Abstract
Assessing cytomegalovirus (CMV)-specific cell-mediated immunity (CMI) represents an appealing strategy for identifying transplant recipients at risk of infection. In this study, we compared two gamma interferon-releasing assays (IGRAs), Quantiferon-CMV and CMV enzyme-linked immunosorbent spot (ELISPOT), to determine the ability of each test to predict protective CMV-specific T-cell responses. Two hundred twenty-one Quantiferon-CMV and ELISPOT tests were conducted on 120 adult kidney transplant recipients (KTRs), including 100 CMV-seropositive transplant recipients (R+) and 20 CMV-seronegative transplant recipients of a CMV-positive donor (D+/R−). As a control cohort, 39 healthy adult subjects (including 33 CMV-seropositive and 6 CMV-seronegative subjects) were enrolled. CMV IgG serology was used as a reference for both tests. In the CMV-seropositive individuals, the ELISPOT and Quantiferon-CMV assays provided 46% concordance with the serology, 12% discordance, 18% disagreement between ELISPOT or Quantiferon-CMV and the serology, and 24% gray areas when one or both tests resulted in weak positives. None of the CMV-seronegative subjects showed detectable responses in the ELISPOT or the Quantiferon-CMV test. In transplant recipients, both the ELISPOT and Quantiferon-CMV assays positively correlated with each other and negatively correlated with CMV DNAemia in a significant way (P < 0.05). During the antiviral prophylaxis, all 20 D+/R− KTRs we examined displayed undetectable Quantiferon-CMV and ELISPOT results, and there was no evidence of CMV seroconversion. The receiving operator curve (ROC) statistical analysis revealed similar specificities and sensitivities in predicting detectable viremia (areas under the curve [AUC], 0.66 and 0.62 for Quantiferon-CMV and ELISPOT, respectively). ELISPOT and Quantiferon-CMV values of >150 spots/200,000 peripheral blood mononuclear cells (PBMCs) and >1 to 6 IU gamma interferon (IFN-γ) were associated with protection from CMV infection (odds ratios [OR], 5 and 8.75, respectively). In transplant recipients, the two tests displayed similar abilities for predicting CMV infection. Both the ELISPOT and Quantiferon-CMV assays require several ameliorations to avoid false-negative results.
INTRODUCTION
Human cytomegalovirus (CMV) represents one of the primary opportunistic pathogens and is a leading cause of morbidity and mortality in transplant recipients. Preemptive or prophylaxis antiviral therapy has effectively reduced the impact and incidence of symptomatic CMV infection; however, drug-related toxicity and the potential emergence of drug-resistant CMV strains discourage a prolonged antiviral regimen.
Cell-mediated immunity (CMI), and specifically CMV-specific CD4+ and CD8+ T-cell responses, is able to control viral replication and thus the onset of symptomatic infections (1–5). In CMV-seronegative transplant recipients of a CMV-positive donor (D+/R−), de novo CMI develops upon primary CMV infection, which often originates from virus reactivation within the allograft, while in CMV-seropositive transplant recipients (R+), CMI recovers from previously existing immunity. The assessment of CMV-specific CMI has also been used to determine the individual risk of infection and as a helpful indicator in making therapeutic decisions, such as whether to initiate or terminate an antiviral treatment (6–12). For this reason, a diagnostic test assessing the status of immune reconstitution has been recommended in the current CMV management guidelines for transplant patients (13).
At present, several methods are available for monitoring the CMI in transplant recipients. Several methods rely on the functional analysis of T-cell-secreted cytokines or the T-cell phenotype (i.e., gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α], interleukin 2 [IL-2], CD107a, programmed cell death protein 1 [PD-1]) upon antigen stimulation, while other methods, such as tetramer assays, are based on the direct detection of antigen-specific T cells or cell proliferation assays (14–17). Moreover, tetramer assays are limited to a few human leukocyte antigen (HLA) haplotypes and thus may not be applicable for all patients. Most of these methods rely on advanced technologies and costly reagents or require a long turnaround time, all of which make the test impractical to use for clinical/diagnostic purposes. These issues and the lack of standardization have limited the use of these methods to highly specialized laboratories. In recent years, an increasing number of reports have focused on gamma interferon-releasing assays (IGRAs) as the diagnostic standard for detecting CMI toward infectious agents in humans (6–8, 11, 18–21). The IGRA provides a practical, standardized, rapid, and cost-effective tool for assessing pathogen-specific CMI (reviewed in references 22, 23, and 24). The most commonly used IGRAs, the T-SPOT TB (enzyme-linked immunosorbent spot [ELISPOT]) and the TB-Gold (Quantiferon), were developed for detecting Mycobacterium tuberculosis (TB) responses. Both the T-SPOT TB and the TB-Gold are FDA-approved tests for use in humans and display similar characteristics in assessing tuberculosis infection in immunocompetent subjects (25, 26). Reports also indicate that the T-SPOT TB test may be useful in an immunosuppressed population since it has a higher sensitivity than that of the TB-Gold test (27, 28).
