Abstract
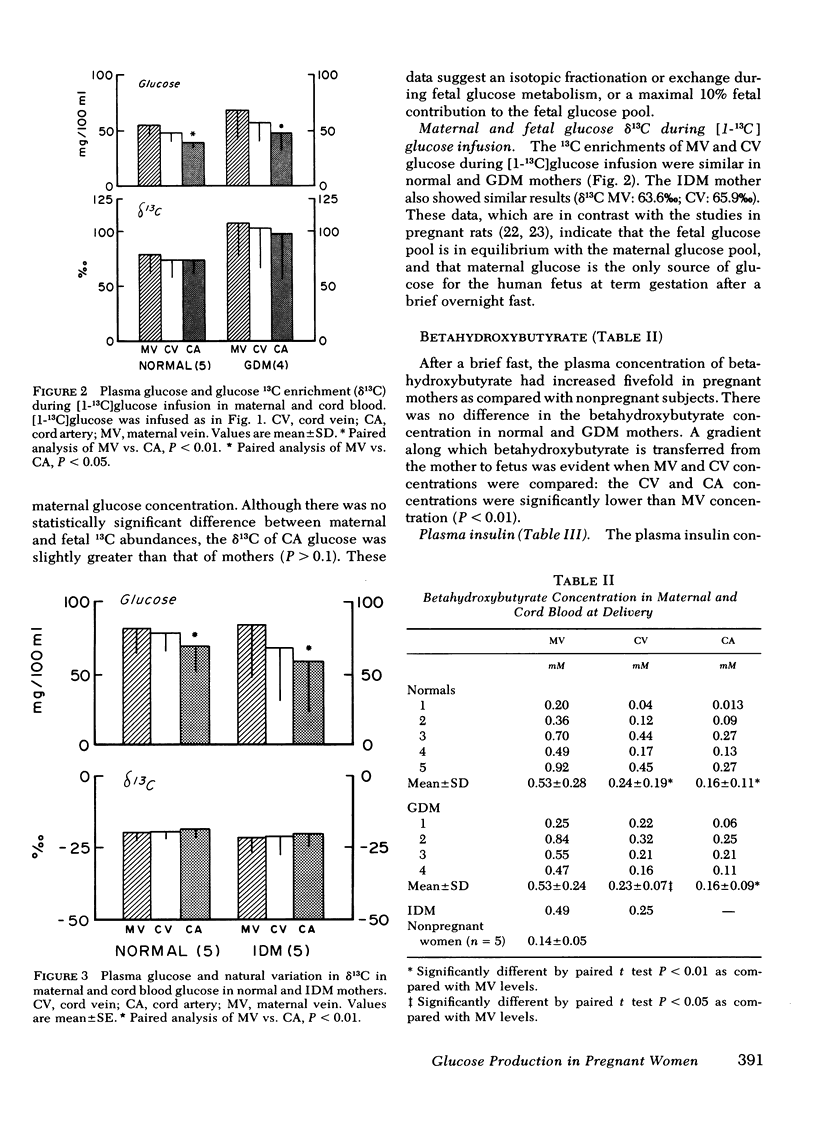
The effects of pregnancy and diabetes on systemic glucose production rates and the sources of glucose for the human fetus in utero were evaluated in five normal, four gestationally diabetic, and one insulin-dependent diabetic subject undergoing elective caesarean section at term gestation. Five normal nonpregnant women were studied for comparison. Systemic glucose production rates were measured with stable tracer [1-13C]glucose according to the prime-constant rate infusion technique. Even though the plasma glucose concentration during normal pregnancy had declined as compared with the nonpregnant subjects (P < 0.0005), the systemic glucose production rate was 16% greater, a rate sufficient to provide the glucose requirement of the fetus at term gestation. The decline in glucose concentration could be the result of an increase in apparent volume of distribution of glucose. Systemic glucose production rates in well-controlled, gestationally diabetic subjects were similar to those in normal pregnant subjects (2.07±0.53 vs. 2.42±0.51 mg/kg·min). The sources of glucose for the human fetus at term gestation were evaluated by comparing (a) natural variation in 13C:12C ratio of plasma glucose and (b) enriched 13C:12C ratio of plasma glucose during [1-13C]glucose infusion in maternal and fetal blood at delivery in both normal and diabetic subjects. These data showed that the fetal glucose pool was in equilibrium with the maternal glucose pool in both normal and diabetic subjects, indicating that a brief maternal fast did not initiate systemic glucose production in human fetus. A materno-fetal gradient was observed for betahydroxybutyrate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam P. A., Schwartz A. L., Rahiala E. L., Kekomäki M. Glucose production in midterm human fetus. I. Autoregulation of glucose uptake. Am J Physiol. 1978 Jun;234(6):E560–E567. doi: 10.1152/ajpendo.1978.234.6.E560. [DOI] [PubMed] [Google Scholar]
- BLEICHER S. J., O'SULLIVAN J. B., FREINKEL N. CARBOHYDRATE METABOLISM IN PREGNANCY. V. THE INTERRELATIONS OF GLUCOSE, INSULIN AND FREE FATTY ACIDS IN LATE PREGNANCY AND POST PARTUM. N Engl J Med. 1964 Oct 22;271:866–872. doi: 10.1056/NEJM196410222711702. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Leake R. D., Haymond M. W., Arnold K. J., Gruenke L. D., Sperling M. A., Kipnis D. M. Measurement of "true" glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977 Nov;26(11):1016–1023. doi: 10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- Bossi E., Greenberg R. E. Sources of blood glucose in the rat fetus. Pediatr Res. 1972 Oct;6(10):765–772. doi: 10.1203/00006450-197210000-00004. [DOI] [PubMed] [Google Scholar]
- DeNiro M. J., Epstein S. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science. 1977 Jul 15;197(4300):261–263. doi: 10.1126/science.327543. [DOI] [PubMed] [Google Scholar]
