Abstract
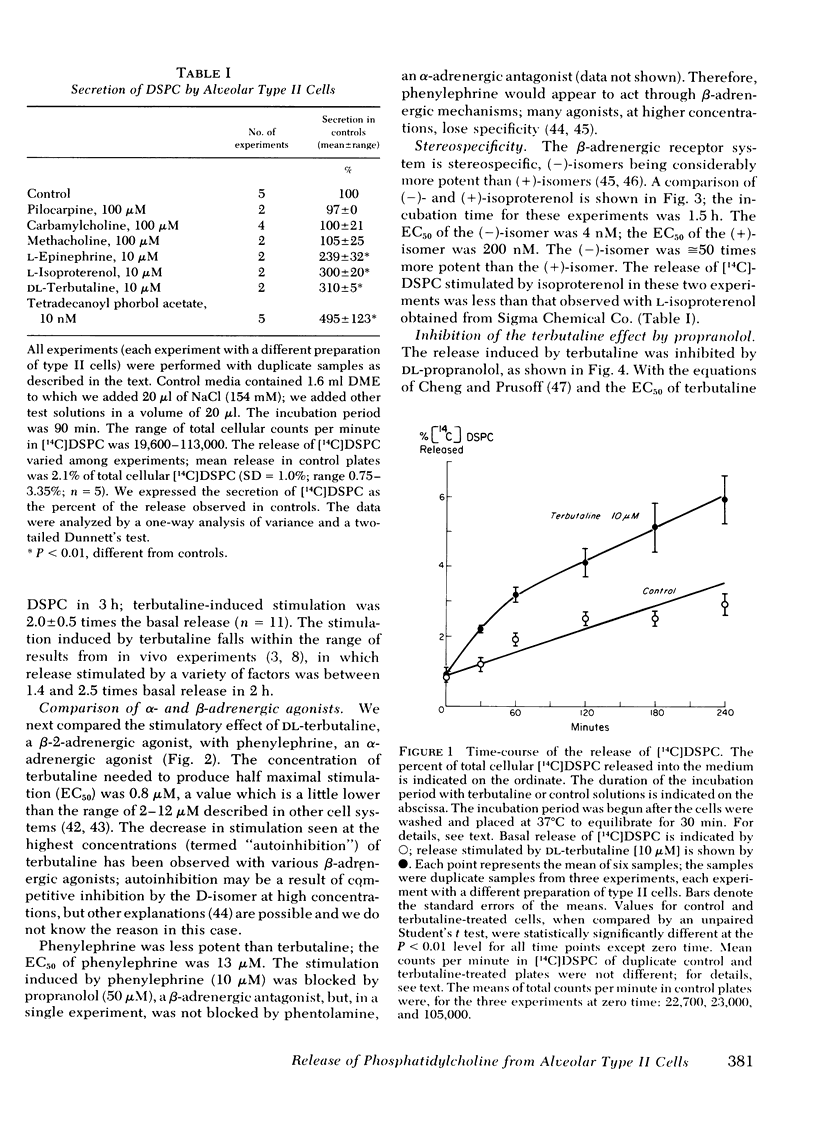
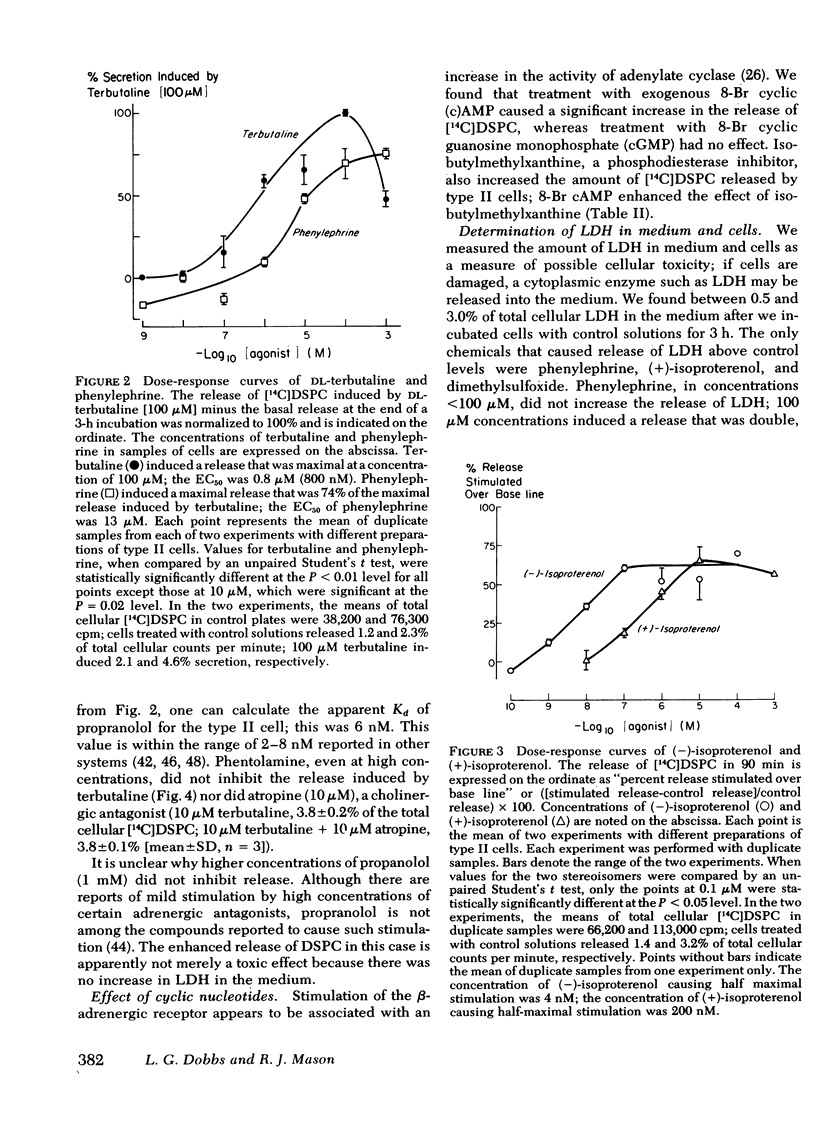
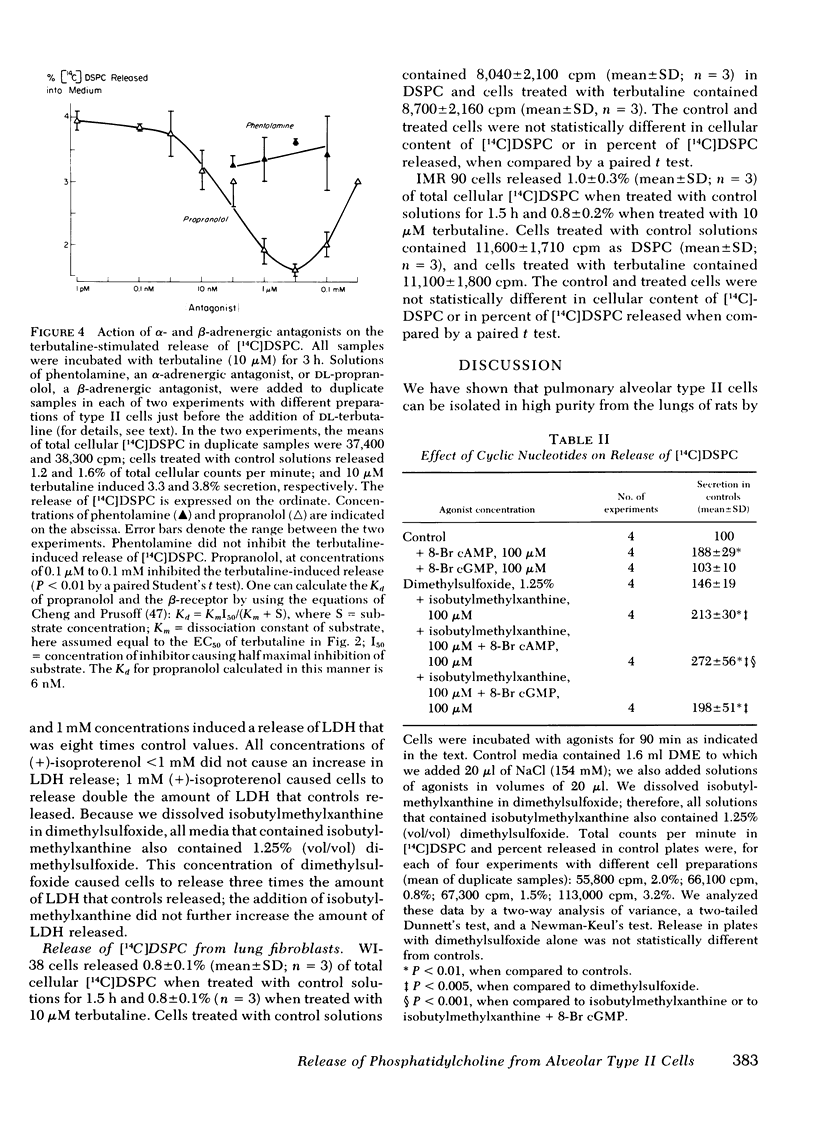
It is unclear what factors control the secretion of pulmonary surface active material from alveolar type II cells in vivo. Other workers have suggested that cholinergic stimuli, adrenergic stimuli, and prostaglandins may all stimulate secretion. We isolated type II cells from the lungs of rats by treatment with elastase, discontinuous density centrifugation, and adherence in primary culture. β-Adrenergic agonists, but not cholinergic agonists, caused an increase in the release of [14C]disaturated phosphatidylcholine, the major component of surface-active material, from type II cells in culture. The β-adrenergic effect was stereo-selective, (−)-isoproterenol being 50 times more potent than (+)-isoproterenol. Terbutaline, 10 μM, a noncatecholamine β-2 adrenergic agonist, caused a release of 2.0±0.5 (mean±SD) times the basal release of [14C]disaturated phosphatidylcholine in 3 h; the concentration of terbutaline causing half maximal stimulation was 800 nM. The terbutaline effect was blocked by propranolol, a β-adrenergic antagonist (calculated Kd = 6 nM), but not by phentolamine, an α-adrenergic antagonist. Isobutylmethylxanthine, a phosphodiesterase inhibitor, and 8-Br cyclic AMP, but not 8-Br cyclic guanosine monophosphate, also stimulated release. We conclude that type II cells secrete disaturated phosphatidylcholine in response to treatment with adrenergic stimulation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber R., Kelly L. A., McGuire R. F., Butcher R. W. Distortion of cyclic AMP responses to catecholamine due to destruction of the hormone. J Cyclic Nucleotide Res. 1977 Aug;3(4):249–261. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Corbet A. J., Flax P., Rudolph A. J. Reduced surface tension in lungs of fetal rabbits injected with pilocarpine. J Appl Physiol. 1976 Jul;41(1):7–14. doi: 10.1152/jappl.1976.41.1.7. [DOI] [PubMed] [Google Scholar]