In this study, we compared the CMV IFN-γ ELISPOT and Quantiferon-CMV tests to assess their grade of agreement, correlations, and abilities to predict CMV infection. Both tests have been used in experimental settings to detect CMV-specific T-cell responses in transplant recipients (8, 19, 29–32).
The main differences between the CMV ELISPOT and Quantiferon-CMV assays are that (i) the stimulus peptide composition is designed to selectively stimulate CD8+ T cells (Quantiferon) or both CD4+ and CD8+ T cells (ELISPOT), (ii) the Quantiferon-CMV test evaluates the IFN-γ production in a volume of 1 ml of whole blood, while the ELISPOT test considers the IFN-γ production in a given number of peripheral blood mononuclear cells (PBMCs) isolated from blood, and (iii) the Quantiferon-CMV assay quantitatively measures IFN-γ as international units (IU), while the ELISPOT test quantifies the spot-forming colonies (SFC) produced by a given number of PBMCs.
MATERIALS AND METHODS
Patients and definitions.
Two hundred twenty-one ELISPOT and Quantiferon-CMV tests were performed on 125 kidney transplant recipients (KTRs) enrolled in the study from September 2009 to September 2012. As a control group, we also enrolled 39 healthy adult subjects, including 33 CMV IgG-seropositive and 6 CMV IgG-seronegative subjects with a median age of 52 years (range, 24 to 72 years), comprising 19 Caucasian males and 20 Caucasian females. One hundred twenty-five KTRs were recruited as volunteers at 30, 60, 90, 180, and 360 days after transplant. The main clinical characteristics of the patients are shown in Table 1. Study exclusion criteria included any preexisting condition or acquired immunodeficiency. The Padua General Hospital Institutional Review Board and Ethical Committee approved all the medical and diagnostic procedures. CMV-seropositive recipients (R+) were treated according to the preemptive antiviral strategy once CMV DNAemia was detected at levels above 10,000 copies/ml of whole blood. CMV-seronegative recipients of a CMV-seropositive allograft (D+/R−) were treated according to an antiviral prophylaxis regimen for 180 days after transplant. The standard antiviral therapy, valganciclovir (Valcyte, Roche) at the standard dose (900 mg per day orally), corrected so as not to impair renal functionality, was administered. No cases of drug-resistant CMV strains occurred during the study. CMV disease was defined as fever, malaise, and/or gastrointestinal symptoms with concurrent CMV DNAemia and an absence of other ongoing infections.
Table 1.
Transplant recipients' characteristics
| Patient characteristics | Value for KTRs |
|---|---|
| Total no. | 120 |
| Gender (no. [%]) | |
| Male | 82 (71) |
| Female | 38 (29) |
| Median age (range) (yr) | 53 (21–82) |
| CMV serostatus (no. [%]) | |
| D+/R+ and D−/R+ | 100 (83) |
| D+/R− | 20 (17) |
| Immunosuppressive regimen: CNI, MMF,a and steroids (no. [%]) | 120 (100) |
| Acute rejection episodes (no. [%]) | 26 (22) |
| Patients who experienced posttransplant CMV DNAemia (no. [%]) | |
| All | 53 (44) |
| R+ | 47 (89) |
| R− | 6 (11) |
| Patients with CMV disease (no. [%]) | |
| All | 2 (2) |
| R+ | 1 (50) |
| R− | 1 (50) |
| Patients who received treatment for CMV infection (no. [%]) | |
| All | 32 (27) |
| R+ | 27 (84) |
| R− | 5 (16) |
CNI, calcineurin inhibitors; MMF, mycophenolate mofetil.
Detection of CMV viremia.
In all cases shown, CMV viremia (DNAemia) was evaluated using real-time PCR with an ABI Prism 7900 HT sequence detection system (Applied Biosystems). PCR primers, probes, and conditions were as described previously (33). The lowest detection limit was defined as <1,000 copies/ml of whole blood. CMV infection was defined as two sequential episodes of CMV DNAemia (>1,000 copies). Routine surveillance for viral reactivation or infection included weekly determination of CMV DNAemia during the first 100 days after transplant and continued thereafter if clinically indicated.
CMV serology.
CMV serostatus was assessed using an IgG enzyme-linked immunosorbent assay (ELISA) (Enzygnost, Dade Behring). An IgM ELISA (Enzygnost) was used to detect primary CMV infection.
Quantiferon-CMV and ELISPOT tests.
Both the Quantiferon-CMV and ELISPOT tests were performed on freshly isolated blood. At the same time, peripheral blood was collected in 3 (3× 1 ml) Quantiferon (Cellestis) tubes (for the positive control, negative control, and CMV stimulus) and 10 ml of peripheral blood was collected in sodium citrate tubes for ELISPOT testing. The Quantiferon blood tubes were incubated overnight at 37°C and further processed according to the manufacturer's instructions. Quantiferon data were acquired using the Personal Lab Workstation (Adaltis). For ELISPOT testing, PBMCs were extracted using Ficoll-Plaque Plus gradient (GE Healthcare), and 200,000 PBMCs/well were seeded onto a 96-well ELISPOT plate (AID Diagnostic) and stimulated as described previously (8). Quantiferon-CMV and ELISPOT tests were performed independently in a double-blind fashion. As indicated by the manufacturer's datasheet, ELISPOT results were considered positive at >20 spots, while Quantiferon-CMV results were considered positive at >0.2 IU IFN-γ. The reported values refer to the presence of CMI, not to protection from infection.
