Abstract
Scrub typhus is a major infectious threat in the Asia-Pacific region. We report an unusual case of scrub typhus in a patient in Singapore who presented with sepsis and acute respiratory distress syndrome but lacked the pathognomonic eschar. The patient recovered after appropriate diagnosis and doxycycline treatment. Rickettsial diseases should be included in the differential diagnosis of febrile illnesses in regions where the diseases are endemic, and absence of eschar should not be the criterion used to rule out scrub typhus.
CASE REPORT
On 3 October 2011, a 35-year-old male became acutely ill and was admitted to a hospital in a neighboring country following 4 days of an undifferentiated febrile illness. On admission, the patient had fever, chills, giddiness, nonspecific abdominal pain, cough with nonpurulent sputum, and lethargy. His condition rapidly deteriorated, requiring mechanical ventilation for respiratory distress, followed by septic shock on the third day of hospitalization. He received empirical intravenous moxifloxacin and meropenem, oral clarithromycin, and vasopressor support. As his condition did not improve, he was transferred to Singapore on day 4 of hospitalization.
On admission in Singapore on 6 October 2011, the patient was febrile (38.0°C) with a blood pressure of 120/63 mmHg supported by 2 μg/kg/min of dopamine. His oxygen saturation was 97% with a fraction of inspired O2 of 60%. Physical examination disclosed no significant rash. His abdomen was flushed, soft, and nontender. Chest auscultation revealed bilateral crepitations.
A chest radiograph showed bilateral alveolar shadows consistent with acute respiratory distress syndrome (ARDS). Abdominal sonography and echocardiography findings were normal. Laboratory data (Table 1) revealed leukocytosis, thrombocytopenia, elevated aspartate aminotransferase and alanine aminotransferase levels, and mild renal impairment. He was diagnosed with sepsis syndrome complicated by ARDS. The differential diagnosis included melioidosis, leptospirosis, rickettsiosis, and viral infections, including influenza virus. Serologic tests for hantavirus, melioidosis, dengue virus, Leptospira, chikungunya virus, Nipah virus, and Mycoplasma were negative. Urine, blood, and sputum cultures also were negative for Legionella and pneumococcal antigens. A Widal, Weil-Felix test (agglutination test for the diagnosis of rickettsial infections) was also performed with a serum sample (taken on 6 October 2011) but was negative (all titers were less than 1:40). He was empirically treated with oral oseltamivir, doxycycline, intravenous amoxicillin-clavulanate, and ceftazidime. He became afebrile 48 h after admission in Singapore. Bronchoalveolar lavage was performed on day 2 after admission, and cultures were sterile. In view of his severe ARDS, intravenous hydrocortisone, beginning with an initial dose of 100 mg every 8 h, was initiated on day 3 after admission and subsequently tapered off. Chest radiography and laboratory marker (C-reactive protein, procalcitonin) level tests on day 6 after admission showed much improvement. He was successfully weaned from mechanical ventilatory support on day 7.
Table 1.
Laboratory profile of the patient on hospital admission in Singapore
| Variable | Value | Reference range |
|---|---|---|
| Hematology | ||
| WBCa count (mm−3) | 12.3 × 103 (↑)e | (4–11) × 103 |
| % Neutrophils | 74 | 54–62 |
| % Lymphocytes | 9 (↓)f | 15–40 |
| Hemoglobin level (g/dl) | 11 (↓) | 11.5–16.5 |
| Prothrombin time (s) | 11.1 | 11–13.5 |
| Partial thromboplastin time (s) | 37.8 | 25–35 |
| Platelet count (μl−1) | 87 × 103 (↓) | (140–440) × 103 |
| Blood chemistry | ||
| ALTb level (U/liter) | 113 (↑) | <37 |
| ASTc level (U/liter) | 140 (↑) | <41 |
| Bilirubin level (μmol/liter) | 22 | 3–24 |
| Albumin level (g/liter) | 24 (↓) | 35–50 |
| Creatinine level (μmol/liter) | 154 (↑) | 44–110 |
| CRPd level (mg/liter) | 181.9 (↑) | <10 |
WBC, white blood cell.
ALT, alanine aminotransferase.
AST, aspartate aminotransferase.
CRP, C-reactive protein.
↑, value above normal reference range.
↓, value below normal reference range.