- Corbet A. J., Flax P., Rudolph A. J. Role of autonomic nervous system controlling surface tension in fetal rabbit lungs. J Appl Physiol Respir Environ Exerc Physiol. 1977 Dec;43(6):1039–1045. doi: 10.1152/jappl.1977.43.6.1039. [DOI] [PubMed] [Google Scholar]
- Dale H. H., Laidlaw P. P. The significance of the suprarenal capsules in the action of certain alkaloids. J Physiol. 1912 Aug 2;45(1-2):1–26. doi: 10.1113/jphysiol.1912.sp001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L. G., Mason R. J. Stimulation of secretion of disaturated phosphatidylcholine from isolated alveolar type II cells by 12-O-tetradecanoyl-13-phorbol acetate. Am Rev Respir Dis. 1978 Oct;118(4):705–733. doi: 10.1164/arrd.1978.118.4.705. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fanestil D. D., Barrows C. H., Jr Aging in the rotifer. J Gerontol. 1965 Oct;20(4):462–469. [PubMed] [Google Scholar]
- Feldberg W., Minz B., Tsudzimura H. The mechanism of the nervous discharge of adrenaline. J Physiol. 1934 Jun 9;81(3):286–304. doi: 10.1113/jphysiol.1934.sp003136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. B., Furia L. Isolation and metabolism of granular pneumocytes from rat lungs. Lung. 1977;154(3):155–165. doi: 10.1007/BF02713531. [DOI] [PubMed] [Google Scholar]
- Goldenberg V. E., Buckingham S., Sommers S. C. Pilocarpine stimulation of granular pneumocyte secretion. Lab Invest. 1969 Feb;20(2):147–158. [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Hanoune J., Stengel D., Lacombe M. L., Feldmann G., Coudrier E. Proteolytic activation of rat liver adenylate cyclase by a contaminant of crude collagenase from Clostridium histolyticum. J Biol Chem. 1977 Mar 25;252(6):2039–2045. [PubMed] [Google Scholar]
- Hung K. S., Hertweck M. S., Hardy J. D., Loosli C. G. Innervation of pulmonary alveoli of the mouse lung: an electron microscopic study. Am J Anat. 1972 Dec;135(4):477–495. doi: 10.1002/aja.1001350404. [DOI] [PubMed] [Google Scholar]
- Jobe A. The labeling and biological half-life of phosphatidylcholine in subcellular fractions of rabbit lung. Biochim Biophys Acta. 1977 Dec 21;489(3):440–453. doi: 10.1016/0005-2760(77)90165-5. [DOI] [PubMed] [Google Scholar]