Statistical analysis.
Stata software (StataCorp) was used to analyze the data. The correlation of ELISPOT to Quantiferon-CMV and CMV DNAemia was obtained by negative binomial regression, where an ELISPOT result was expressed as the number of spots, a Quantiferon-CMV result was expressed as the IFN-γ cytokine concentration, and CMV DNAemia was a binary (0, 1) variable.
The ELISPOT and Quantiferon-CMV assays were evaluated by receiving operator curve (ROC) analysis, using as the endpoint (reference variable) the protection against the emergence of an episode of detectable CMV viremia. The sensitivity and specificity were obtained for every possible cutoff of the ELISPOT or Quantiferon-CMV test. The overall odds ratio (OR) was calculated as sensitivity × specificity/[(1-sensitivity) × (1-specificity)].
We considered ELISPOT and Quantiferon-CMV levels protective if no detectable events of CMV DNAemia occurred within 60 days after the ELISPOT and Quantiferon-CMV determinations. Given the low number of D+/R− patients analyzed, statistical analysis to assess the abilities of the Quantiferon-CMV and ELISPOT assays to predict viremia was not possible.
RESULTS
Quantiferon-CMV and ELISPOT with respect to CMV IgG serology in healthy adults.
We compared the results of the Quantiferon-CMV and ELISPOT assays in a cohort of 39 controls, including 33 CMV IgG-positive and 6 IgG-negative subjects. None of these subjects was positive for CMV IgM. In 6/6 CMV IgG-seronegative controls, both the Quantiferon-CMV and the ELISPOT tests displayed undetectable values, in accordance with negative serology.
Of the 33 healthy IgG-seropositive subjects analyzed, 4/33 (12%) displayed undetectable CMI with both the Quantiferon-CMV and ELISPOT tests, 15/33 (46%) were positive for CMI with both the Quantiferon-CMV and ELISPOT tests (>40 spots and >0.3 IU IFN-γ, respectively), while in 6/33 (18%) the ELISPOT and Quantiferon results were discordant (4 ELISPOT+/Quantiferon− and 2 ELISPOT−/Quantiferon+), and in 8/33 (24%) either the ELISPOT or the Quantiferon-CMV test displayed a borderline weak positive value (Table 2). The 4/33 (12%) subjects displaying undetectable CMI in the Quantiferon-CMV and ELISPOT assays in disagreement with positive CMV IgG serology produced detectable high responses in ELISPOT when stimulated with whole CMV virion lysate (data not shown).
Table 2.
ELISPOT and Quantiferon-CMV tests in CMV IgG+ individuals
| No. (%) of HSa | ELISPOT resultc | Quantiferon-CMV resultd |
|---|---|---|
| 15 (46) | Pos.b | Pos. |
| 4 (12) | Neg. | Neg. |
| 4 (12) | Pos. | Neg. |
| 2 (6) | Neg. | Pos. |
| 4 (12) | Weak Pos. | Pos. |
| 1 (3) | Weak Pos. | Weak Pos. |
| 3 (9) | Pos. | Weak Pos. |
HS, healthy subjects.
Positive values: ELISPOT > 40, Quantiferon-CMV > 0.3; negative values: ELISPOT < 20, Quantiferon-CMV < 0.2; weak positives: ELISPOT = >20 to <40; Quantiferon-CMV = >0.2 to <0.3.
ELISPOT results are expressed as the number of spots/200,000 PBMCs.
Quantiferon-CMV results are expressed as IU IFN-γ.
Quantiferon-CMV and ELISPOT in kidney transplant patients.
A total of 221 Quantiferon-CMV and ELISPOT tests were performed in a cohort of 120 kidney transplant patients, including 100 adult CMV R+ and 20 adult CMV D+/R−. ELISPOT and Quantiferon-CMV analyses were performed at 30, 60, 90, 180, and 360 days after transplant.
In the group of 20 D+/R−, all subjects analyzed displayed undetectable Quantiferon-CMV and ELISPOT results and had negative CMV IgG and IgM serology throughout the antiviral prophylaxis regimen. In the group of 100 CMV R+, the time courses of the ELISPOT and Quantiferon-CMV tests revealed a consistent increase in CMI over time that peaked at 180 days after transplant, followed by a slight decrease at 360 days (Fig. 1).
Fig 1.