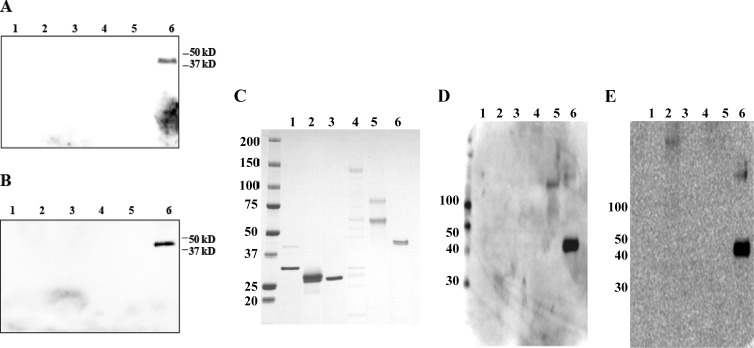
Serum samples taken on day 9 of illness were tested with a recently developed Western blot assay (developed at the U.S. Naval Medical Research Center [NMRC]) that can recognize and differentiate various febrile illnesses of bacterial origin (our unpublished data). The serum testing was performed at the Emerging Infectious Diseases Division, Duke-NUS Graduate Medical School, Singapore, for murine typhus, spotted fever, scrub typhus, Q fever, and leptospirosis. The assay uses recombinant antigens/whole-cell antigens from various pathogens (Rickettsia typhi, R. conorii, Orientia tsutsugamushi, Coxiella burnetii, and Leptospira interrogans). The serum was negative for all of the pathogens tested for, except O. tsutsugamushi (Fig. 1A and B), and a mixture of recombinant 56-kDa antigens (1) derived from the most prevalent serotypes of O. tsutsugamushi (Karp, Kato, Gilliam, and TA763) was detected. Figure 1C represents the protein gel showing the presence of various recombinant/whole-cell antigens used in the Western blot assay. Figure 1D and E show the positive-control Western blot assays detecting these antigens in the serum of a confirmed scrub typhus patient. The results were also confirmed by using a prototype rapid chromatographic immunoassay kit developed by InBios in collaboration with NMRC for scrub typhus detection.
Fig 1.
Western immunoblot assays of patient serum to detect levels of IgG (A) and IgM (B) antibodies to various pathogens. Lanes: 1, L. interrogans (recombinant LipL32 and LipL41 proteins); 2, Rickettsia conorii (recombinant OmpA protein fragments); 3, C. burnetii (recombinant Com-1 protein); 4, R. typhi (whole-cell antigen from R. typhi strain Wilmington); 5, R. typhi (recombinant OmpB protein fragments); 6, O. tsutsugamushi (recombinant R56 proteins from O. tsutsugamushi strains Karp, Kato, Gilliam, and TA763). The recombinant antigens/whole-cell antigens derived from various pathogens were separated on a 4 to 15% polyacrylamide gel, transferred to a nylon membrane, and probed with patient serum (1:100 dilution) overnight at 4°C. The blot was probed with a horseradish peroxidase-conjugated anti-human IgG or IgM secondary antibody (1:1,000 dilution) and visualized with a chemiluminescence-based detection kit (Amersham). (C) Corresponding protein gel stained with SimplyBlue SafeStain (Invitrogen) showing the presence of recombinant/whole-cell antigens used in a Western blot assay loaded in the same order as in panels A and B. (D and E) Positive-control Western blot assays detecting the antigens (loaded in the same order as in panels A and B) in the serum of a confirmed scrub typhus patient. The patient's scrub typhus was confirmed by a 4-fold titer increase and isolation of O. tsutsugamushi from a serum sample. Panel D shows IgG results, and panel E shows IgM results. The values to the left of panels C to E are molecular sizes in kilodaltons.
