Abstract
In immunocompromized patients, including hematopoietic stem cell transplant (HSCT) recipients, life-threatening toxoplasmosis may result from reactivation of previous infection. We report a case of severe disseminated toxoplasmosis that developed early after allogeneic HSCT for T-cell lymphoblastic leukemia/lymphoma in a 15-year-old Toxoplasma gondii-seropositive boy with Nijmegen breakage syndrome, a rare genetic DNA repair disorder associated with immunodeficiency. The donor was the patient's HLA-identical brother. Prophylaxis with cotrimoxazole was discontinued a day before the HSCT procedure. Signs of lung infection appeared as early as day 14 post-HSCT. The presence of tachyzoite-like structures on Giemsa-stained bronchoalveolar lavage (BAL) fluid smears suggested toxoplasmosis. Real-time PCR targeted at the T. gondii AF146527 gene revealed extremely high parasite burdens in both blood and BAL fluid. Although immediate introduction of specific treatment resulted in a marked reduction of the parasite load and transient clinical improvement, the patient deteriorated and died of multiple organ failure on day 39 post-HSCT. Direct genotyping of T. gondii DNA from blood and BAL fluid with the PCR-restriction fragment length polymorphism method revealed type II alleles with SAG1, SAG2, and GRA6 markers but alleles of both type I and type II with GRA7. Additional analysis with 15 microsatellite markers showed that the T. gondii DNA was atypical and genetically divergent from that of the clonal type I, II, and III strains. This is the first report of increased clinical severity of toxoplasmosis associated with an atypical strain in the setting of immunosuppression, which emphasizes the need to diagnose and monitor toxoplasmosis by quantitative molecular methods in cases of reactivation risk.
INTRODUCTION
Toxoplasma gondii is a protozoan parasite distributed worldwide and infecting one-third of the global population. Infection is acquired by ingestion of parasites via consumption of undercooked meat containing tissue cysts or of water, fruits, or vegetables contaminated with oocysts. In immunocompetent individuals, primary infection results in the formation of tissue cysts and in serological evidence of infection. However, in immunocompromized patients, such as hematopoietic stem cell transplant (HSCT) recipients, toxoplasmosis is often a life-threatening opportunistic infection arising either from transmission of the parasites via a graft from a seropositive donor to a seronegative recipient or, far more frequently, from reactivation of a preexisting latent infection in a seropositive recipient, regardless of the donor's serological status (1). After allogeneic HSCT, studies have shown an incidence of invasive toxoplasmosis among seropositive recipients of 4% to 6%, with an estimated mortality rate of 60% to 90% (2, 3).
Nijmegen breakage syndrome (NBS) is a rare, autosome-recessive DNA repair disorder characterized by microcephaly with normal intelligence, facial dysmorphia (bird-like facial features), primary immunodeficiency, and a predisposition to lymphoid malignancies at a young age (4, 5). Treatment of NBS-related malignancies is challenging due to the chromosomal instability and immunodeficiency which make the patients more susceptible to the toxic effects of standard chemotherapy and radiation. Although lymphoid malignancies occurring in NBS patients can be successfully brought into remission using standard chemotherapy regimens with minor dose modifications (6, 7), a high rate of treatment failure and relapse has been observed (8). An alternative treatment option for malignancies in NBS is HSCT from HLA-identical donors, which has been shown not only to correct humoral and cellular immunodeficiency but also to lower the secondary malignancy rate (9).
We here present a case of an early and fulminant post-HSCT reactivation of toxoplasmosis caused by an atypical T. gondii strain in a young patient with NBS that contributed to a subsequent fatal outcome within 40 days after transplantation. This case is the first report of increased clinical severity of toxoplasmosis caused by an atypical strain in the setting of immunosuppression. It also stresses the value and necessity of the use of quantitative molecular methods to monitor T. gondii reactivation in cases of reactivation risk.
(The results of this study have been presented in part at the 11th European Multicolloquium of Parasitology [EMOP XI], Cluj-Napoca, Romania, 25 to 29 July 2012.)
CASE REPORT
A 15-year-old male patient with NBS was admitted at the Mother and Child Health Care Institute of Serbia for planned HSCT from a HLA-identical sibling. His past medical history revealed no recurrent infections during childhood.
The first hospitalization at the Institute was in October 2010, when a diagnosis of T-cell lymphoblastic leukemia/lymphoma (TLBL/ALL) was established. The patient received modified chemotherapy leading to complete clinical and laboratory remission of TLBL/ALL. At the time, microcephaly along with characteristic facial features, including large ears, prominent midface, and receding mandible, raised suspicion of NBS. Diagnosis of NBS was confirmed by a mutation analysis of the NBN(NBS1) gene which revealed a homozygous state for a typical 5-bp deletion (g.657del.5). Immunodeficiency was characterized by low serum IgA (0.57 g/liter) and IgM (0.36 g/liter) concentrations, while IgG was within the normal range for his age.
HSCT was performed in November 2011. Before HSCT, he received a standard reduced-intensity fludarabine-based conditioning regimen according to the European Blood and Marrow Transplantation-Inborn Errors Working Party guidelines, without irradiation. Acute graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine. Pretransplantation serological screening for toxoplasmosis revealed a donor-recipient mismatch, i.e., the donor was seronegative, while the recipient had serological (enzyme-linked immunosorbent assay [ELISA]) (Enzygnost Toxo IgG and Enzygnost Toxo IgM tests; Siemens Healthcare Diagnostics, Marburg, Germany) evidence of past infection (Table 1). Trimethoprim-sulfamethoxazole (TMP-SMX) administered for prophylaxis of Pneumocystis jirovecii pneumonia was considered to cover toxoplasmosis as well but was discontinued a day before HSCT, following the guidelines to avoid an early graft failure (10).