- Kero P., Hirvonen T., Välimäki I. Prenatal and postnatal isoxsuprine and respiratory-distress syndrome. Lancet. 1973 Jul;2(7822):198–passim. doi: 10.1016/s0140-6736(73)93024-9. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y., Yoneda K. The type II epithelial cell of the lung. I. Method of isolation. Lab Invest. 1974 Jan;30(1):76–84. [PubMed] [Google Scholar]
- King R. J. Metabolic fate of the apoproteins of pulmonary surfactant. Am Rev Respir Dis. 1977 Jun;115(6 Pt 2):73–79. doi: 10.1164/arrd.1977.115.S.73. [DOI] [PubMed] [Google Scholar]
- Kono T. Destruction and restoration of the insulin effector system of isolated fat cells. J Biol Chem. 1969 Nov 10;244(21):5777–5784. [PubMed] [Google Scholar]
- Lefkowitz R. J., Mukherjee C., Limbird L. E., Caron M. G., Williams L. T., Alexander R. W., Mickey J. V., Tate R. Regulation of adenylate cyclase coupled beta-adrenergic receptors. Recent Prog Horm Res. 1976;32:597–632. doi: 10.1016/b978-0-12-571132-6.50033-6. [DOI] [PubMed] [Google Scholar]
- Maguire M. E., Van Arsdale P. M., Gilman A. G. An agonist-specific effect of guanine nucleotides on binding to the beta adrenergic receptor. Mol Pharmacol. 1976 Mar;12(2):335–339. [PubMed] [Google Scholar]
- Malbon C. C., Moreno F. J., Cabelli R. J., Fain J. N. Fat cell adenylate cyclase and beta-adrenergic receptors in altered thyroid states. J Biol Chem. 1978 Feb 10;253(3):671–678. [PubMed] [Google Scholar]
- Mangos J. A., McSherry N. R., Barber T., Arvanitakis S. N., Wagner V. Dispersed rat parotid acinar cells. II. Characterization of adrenergic receptors. Am J Physiol. 1975 Sep;229(3):560–565. doi: 10.1152/ajplegacy.1975.229.3.560. [DOI] [PubMed] [Google Scholar]
- Mangos J. A., McSherry N. R., Butcher F., Irwin K., Barber T. Dispersed rat parotid acinar cells. I. Morphological and functional characterization. Am J Physiol. 1975 Sep;229(3):553–559. doi: 10.1152/ajplegacy.1975.229.3.553. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Huber G., Vaughan M. Synthesis of dipalmitoyl lecithin by alveolar macrophages. J Clin Invest. 1972 Jan;51(1):68–73. doi: 10.1172/JCI106798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. J., Nellenbogen J., Clements J. A. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976 May;17(3):281–284. [PubMed] [Google Scholar]