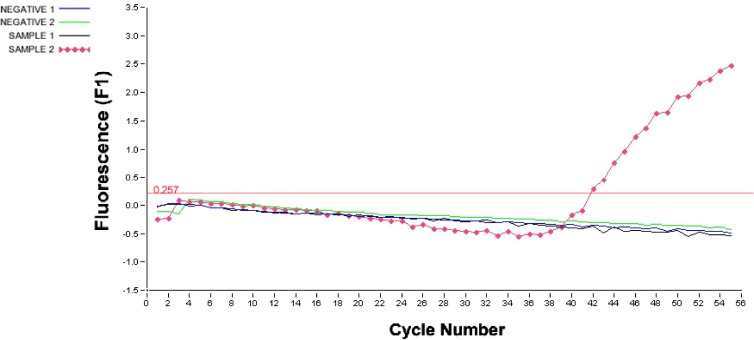
O. tsutsugamushi infection was further verified by an in-house-developed real-time PCR assay that detects the 56-kDa protein-encoding gene. This real-time PCR assay was sensitive enough to detect as few as 10 genomic equivalents of O. tsutsugamushi and had been tested for specificity against 15 different bacterial species with no cross-reactivity, demonstrating 100% specificity for O. tsutsugamushi (our unpublished data). PCR is considered a very sensitive and specific method for the detection of O. tsutsugamushi rickettsial DNA (2, 3). The PCR was performed with whole blood taken from the patient on day 9 of illness (at DSO National Laboratories, Singapore). The primers and probe used were forward primer 5′-GTTGTCGTTGCCGTTTTCA-3′, reverse primer 5′-TGGATCCAAGCGAGCAGA-3′, and TaqMan probe 5′-(6-carboxyfluorescein)-TAGTGCGATAGAATTGGATGATGAAGGA-(Black Hole Quencher 1)-3′. The real-time PCR conditions used were 95°C for 2 min and 50 cycles of 95°C for 0 s, 60°C for 6 s, and 72°C for 12 s. Figure 2 shows the fluorescence profile (relative fluorescence units) representing positive detection of the O. tsutsugamushi 56-kDa rickettsial antigen gene in a whole-blood sample (sample 2); a serum sample (sample 1) was negative. We also confirmed the sequence of the PCR product obtained in this study as that of the 56-kDa rickettsial antigen gene.
Fig 2.
Results of a real-time PCR used to detect the presence of O. tsutsugamushi in the patient's blood. The total nucleic acids were extracted from the whole-blood sample (200 μl) and the serum sample (200 μl) with the QIAamp DNA minikit (Qiagen) according to manufacturer's instructions. The samples were then tested with an in-house-developed real-time PCR assay targeting the gene encoding the 56-kDa antigen of O. tsutsugamushi. Shown is the fluorescence profile (relative fluorescence units plotted against cycle numbers) representing positive detection of the O. tsutsugamushi 56-kDa antigen gene in a whole blood sample (sample 2). A serum sample (sample 1) was negative. Negative 1 is the negative control for the serum sample (sample 1), and negative 2 is the negative control for the blood sample (sample 2). The threshold line is shown in red, and the amplification threshold value of 0.257 is indicated.
In view of the confirmed scrub typhus diagnosis, oseltamivir, intravenous amoxicillin-clavulanate, and ceftazidime were stopped and the patient received 2 weeks of oral doxycycline (100 mg twice daily) and recovered completely. He was discharged from the hospital on day 11 following admission.
In this report, we present the case of a patient with clinically severe scrub typhus who was hospitalized in Singapore with the complication of sepsis syndrome with ARDS. However, no characteristic eschar was present and the patient recovered completely with timely diagnosis and treatment. Improvement in the health of the patient was observed from admission, as doxycycline therapy was initiated on day 1 after his transfer to Singapore. While most patients with scrub typhus present with a relatively mild illness, this case illustrates its potential to cause severe clinical manifestations.
Scrub typhus is an acute febrile illness caused by the obligate mite-borne Gram-negative bacterium O. tsutsugamushi (family Rickettsiaceae). It is endemic to the Tsutsugamushi Triangle of the Asia-Pacific region (4), accounts for 23% of all febrile illness in that region, and can have a mortality rate of up to 35% if left untreated (1). The disease is an important public health problem (5–9), and an estimated one billion people live in areas at risk for scrub typhus with an estimated one million annual cases (10). Scrub typhus was the most notable rickettsiosis affecting U.S. troops in WWII in the China-Burma-India theater of operations and had a higher mortality rate than any other infectious disease there (8). Many new or imported cases of scrub typhus have also been reported from regions outside the traditional Asia-Pacific region (11–13).
Scrub typhus, like most rickettsial diseases, is generally difficult to diagnose because it produces clinical characteristics similar to those of many other tropical febrile illnesses. The clinical severity of scrub typhus ranges from mild febrile illness to a fatal outcome. Complications may include encephalitis, interstitial pneumonia, hemolysis in patients with glucose-6-phosphate dehydrogenase deficiency, ARDS, acute renal failure, myocarditis, septic shock, and death (14, 15). Pulmonary involvement frequently occurs in mild cases and is the principal cause of death in severe disease (6, 7). Severe complications such as sepsis and ARDS are typically the results of delayed diagnosis and treatment. When treated with an appropriate antibiotic (doxycycline, tetracycline, or chloramphenicol), patients typically become afebrile within 48 h.