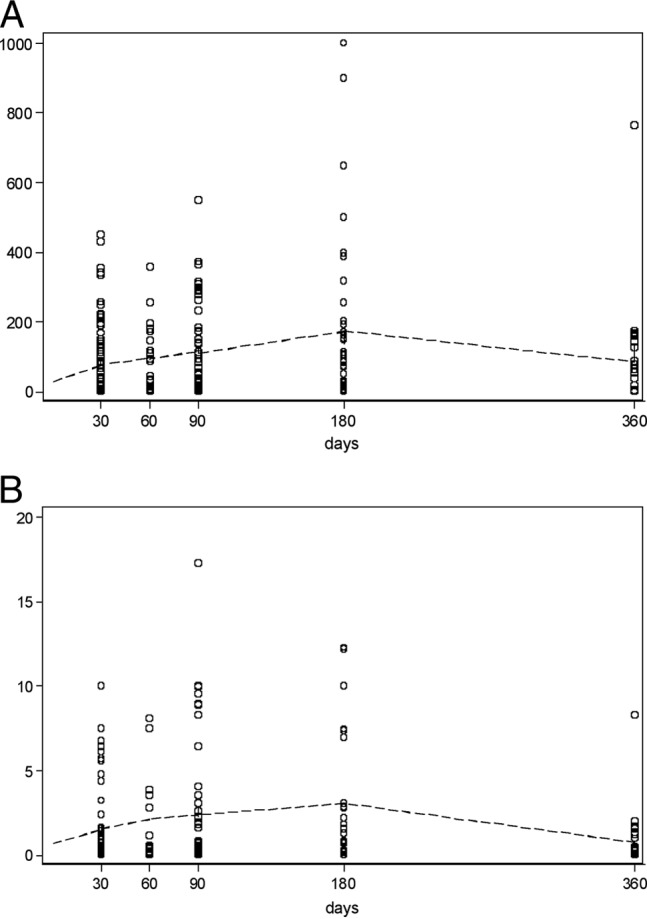
Regression of ELISPOT (top) and Quantiferon-CMV (bottom) data over time after transplant in CMV R+ subjects. Hollow circles represent single observations. A “Lowess smoother” (a locally weighted regression line [dashed line]) was added for clarity.
Correlation of Quantiferon-CMV and ELISPOT and the development of CMV DNAemia.
In order to assess the correlation of the Quantiferon-CMV and ELISPOT tests in transplant patients and their relationship with CMV DNAemia, we employed a negative binomial regression statistical test. The results show that there is a statistically significant correlation between the Quantiferon-CMV and ELISPOT assays (P < 0.037) and a statistically significant inverse correlation between the two tests and the development of CMV DNAemia (P < 0.017) (Table 3 and Fig. 2). Since the exponentiated coefficient of the Quantiferon-CMV test is 100.0740 = 1.076781, a one-unit increase of a Quantiferon-CMV result predicts a 7.7% increase of the ELISPOT result.
Table 3.
Negative binomial regression of ELISPOT versus Quantiferon-CMV results and CMV DNAemia in kidney transplant recipients
| ELISPOTa | Coefficient | SE | z | P | 95% confidence interval |
|---|---|---|---|---|---|
| Quantiferon | 0.0740 | 0.0355 | 2.09 | 0.037 | 0.0045 to 0.1435 |
| CMV DNAemia | −0.7020 | 0.2946 | −2.38 | 0.017 | −1.2794 to −0.1245 |
| Incept | 4.6125 | 0.1448 | 31.85 | 0.000 | 4.3287 to 4.8963 |
| Alpha | 2.674709 | 0.2339 | 2.253408 to 3.174776 |
Log likelihood = −1168.0423; likelihood ratio χ2(2) = 11.00; probability > χ2 = 0.0041.
Fig 2.
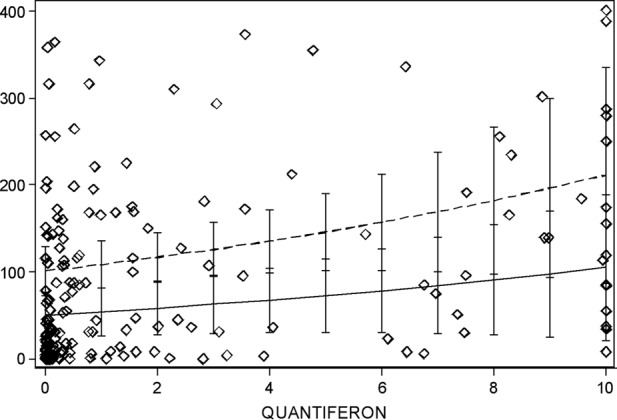
The fitted values of ELISPOT scores (predictions of the negative binomial model) are indicated as two lines, assuming undetectable CMV DNAemia (dashed line) or detectable CMV DNAemia (solid line). Hollow diamonds indicate the individual observations. 95% confidence intervals for the predictions are also indicated. ELISPOT values were limited to a scale of 0 to 400, and Quantiferon-CMV values to a scale of 0 to 10.
We also attempted to correlate ELISPOT and Quantiferon-CMV results with the magnitude (peak) and duration (days) of viremia using a linear regression approach, but no significant differences were found (data not shown). The statistical analysis showed that the low number of cases studied might have caused this lack of significance.