- Mason R. J., Williams M. C., Greenleaf R. D., Clements J. A. Isolation and properties of type II alveolar cells from rat lung. Am Rev Respir Dis. 1977 Jun;115(6):1015–1026. doi: 10.1164/arrd.1977.115.6.1015. [DOI] [PubMed] [Google Scholar]
- Massaro D. In vivo protein secretion by lung. Evidence for active secretion and interspecies differences. J Clin Invest. 1975 Aug;56(2):263–271. doi: 10.1172/JCI108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClenahan J. B., Urtnowski A. Effect of ventilation on surfactant, and its turnover rate. J Appl Physiol. 1967 Aug;23(2):215–220. doi: 10.1152/jappl.1967.23.2.215. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. Nerves in rat intra-acinar alveoli: an electron microscopic study. Respir Physiol. 1971 Mar;11(3):367–377. doi: 10.1016/0034-5687(71)90010-7. [DOI] [PubMed] [Google Scholar]
- Nichols W. W., Murphy D. G., Cristofalo V. J., Toji L. H., Greene A. E., Dwight S. A. Characterization of a new human diploid cell strain, IMR-90. Science. 1977 Apr 1;196(4285):60–63. doi: 10.1126/science.841339. [DOI] [PubMed] [Google Scholar]
- Oyarzun M. J., Clements J. A. Control of lung surfactant by ventilation, adrenergic mediators, and prostaglandins in the rabbit. Am Rev Respir Dis. 1978 May;117(5):879–891. doi: 10.1164/arrd.1978.117.5.879. [DOI] [PubMed] [Google Scholar]
- Oyarzún M. J., Clements J. A. Ventilatory and cholinergic control of pulmonary surfactant in the rabbit. J Appl Physiol Respir Environ Exerc Physiol. 1977 Jul;43(1):39–45. doi: 10.1152/jappl.1977.43.1.39. [DOI] [PubMed] [Google Scholar]
- Patil P. N., Miller D. D., Trendelenburg U. Molecular geometry and adrenergic drug activity. Pharmacol Rev. 1974 Dec;26(4):323–392. [PubMed] [Google Scholar]
- Persson K., Persson K. The metabolism of terbutaline in vitro by rat and human liver O-methyltransferases and monoamine oxidases. Xenobiotica. 1972 Jul;2(4):375–382. doi: 10.3109/00498257209111064. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips H. J. Dissociation of single cells from lung or kidney tissue with elastase. In Vitro. 1972 Sep-Oct;8(2):101–105. doi: 10.1007/BF02615967. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Fleming R. M. A new, highly sensitive and specific assay for chymotrypsin. Clin Chim Acta. 1975 Mar 10;59(2):139–146. doi: 10.1016/0009-8981(75)90021-2. [DOI] [PubMed] [Google Scholar]
