC-PD patients. In fact, many NTM organisms, including MAC, MAB, M. fortuitum, M. chelonae, M. simiae, M. peregrinum, M. senegalense, M. porcinum, M. smegmatis, and M. butyricum, possess GPL (14, 15). A previous study from Japan reported that this EIA kit could discriminate MAC-PD from M. kansasii PD (1). Because GPL is not located on the surface of the M. kansasii cell wall (16), this finding seems reasonable. Therefore, this EIA kit is not useful for prediction of NTM etiology, especially in countries in which MAB is a relatively common etiology of NTM lung disease.
Antibody titers in our patients with MAC-PD were higher than those in Caucasian patients (4) and similar to those in Japanese patients (3, 5). Differences in race, disease status, and etiological proportion may affect these results and the best cutoff value. Finally, because of high PPV and low NPV (Fig. 1D), a high value of serum IgA antibody could be in favor of NTM infection but a low value could not exclude a possibility of NTM infection.
In summary, this EIA kit that detects serum IgA antibody to the GPL core antigen cannot differentiate MAC-PD from MAB-PD. Therefore, caution in the interpretation of the test results should be required in countries such as the United States and South Korea in which MAB is a common etiology of NTM lung disease. In addition, this kit could be useful for differentiating NTM-PD, including MAC- and MAB-PD, from PTB instead of for differentiating MAC-PD from MAB-PD.
ACKNOWLEDGMENTS
This work was supported by the Mid-career Researcher Program through a National Research Foundation grant funded by the Ministry of Education, Science and Technology (2011-0015546).
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 5 June 2013
REFERENCES
- 1. Kitada S, Maekura R, Toyoshima N, Fujiwara N, Yano I, Ogura T, Ito M, Kobayashi K. 2002. Serodiagnosis of pulmonary disease due to Mycobacterium avium complex with an enzyme immunoassay that uses a mixture of glycopeptidolipid antigens. Clin. Infect. Dis. 35:1328–1335 [DOI] [PubMed] [Google Scholar]
- 2. Kitada S, Kobayashi K, Ichiyama S, Takakura S, Sakatani M, Suzuki K, Takashima T, Nagai T, Sakurabayashi I, Ito M, Maekura R. 2008. Serodiagnosis of Mycobacterium avium-complex pulmonary disease using an enzyme immunoassay kit. Am. J. Respir. Crit. Care Med. 177:793–797 [DOI] [PubMed] [Google Scholar]
- 3. Kitada S, Kobayashi K, Nishiuchi Y, Fushitani K, Yoshimura K, Tateishi Y, Miki K, Miki M, Hashimoto H, Motone M, Fujikawa T, Hiraga T, Maekura R. 2010. Serodiagnosis of pulmonary disease due to Mycobacterium avium complex proven by bronchial wash culture. Chest 138:236–237 [DOI] [PubMed] [Google Scholar]
- 4. Kitada S, Levin A, Hiserote M, Harbeck RJ, Czaja CA, Huitt G, Kasperbauer SH, Daley CL. 25 October 2012. Serodiagnosis of Mycobacterium avium complex pulmonary disease in the United States. Eur. Respir. J. [Epub ahead of print.] 10.1183/09031936.00098212 [DOI] [PubMed] [Google Scholar]
- 5. Kobashi Y, Mouri K, Obase Y, Kato S, Oka M. 2013. Serological assay by use of glycopeptidolipid core antigen for Mycobacterium avium complex. Scand. J. Infect. Dis. 45:241–249 [DOI] [PubMed] [Google Scholar]
- 6. Kartalija M, Ovrutsky AR, Bryan CL, Pott GB, Fantuzzi G, Thomas J, Strand MJ, Bai X, Ramamoorthy P, Rothman MS, Nagabhushanam V, McDermott M, Levin AR, Frazer-Abel A, Giclas PC, Korner J, Iseman MD, Shapiro L, Chan ED. 2013. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am. J. Respir. Crit. Care Med. 187:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am. J. Respir. Crit. Care Med. 183:405–410 [DOI] [PubMed] [Google Scholar]
- 8. Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, Shin SJ, Kwon OJ. 2012. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M. avium complex lung disease. Chest 142:1482–1488 [DOI] [PubMed] [Google Scholar]
- 9. Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, Olivier KN. 2010. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am. J. Respir. Crit. Care Med. 182:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ripoll F, Deshayes C, Pasek S, Laval F, Beretti JL, Biet F, Risler JL, Daffe M, Etienne G, Gaillard JL, Reyrat JM. 2007. Genomics of glycopeptidolipid biosynthesis in Mycobacterium abscessus and M. chelonae. BMC Genomics 8:114. 10.1186/1471-2164-8-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416 [DOI] [PubMed] [Google Scholar]
- 12. Bewick V, Cheek L, Ball J. 2004. Statistics review 13: receiver operating characteristic curves. Crit. Care 8:508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maiga M, Siddiqui S, Diallo S, Diarra B, Traore B, Shea YR, Zelazny AM, Dembele BP, Goita D, Kassambara H, Hammond AS, Polis MA, Tounkara A. 2012. Failure to recognize nontuberculous mycobacteria leads to misdiagnosis of chronic pulmonary tuberculosis. PLoS One 7:e36902. 10.1371/journal.pone.0036902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chatterjee D, Khoo KH. 2001. The surface glycopeptidolipids of mycobacteria: structures and biological properties. Cell. Mol. Life Sci. 58:2018–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schorey JS, Sweet L. 2008. The mycobacterial glycopeptidolipids: structure, function, and their role in pathogenesis. Glycobiology 18:832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puzo G. 1990. The carbohydrate- and lipid-containing cell wall of mycobacteria, phenolic glycolipids: structure and immunological properties. Crit. Rev. Microbiol. 17:305–327 [DOI] [PubMed] [Google Scholar]