Sensitivities and specificities of Quantiferon-CMV and ELISPOT.
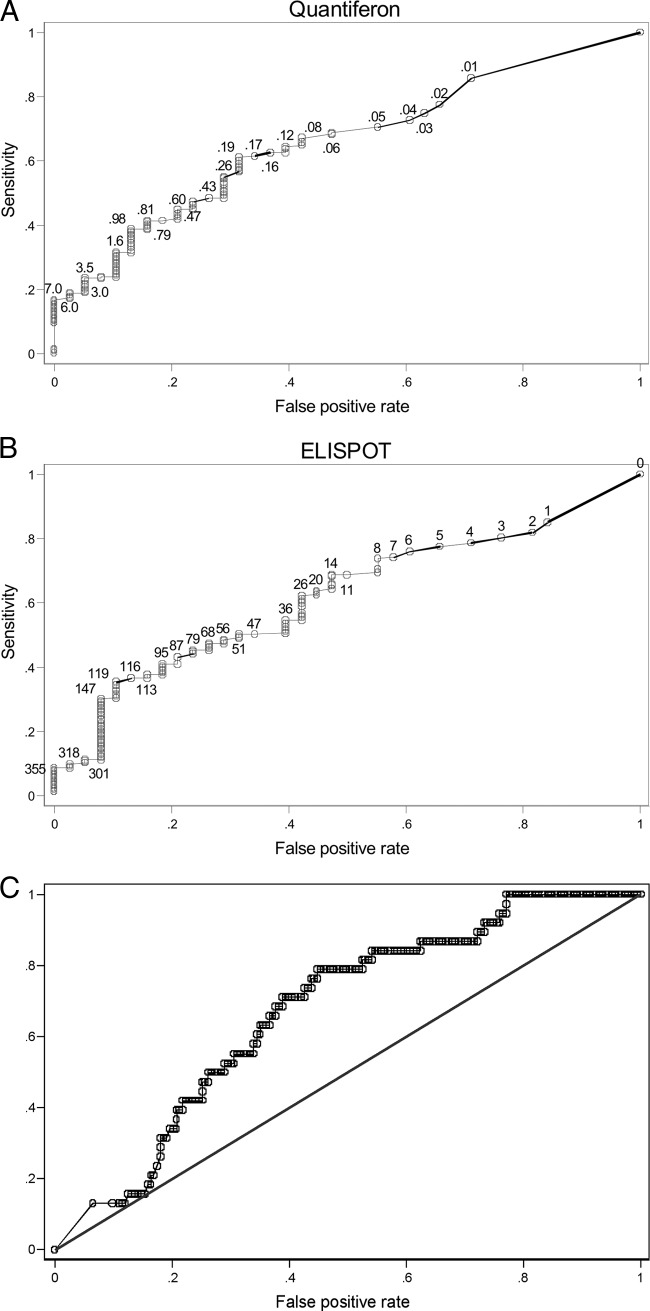
In order to assess the sensitivities and specificities of the Quantiferon-CMV and ELISPOT assays in detecting the onset of CMV DNAemia, we employed ROC statistical analysis (Fig. 3A and B). The calculated OR for the ELISPOT test was 2.12. The maximum OR value observed for the ELISPOT was 5.01 at cutoff values of ≥147. However, the OR mostly exceeded 4 in the cutoff area of ≥119 to ≥165. The Quantiferon-CMV calculated OR was 2.52. The maximum value observed for the OR was 8.75 at a cutoff of ≥6.1. However, this cutoff is very high and presumably not really useful, implying a low sensitivity of 19.13%. The ROC areas, standard errors, and 95% confidence intervals for Quantiferon-CMV and ELISPOT results are reported in Table 4. We also tested if combining the ELISPOT and the Quantiferon-CMV tests increases the ROC area. As shown in Fig. 3C, the combination of the two tests resulted in a modest increase in the ROC area (0.67).
Fig 3.
ROC analysis using only the Quantiferon-CMV (black solid dots, AUC = 0.6604) (A), only the ELISPOT (gray hollow dots, AUC = 0.6203) (B), or both tests in combination (AUC = 0.6731) (C). The endpoint (reference variable) was the protection against the emergence of an episode of CMV viremia. The Quantiferon-CMV or ELISPOT scores were the classifying variables. Numbers on the curve represent the absolute Quantiferon-CMV or ELISPOT values.
Table 4.
ELISPOT and Quantiferon ROC resultsa
| Test | ROC |
Asymptotic normal distribution (95% confidence interval) | ||
|---|---|---|---|---|
| Area | SE | |||
| ELISPOT | 0.6203 | 0.0458 | 0.53048 | 0.7101 |
| Quantiferon | 0.6604 | 0.0447 | 0.57285 | 0.74797 |
ELISPOT and Quantiferon-CMV scores as predictors of CMV viremia episode avoidance (protection), according to the ROC method. The area under the curve (AUC) is reported along with the standard error and the 95% confidence interval.