- SNYDER F. RADIOASSAY OF THIN-LAYER CHROMATOGRAMS: A HIGH-RESOLUTION ZONAL SCRAPER FOR QUANTITATIVE C14 AND H3 SCANNING OF THIN-LAYER CHROMATOGRAMS. Anal Biochem. 1964 Oct;9:183–196. doi: 10.1016/0003-2697(64)90102-2. [DOI] [PubMed] [Google Scholar]
- Smith B. T. Cell line A549: a model system for the study of alveolar type II cell function. Am Rev Respir Dis. 1977 Feb;115(2):285–293. doi: 10.1164/arrd.1977.115.2.285. [DOI] [PubMed] [Google Scholar]
- Vulliemoz Y., Verosky M., Triner L. Effect of albuterol and terbutaline, synthetic beta adrenergic stimulants, on the cyclic 3',5'-adenosine monophosphate system in smooth muscle. J Pharmacol Exp Ther. 1975 Dec;195(3):549–556. [PubMed] [Google Scholar]
- Wallach D., Anderson W., Pastan I. Activation of adenylate cyclase in cultured fibroblasts by trypsin. J Biol Chem. 1978 Jan 10;253(1):24–26. [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Lefkowitz R. J. Identification of beta-adrenergic receptors in human lymphocytes by (-) (3H) alprenolol binding. J Clin Invest. 1976 Jan;57(1):149–155. doi: 10.1172/JCI108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszogrodski I., Kyei-Aboagye K., Taeusch H. W., Jr, Avery M. E. Surfactant inactivation by hyperventilation: conservation by end-expiratory pressure. J Appl Physiol. 1975 Mar;38(3):461–466. doi: 10.1152/jappl.1975.38.3.461. [DOI] [PubMed] [Google Scholar]
- Wyszogrodski I., Taeusch H. W., Jr, Avery M. E. Isoxsuprine-induced alterations of pulmonary pressure-volume relationships in premature rabbits. Am J Obstet Gynecol. 1974 Aug 15;119(8):1107–1111. doi: 10.1016/0002-9378(74)90267-1. [DOI] [PubMed] [Google Scholar]
- Young S. L., Tierney D. F. Dipalmitoyl lecithin secretion and metabolism by the rat lung. Am J Physiol. 1972 Jun;222(6):1539–1544. doi: 10.1152/ajplegacy.1972.222.6.1539. [DOI] [PubMed] [Google Scholar]