- FOLKART G. R., DANCIS J., MONEY W. L. Transfer of carbohydrates across guinea pig placenta. Am J Obstet Gynecol. 1960 Aug;80:221–223. doi: 10.1016/0002-9378(60)90116-2. [DOI] [PubMed] [Google Scholar]
- Felig P. Body fuel matabolism and diabetes mellitus in pregnancy. Med Clin North Am. 1977 Jan;61(1):43–66. doi: 10.1016/s0025-7125(16)31348-7. [DOI] [PubMed] [Google Scholar]
- Felig P., Kim Y. J., Lynch V., Hendler R. Amino acid metabolism during starvation in human pregnancy. J Clin Invest. 1972 May;51(5):1195–1202. doi: 10.1172/JCI106913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P. Maternal and fetal fuel homeostasis in human pregnancy. Am J Clin Nutr. 1973 Sep;26(9):998–1005. doi: 10.1093/ajcn/26.9.998. [DOI] [PubMed] [Google Scholar]
- Freinkel N., Metzger B. E., Nitzan M., Hare J. W., Shambaugh G. E., 3rd, Marshall R. T., Surmaczynska B. Z., Nagel T. C. "Accelerated starvation" and mechanisms for the conservation of maternal nitrogen during pregnancy. Isr J Med Sci. 1972 Mar;8(3):426–439. [PubMed] [Google Scholar]
- Glinsmann W. H., Eisen H. J., Lynch A., Chez R. A. Glucose regulation by isolated near term fetal monkey liver. Pediatr Res. 1975 Jul;9(7):600–604. doi: 10.1203/00006450-197507000-00009. [DOI] [PubMed] [Google Scholar]
- Goodner C. J., Conway M. J., Werrbach J. H. Relation between plasma glucose levels of mother and fetus during maternal hyperglycemia, hypoglycemia, and fasting in the rat. Pediatr Res. 1969 Mar;3(2):121–127. doi: 10.1203/00006450-196903000-00003. [DOI] [PubMed] [Google Scholar]
- Goodner C. J., Thompson D. J. Glucose metabolism in the fetus in utero: the effect of maternal fasting and glucose loading in the rat. Pediatr Res. 1967 Nov;1(6):443–451. doi: 10.1203/00006450-196711000-00003. [DOI] [PubMed] [Google Scholar]
- HOLMBERG N. G., KAPLAN B., KARVONEN M. J., LIND J., MALM M. Permeability of human placenta to glucose, fructose, and xylose. Acta Physiol Scand. 1956 May 31;36(4):291–299. doi: 10.1111/j.1748-1716.1956.tb01326.x. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Herrera E., Knopp R. H., Freinkel N. Carbohydrate metabolism in pregnancy. VI. Plasma fuels, insulin, liver composition, gluconeogenesis, and nitrogen metabolism during late gestation in the fed and fasted rat. J Clin Invest. 1969 Dec;48(12):2260–2272. doi: 10.1172/JCI106192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler K. Y., Williams H. R., Shreeve W. W., Landau B. R. Conversion of specifically 14 C-labeled lactate and pyruvate to glucose in man. J Biol Chem. 1969 Apr 25;244(8):2075–2077. [PubMed] [Google Scholar]
- KARVONEN M. J., RAIHA N. Permeability of placenta of the guinea pig to glucose and fructose. Acta Physiol Scand. 1954 Jul 18;31(2-3):194–202. doi: 10.1111/j.1748-1716.1954.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Kalhan S. C., Savin S. M., Adam P. A. Attenuated glucose production rate in newborn infants of insulin-dependent diabetic mothers. N Engl J Med. 1977 Feb 17;296(7):375–376. doi: 10.1056/NEJM197702172960706. [DOI] [PubMed] [Google Scholar]