This patient was a vegetable farm supervisor with no significant medical or travel history. An increase in rat infestation was noted at the farm where he worked in the weeks leading to his illness. He subsequently reported having noticed a dead rat in his car, which he removed with his bare hands about a week prior to falling ill. While the patient's history and clinical presentation were suggestive of a scrub typhus diagnosis, he did not have the pathognomonic eschar.
The patient did not respond to quinolone and macrolide therapy. Scrub typhus is well known to be unresponsive or show only a suboptimal response to quinolones, beta-lactams, penicillins, clarithromycin, and cephalosporins (8, 16, 17), and in northern Thailand, there has been a reported case of resistance to chloramphenicol and doxycycline (18). It is also notable that the Widal, Weil-Felix test, a generally used diagnostic test for rickettsial diseases, showed a negative result with the patient sample. This result is not surprising, as it is well known that the Widal, Weil-Felix test is not very sensitive for the diagnosis of scrub typhus (19).
Although Singapore is a major economic hub attracting a large number of travelers, there are only scant published reports of the prevalence of rickettsial disease in Singapore (13, 20–23). Because scrub typhus is endemic in countries neighboring Singapore, health care providers must be aware of a patient's travel history. While clinical signs such as lymphadenopathy, splenomegaly, rash, and fever are nonspecific, an eschar, if present, is pathognomonic in the diagnosis of scrub typhus. However, eschars are rare among Southeast Asian patients (24). The nonspecific presentation of patients and the absence of a characteristic eschar make misdiagnosis and underreporting of scrub typhus common.
This study underscores the importance of including scrub typhus and other rickettsial diseases in the differential diagnosis of patients with acute febrile illnesses of unknown origin in this region. Although eschar is a pathognomonic feature of scrub typhus, its absence does not rule out scrub typhus. Awareness of unusual manifestations of scrub typhus such as sepsis syndrome and ARDS and the timely administration of empirical treatment with doxycycline in suspicious cases can greatly help to decrease the morbidity and mortality associated with rickettsial diseases.
ACKNOWLEDGMENTS
This study was supported in part by a Duke-NUS signature research program funded by the Agency for Science, Technology and Research (A*STAR), Singapore, and the Ministry of Health, Singapore, and by work unit no. 6000.RAD1.J.A0310 (NMRC).
The opinions and assertions contained herein are ours and are not to be construed as official or as reflecting the views of the Department of the Navy, the Naval Service at large, the Department of Defense, or the U.S. Government. C.-C.C. and W.M.C. are employees of the U.S. Government. This work was prepared as part of official duties. Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by an employee of the U.S. Government as part of that person's official duties, and “copyright protection under this title is not available for any work of the United States Government.”
We thank Goh Liang Kee (Duke-NUS) for assistance with statistical analysis. C.-C.C. and W.M.C. thank Zhiwen Zhang for her effort in developing the Western blot assay using recombinant proteins.
We have no conflict of interest to declare.