DISCUSSION
Predicting the risk of infection in transplant recipients represents an innovative and promising strategy for improving the clinical management of transplant recipients. In this study, we present the results of a comparative analysis of two IGRAs, the ELISPOT and the Quantiferon-CMV, widely used to assess CMI in transplant recipients.
For healthy adult CMV-seronegative subjects, both the Quantiferon-CMV and the ELISPOT results were in agreement with the negative IgG serology, while for the CMV IgG-seropositive adults, 12% of the subjects tested negative in both tests. This finding was unexpectedly high given previous reports (32) showing a 97% agreement with serology. The negative results of the Quantiferon-CMV and the ELISPOT opposing the positive CMV serology in healthy individuals probably depend on the previously shown inability of certain individuals to recognize the pp65 (ppUL83) stimulus peptide (34) or on the atypical or uncommon HLA haplotypes of these subjects (D. Abate, A. Saldan, D. Tinto, A. Bianchin, and G. Palù, unpublished data). Indeed, CMV IgG+ individuals with negative CMI in the Quantiferon-CMV and ELISPOT tests produced positive, detectable high responses when stimulated with non-HLA-restricted whole CMV virion lysate (data not shown), suggesting a limitation of the currently used stimuli in being recognized from heterogeneous HLA types. The consistency of the results from healthy subjects should be taken into account when immune monitoring is performed after transplants, in particular when a negative result is obtained. In transplant recipients, we have found that none of the D+/R− receiving antiviral prophylaxis therapy tested positive with the Quantiferon-CMV, the ELISPOT, or CMV IgG or IgM. This finding suggests that the current antiviral prophylaxis scheme is highly effective in suppressing CMV reactivation from the allograft. This finding is also consistent with previously published data on D+/R− with kidney transplant patients being unable to mount virus-specific immune responses during the antiviral prophylaxis regimen (8). In transplant patients, the Quantiferon-CMV and ELISPOT assays displayed a positive statistically significant correlation with each other. The Quantiferon-CMV and the ELISPOT also showed a consistent increase in CMV immunity over time, peaking at 180 days after transplant, and decreased at 360 days; this may suggest a generally slow process of immune reconstitution boosted by early posttransplant episodes of CMV viremias, achieving a steady-state level of antiviral immunity.
In transplant recipients, the Quantiferon-CMV and the ELISPOT tests displayed similar robustness, sensitivities, specificities and an inverse correlation with the development of CMV viremia, suggesting that values of >150 spots/200,000 PBMCs for the ELISPOT and >1 to 6 IU IFN-γ for the Quantiferon-CMV may have good predictive value for protection from CMV viremia. The proposed cutoffs refer to protection from CMV viremia and are different from the cutoffs of presence/absence of CMI proposed from the manufacturers of the Quantiferon-CMV and the ELISPOT. This study, to our knowledge, is the first comparison of the Quantiferon-CMV and the ELISPOT in transplant recipients, and the results may better aid clinicians in understanding the limitations and advantages of the two IGRAs analyzed. This study has also elucidated some critical aspects that may be improved for both tests. There is an urgent need to overcome the unexpectedly high number of false-negative results due to the inability of some HLA types to recognize the CMV stimulus composition.
ACKNOWLEDGMENTS
We thank Padua General Hospital-Azienda Ospedaliera di Padova for providing us with the IFN-γ ELISPOT plates and Cellestis and A.D.A., Italy, for providing Quantiferon and control tubes and reagents. We thank Daniel Tinto, Alice Bianchin, Angela Bozza, Simona Cofano, and Valentina Fornasiero for the technical guidance. We also thank Elisa Sefora Pierobon and Margherita Moro for medical advice.
Authors' contributions: D. Abate, C. Mengoli, D. Sgarabotto, P. Rigotti, and G. Palù analyzed the statistical data and wrote the manuscript, A. Saldan, L. Fallico, M. Peracchi, and M. Fiscon performed the tests and collected the experimental data, and D. Abate, L. Furian, C. Silvestre, P. Rigotti, R. Cusinato, B. Rossi, M. Fiscon, D. Sgarabotto, C. Mengoli, and G. Palù supervised the clinical study.
The authors declare no conflicts of interest.