- Kalhan S. C., Savin S. M., Adam P. A. Estimation of glucose turnover with stable tracer glucose-1-13C. J Lab Clin Med. 1977 Feb;89(2):285–294. [PubMed] [Google Scholar]
- Kalhan S. C., Savin S. M., Adam P. A. Measurement of glucose turnover in the human newborn with glucose-1-13C. J Clin Endocrinol Metab. 1976 Sep;43(3):704–707. doi: 10.1210/jcem-43-3-704. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Felig P. Maternal and amniotic fluid substrate levels during caloric deprivation in human pregnancy. Metabolism. 1972 Jun;21(6):507–512. doi: 10.1016/0026-0495(72)90094-7. [DOI] [PubMed] [Google Scholar]
- Metzger B. E., Agnoli F. S., Freinkel N. Effect of sex and pregnancy on formation of urea and ammonia during gluconeogenesis in the perfused rat liver. Horm Metab Res. 1970 Nov;2(6):367–368. doi: 10.1055/s-0028-1096820. [DOI] [PubMed] [Google Scholar]
- Metzger B. E., Agnoli F. S., Hare J. W., Freinkel N. Carbohydrate metabolism in pregnancy. X. Metabolic disposition of alanine by the perfused liver of the fasting pregnant rat. Diabetes. 1973 Aug;22(8):601–612. doi: 10.2337/diab.22.8.601. [DOI] [PubMed] [Google Scholar]
- Metzger B. E., Hare J. W., Freinkel N. Carbohydrate metabolism in pregnancy. IX. Plasma levels of gluconeogenic fuels during fasting in the rat. J Clin Endocrinol Metab. 1971 Nov;33(5):869–872. doi: 10.1210/jcem-33-5-869. [DOI] [PubMed] [Google Scholar]
- Paterson P., Sheath J., Taft P., Wood C. Maternal and foetal ketone concentrations in plasma and urine. Lancet. 1967 Apr 22;1(7495):862–865. doi: 10.1016/s0140-6736(67)91426-2. [DOI] [PubMed] [Google Scholar]
- Persson B. Determination of plasma acetoacetate and D-beta-hydroxybutyrate in new-born infants by an enzymatic fluorometric micro-method. Scand J Clin Lab Invest. 1970 Jan;25(1):9–18. doi: 10.3109/00365517009046184. [DOI] [PubMed] [Google Scholar]
- Räihä N. C., Lindros K. O. Development of some enzymes involved in gluconeogenesis in human liver. Ann Med Exp Biol Fenn. 1969;47(2):146–150. [PubMed] [Google Scholar]
- SCOW R. O., CHERNICK S. S., SMITH B. B. Ketosis in the rat fetus. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):833–835. doi: 10.3181/00379727-98-24199. [DOI] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Sabata V., Wolf H., Lausmann S. The role of free fatty acids, glycerol, ketone bodies and glucose in the energy metabolism of the mother and fetus during delivery. Biol Neonat. 1968;13(1):7–17. doi: 10.1159/000240128. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Rall T. W. Hormonal regulation of incorporation of alanine-U-14C into glucose in human fetal liver explants. Effect of dibutyryl cyclic AMP, glucagon, insulin, and triamcinolone. Diabetes. 1975 Jul;24(7):650–657. doi: 10.2337/diab.24.7.650. [DOI] [PubMed] [Google Scholar]
- Victor A. Normal blood sugar variation during pregnancy. Acta Obstet Gynecol Scand. 1974;53(1):37–40. doi: 10.3109/00016347409156886. [DOI] [PubMed] [Google Scholar]