Footnotes
Published ahead of print 12 June 2013
REFERENCES
- 1. Ching WM, Rowland D, Zhang Z, Bourgeois AL, Kelly D, Dasch GA, Devine PL. 2001. Early diagnosis of scrub typhus with a rapid flow assay using recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi. Clin. Diagn. Lab. Immunol. 8:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horinouchi H, Murai K, Okayama A, Tachibana N, Tsubouchi H. 1996. Genotypic identification of Rickettsia tsutsugamushi by restriction fragment length polymorphism analysis of DNA amplified by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 54:647–651 [DOI] [PubMed] [Google Scholar]
- 3. Tay ST, Nazma S, Rohani MY. 1996. Diagnosis of scrub typhus in Malaysian aborigines using nested polymerase chain reaction. Southeast Asian J. Trop. Med. Public Health 27:580–583 [PubMed] [Google Scholar]
- 4. Devine J. 2003. A review of scrub typhus management in 2000-2001 and implications for soldiers. J. Rural Remote Environ. Health 2:14–20 [Google Scholar]
- 5. Audy JR. 1949. A summary topographical account of scrub typhus 1908-1946 (studies in the distribution and topography of scrub typhus). Government Press, Kuala Lumpur, Malaysia [Google Scholar]
- 6. Watt G. 2000. Scrub typhus, p 1698–1700 In Ledingham JGG, Warrell DA. (ed), Concise Oxford textbook of medicine. Oxford University Press, New York, NY [Google Scholar]
- 7. Singharaj P, Watt G. 1997. Scrub typhus. J. Trop. Med. Parasitol. 20:23–27 [Google Scholar]
- 8. Kelly DJ, Richards AL, Temenak J, Strickman D, Dasch GA. 2002. The past and present threat of rickettsial diseases to military medicine and international public health. Clin. Infect. Dis. 34(Suppl 4):S145–S169 [DOI] [PubMed] [Google Scholar]
- 9. Kawamura A, Tanaka H, Tamura A, ed. 1995. Tsutsugamushi disease. University of Tokyo Press, Tokyo, Japan [Google Scholar]
- 10. Watt G, Parola P. 2003. Scrub typhus and tropical rickettsioses. Curr. Opin. Infect. Dis. 16:429–436 [DOI] [PubMed] [Google Scholar]
- 11. Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, Aukkanit N, Nguyen C, Jiang J, Fenwick S, Day NP, Graves S, Stenos J. 2010. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J. Clin. Microbiol. 48:4404–4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balcells ME, Rabagliati R, García P, Poggi H, Oddó D, Concha M, Abarca K, Jiang J, Kelly DJ, Richards AL, Fuerst PA. 2011. Endemic scrub typhus-like illness, Chile. Emerg. Infect. Dis. 17:1659–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis MD, Yousuf AA, Lerdthusnee K, Razee A, Chandranoi K, Jones JW. 2003. Scrub typhus reemergence in the Maldives. Emerg. Infect. Dis. 9:1638–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang CC, Liu SF, Liu JW, Chung YH, Su MC, Lin MC. 2007. Acute respiratory distress syndrome in scrub typhus. Am. J. Trop. Med. Hyg. 76:1148–1152 [PubMed] [Google Scholar]
- 15. Tsay RW, Chang FY. 1998. Serious complications in scrub typhus. J. Microbiol. Immunol. Infect. 31:240–244 [PubMed] [Google Scholar]
- 16. Tantibhedhyangkul W, Angelakis E, Tongyoo N, Newton PN, Moore CE, Phetsouvanh R, Raoult D, Rolain JM. 2010. Intrinsic fluoroquinolone resistance in Orientia tsutsugamushi. Int. J. Antimicrob. Agents 35:338–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee N, Ip M, Wong B, Lui G, Tsang OT, Lai JY, Choi KW, Lam R, Ng TK, Ho J, Chan YY, Cockram CS, Lai ST. 2008. Risk factors associated with life-threatening rickettsial infections. Am. J. Trop. Med. Hyg. 78:973–978 [PubMed] [Google Scholar]
- 18. Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P, Bhodhidatta D, Corcoran KD, Dasch GA, Strickman D. 1996. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet 348:86–89 [DOI] [PubMed] [Google Scholar]
- 19. Mahajan SK, Kashyap R, Kanga A, Sharma V, Prasher BS, Pal LS. 2006. Relevance of Weil-Felix test in diagnosis of scrub typhus in India. J. Assoc. Physicians India. 54:619–621 [PubMed] [Google Scholar]
- 20. Wong SY, Lam MS. 2001. Rickettsioses: the new and old diseases. Singapore Med. J. 42:546–548 [PubMed] [Google Scholar]
- 21. Ong AK, Tambyah PA, Ooi S, Kumarasinghe G, Chow C. 2001. Endemic typhus in Singapore—a re-emerging infectious disease? Singapore Med. J. 42:549–552 [PubMed] [Google Scholar]
- 22. Chen MI, Chua JK, Lee CC, Leo YS, Kumarasinghe G. 2001. Epidemiological, clinical and laboratory characteristics of 19 serologically confirmed rickettsial disease in Singapore. Singapore Med. J. 42:553–558 [PubMed] [Google Scholar]
- 23. Loh KC, Leo YS, Heng MK, Goh BC. 1996. Murine typhus: a forgotten cause of febrile illness in Singapore. Singapore Med. J. 37:39–43 [PubMed] [Google Scholar]
- 24. Mahajan SK. 2005. Scrub typhus. J. Assoc. Physicians India 53:954–958 [PubMed] [Google Scholar]