Footnotes
Published ahead of print 15 May 2013
REFERENCES
- 1. Crough T, Fazou C, Weiss J, Campbell S, Davenport MP, Bell SC, Galbraith A, McNeil K, Khanna R. 2007. Symptomatic and asymptomatic viral recrudescence in solid-organ transplant recipients and its relationship with the antigen-specific CD8(+) T-cell response. J. Virol. 81:11538–11542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danziger-Isakov L, Heeger PS. 2009. Clinical utility of measuring T-cell immunity to CMV in transplant recipients. Am. J. Transplant. 9:987–988 [DOI] [PubMed] [Google Scholar]
- 3. Gerna G, Lilleri D. 2006. Monitoring transplant patients for human cytomegalovirus: diagnostic update. Herpes 13:4–11 [PubMed] [Google Scholar]
- 4. Kotton CN. 2013. CMV: prevention, diagnosis and therapy. Am. J. Transplant. 13(Suppl. 3):24–40 [DOI] [PubMed] [Google Scholar]
- 5. Nebbia G, Mattes FM, Smith C, Hainsworth E, Kopycinski J, Burroughs A, Griffiths PD, Klenerman P, Emery VC. 2008. Polyfunctional cytomegalovirus-specific CD4+ and pp65 CD8+ T cells protect against high-level replication after liver transplantation. Am. J. Transplant. 8:2590–2599 [DOI] [PubMed] [Google Scholar]
- 6. Abate D, Cesaro S, Cofano S, Fiscon M, Saldan A, Varotto S, Mengoli C, Pillon M, Calore E, Biasolo MA, Cusinato R, Barzon L, Messina C, Carli M, Palu G. 2012. Diagnostic utility of human cytomegalovirus-specific T-cell response monitoring in predicting viremia in pediatric allogeneic stem-cell transplant patients. Transplantation 93:536–542 [DOI] [PubMed] [Google Scholar]
- 7. Abate D, Fiscon M, Saldan A, Cofano S, Mengoli C, Sgarabotto D, d'Agostino C, Barzon L, Cusinato R, Toscano G, Feltrin G, Gambino A, Gerosa G, Palu G. 2012. Human cytomegalovirus-specific T-cell immune reconstitution in preemptively treated heart transplant recipients identifies subjects at critical risk for infection. J. Clin. Microbiol. 50:1974–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abate D, Saldan A, Fiscon M, Cofano S, Paciolla A, Furian L, Ekser B, Biasolo MA, Cusinato R, Mengoli C, Bonfante L, Rossi B, Rigotti P, Sgarabotto D, Barzon L, Palu G. 2010. Evaluation of cytomegalovirus (CMV)-specific T cell immune reconstitution revealed that baseline antiviral immunity, prophylaxis, or preemptive therapy but not antithymocyte globulin treatment contribute to CMV-specific T cell reconstitution in kidney transplant recipients. J. Infect. Dis. 202:585–594 [DOI] [PubMed] [Google Scholar]
- 9. Baldanti F, Lilleri D, Gerna G. 2008. Monitoring human cytomegalovirus infection in transplant recipients. J. Clin. Virol. 41:237–241 [DOI] [PubMed] [Google Scholar]
- 10. Cummins NW, Deziel PJ, Abraham RS, Razonable RR. 2009. Deficiency of cytomegalovirus (CMV)-specific CD8+ T cells in patients presenting with late-onset CMV disease several years after transplantation. Transpl. Infect. Dis. 11:20–27 [DOI] [PubMed] [Google Scholar]
- 11. Egli A, Humar A, Kumar D. 2012. State-of-the-art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clin. Infect. Dis. 55:1678–1689 [DOI] [PubMed] [Google Scholar]
- 12. Sester U, Gartner BC, Wilkens H, Schwaab B, Wossner R, Kindermann I, Girndt M, Meyerhans A, Mueller-Lantzsch N, Schafers HJ, Sybrecht GW, Kohler H, Sester M. 2005. Differences in CMV-specific T-cell levels and long-term susceptibility to CMV infection after kidney, heart and lung transplantation. Am. J. Transplant. 5:1483–1489 [DOI] [PubMed] [Google Scholar]
- 13. Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Snydman DR, Allen U, Humar A. 2010. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 89:779–795 [DOI] [PubMed] [Google Scholar]
- 14. Borchers S, Bremm M, Lehrnbecher T, Dammann E, Pabst B, Wolk B, Esser R, Yildiz M, Eder M, Stadler M, Bader P, Martin H, Jarisch A, Schneider G, Klingebiel T, Ganser A, Weissinger EM, Koehl U. 2012. Sequential anti-cytomegalovirus response monitoring may allow prediction of cytomegalovirus reactivation after allogeneic stem cell transplantation. PLoS One 7:e50248. 10.1371/journal.pone.0050248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ravkov EV, Pavlov IY, Hanson KE, Delgado JC. 2012. Validation of cytomegalovirus immune competence assays for the characterization of CD8(+) T cell responses posttransplant. Clin. Dev. Immunol. 2012:451059. 10.1155/2012/451059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ritter M, Schmidt T, Dirks J, Hennes P, Juhasz-Boss I, Solomayer EF, Gortner L, Gartner B, Rohrer T, Sester U, Sester M. 2013. Cytomegalovirus-specific T cells are detectable in early childhood and allow assignment of the infection status in children with passive maternal antibodies. Eur. J. Immunol. 43:1099–1108 [DOI] [PubMed] [Google Scholar]
- 17. Sester U, Presser D, Dirks J, Gartner BC, Kohler H, Sester M. 2008. PD-1 expression and IL-2 loss of cytomegalovirus-specific T cells correlates with viremia and reversible functional anergy. Am. J. Transplant. 8:1486–1497 [DOI] [PubMed] [Google Scholar]
- 18. Clari MA, Munoz-Cobo B, Solano C, Benet I, Costa E, Remigia MJ, Bravo D, Amat P, Navarro D. 2012. Performance of the QuantiFERON-cytomegalovirus (CMV) assay for detection and estimation of the magnitude and functionality of the CMV-specific gamma interferon-producing CD8(+) T-cell response in allogeneic stem cell transplant recipients. Clin. Vaccine Immunol. 19:791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar D, Chernenko S, Moussa G, Cobos I, Manuel O, Preiksaitis J, Venkataraman S, Humar A. 2009. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am. J. Transplant. 9:1214–1222 [DOI] [PubMed] [Google Scholar]
- 20. Lisboa LF, Kumar D, Wilson LE, Humar A. 2012. Clinical utility of cytomegalovirus cell-mediated immunity in transplant recipients with cytomegalovirus viremia. Transplantation 93:195–200 [DOI] [PubMed] [Google Scholar]
- 21. Manuel O, Husain S, Kumar D, Zayas C, Mawhorter S, Levi ME, Kalpoe J, Lisboa L, Ely L, Kaul DR, Schwartz BS, Morris MI, Ison MG, Yen-Lieberman B, Sebastian A, Assi M, Humar A. 2013. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin. Infect. Dis. 56:817–824 [DOI] [PubMed] [Google Scholar]
- 22. Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, Kampmann B, Lange C, Losi M, Markova R, Migliori GB, Nienhaus A, Ruhwald M, Wagner D, Zellweger JP, Huitric E, Sandgren A, Manissero D. 2011. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systemic review and meta-analysis. Eur. Respir. J. 37:88–89 [DOI] [PubMed] [Google Scholar]
- 23. Manuel O, Kumar D. 2008. QuantiFERON-TB Gold assay for the diagnosis of latent tuberculosis infection. Expert Rev. Mol. Diagn. 8:247–256 [DOI] [PubMed] [Google Scholar]
- 24. Pai M, Riley LW, Colford JM., Jr 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4:761–776 [DOI] [PubMed] [Google Scholar]
- 25. Chee CB, Gan SH, Khinmar KW, Barkham TM, Koh CK, Liang S, Wang YT. 2008. Comparison of sensitivities of two commercial gamma interferon release assays for pulmonary tuberculosis. J. Clin. Microbiol. 46:1935–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, Bossink A, Dheda K, Diel R, Dominguez J, Lipman M, Nemeth J, Ravn P, Winkler S, Huitric E, Sandgren A, Manissero D. 2011. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur. Respir. J. 37:100–111 [DOI] [PubMed] [Google Scholar]
- 27. Morris MI, Daly JS, Blumberg E, Kumar D, Sester M, Schluger N, Kim SH, Schwartz BS, Ison MG, Humar A, Singh N, Michaels M, Orlowski JP, Delmonico F, Pruett T, John GT, Kotton CN. 2012. Diagnosis and management of tuberculosis in transplant donors: a donor-derived infections consensus conference report. Am. J. Transplant. 12:2288–2300 [DOI] [PubMed] [Google Scholar]
- 28. Pai M, Zwerling A, Menzies D. 2008. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann. Intern. Med. 149:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Costa C, Saldan A, Sinesi F, Sidoti F, Balloco C, Simeone S, Piceghello A, Mantovani S, Di Nauta A, Solidoro P, Cavallo R. 2012. The lack of cytomegalovirus-specific cellular immune response may contribute to the onset of organ infection and disease in lung transplant recipients. Int. J. Immunopathol. Pharmacol. 25:1003–1009 [DOI] [PubMed] [Google Scholar]
- 30. Gerna G, Lilleri D, Fornara C, Comolli G, Lozza L, Campana C, Pellegrini C, Meloni F, Rampino T. 2006. Monitoring of human cytomegalovirus-specific CD4 and CD8 T-cell immunity in patients receiving solid organ transplantation. Am. J. Transplant. 6:2356–2364 [DOI] [PubMed] [Google Scholar]
- 31. Schmidt T, Ritter M, Dirks J, Gartner BC, Sester U, Sester M. 2012. Cytomegalovirus-specific T-cell immunity to assign the infection status in individuals with passive immunity: a proof of principle. J. Clin. Virol. 54:272–275 [DOI] [PubMed] [Google Scholar]
- 32. Walker S, Fazou C, Crough T, Holdsworth R, Kiely P, Veale M, Bell S, Gailbraith A, McNeil K, Jones S, Khanna R. 2007. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl. Infect. Dis. 9:165–170 [DOI] [PubMed] [Google Scholar]
- 33. Mengoli C, Cusinato R, Biasolo MA, Cesaro S, Parolin C, Palu G. 2004. Assessment of CMV load in solid organ transplant recipients by pp65 antigenemia and real-time quantitative DNA PCR assay: correlation with pp67 RNA detection. J. Med. Virol. 74:78–84 [DOI] [PubMed] [Google Scholar]
- 34. Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]