Table 1.
Results of T. gondii serological and molecular diagnosis and follow-up
| Sampling day vs HSCT | Serologya |
T. gondii burden (parasites/ml)b |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| IgG |
IgM |
||||||||
| ELISA (IU/ml) | HSDA (titer) | TXG (IU/ml) | TXGA index | ELISA absorbance | ISAgA index | TXM index | Blood | BAL fluid | |
| −21 | 578 | ND | ND | ND | neg. | ND | neg. | ND | ND |
| +21 | ND | 1:640 | 144 | 0.566 | ND | 3 | 0.09 | 15,000 | 100,000 |
| +30 | ND | 1:1,280 | 287 | ND | ND | 0 | 0.10 | 200 | ND |
Positivity thresholds for IgG, ≥5 IU/ml for ELISA (Enzygnost Toxo IgG, Siemens), ≥1:40 for HSDA, and ≥8 IU/ml for TXG Vidas (bioMérieux). For IgM, ELISA (Enzygnost Toxo IgM, Siemens) absorbance values, ≥0.3; TXM Vidas (bioMérieux) index, ≥0.65; ISAgA (bioMérieux) index, 9 to 12. For TXGA Vidas (bioMérieux), low index (I), I < 0.200; intermediate index, 0.200 ≤ I < 0.300; high index, I ≥ 0.300. ND, not done; neg., negative.
Number of parasites/ml of sample quantified by real-time PCR.
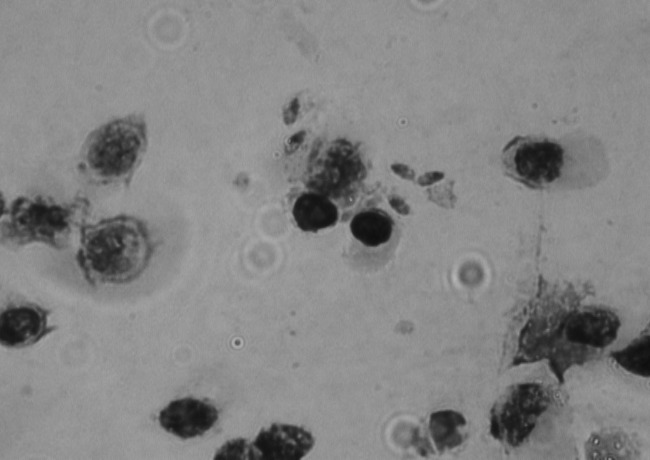
The HSCT procedure performed using his HLA-identical male sibling was uneventful. On day 6 after HSCT, the patient developed mild skin and gut GVHD stage I, for which prednisone at a dose of 1 mg/kg of body weight/day was given. On day 14 post-HSCT, intensive chest pain appeared. A chest X-ray showed left-sided pleural effusion. Bronchoscopy was performed on day 20, and a bronchoalveolar lavage (BAL) fluid sample was obtained. The initial workup ruled out common infection (all bacterial and fungal cultures were negative). However, visualization of tachyzoite-like structures on Giemsa-stained BAL fluid smears suggested toxoplasmosis (Fig. 1). The patient was immediately started on intravenous TMP-SMX (high-dosage regimen of TMP of 15 mg/kg/day) and clindamycin (CLI). On day 21 post-HSCT, the patient's blood and BAL fluid samples were referred to the Serbian National Reference Laboratory for Toxoplasmosis (NRLToxo) for confirmation. Serological findings merely confirmed the seropositive status of the patient, but using molecular methods, T. gondii dissemination was diagnosed, with extremely high parasite burdens in both blood and BAL fluid (Table 1). By that time, however, the patient had developed acute respiratory distress syndrome (ARDS) requiring mechanical ventilation. His condition was further exacerbated by acute renal failure necessitating continuous veno-venous hemodiafiltration (CVVHDF). TMP-SMX and CLI treatment led to gradual clinical and radiological improvement of both respiratory and renal functions, i.e., to reduced pleural effusion (although mechanical ventilation was still required due to respiratory insufficiency), and to establishment of diuresis at 1 ml/kg/h followed by reduction of urea and creatinine levels, which allowed the discontinuation of CVVHDF.
Fig 1.
T. gondii tachyzoites on Giemsa-stained BAL fluid smears on post-HSCT day 20.
Subsequent serological testing performed on day 30 post-HSCT showed a 2-fold rise in specific IgG antibody while specific IgM remained undetectable, whereas control real-time PCR (RT-PCR) at this time point reflected a dramatic decrease in the parasite burden in the blood (Table 1).
However, after a transient improvement (day 25 to day 32 post-HSCT), fever and further deterioration of the respiratory function ensued, along with an increase in inflammatory markers. Multidrug-resistant strains of Acinetobacter baumannii, Klebsiella pneumoniae, and Achromobacter xylosoxidans were isolated from the endotracheal aspirate on day 35 and Acinetobacter baumannii was isolated from blood culture on day 37 post-HSCT. Despite immediate introduction of appropriate antimicrobial treatment, the patient continued to deteriorate and died of sepsis complicated by multiple organ failure on day 39 post-HSCT.
MATERIALS AND METHODS
Clinical samples.
Peripheral blood and BAL fluid were sampled for serology (blood), microscopic detection of the parasite in Giemsa-stained preparations (BAL fluid), and detection of parasite DNA (blood and BAL fluid). Whole blood was centrifuged, and the serum and buffy coat were used for serological testing and DNA extraction, respectively. BAL fluid was centrifuged and the pellet used in part for staining with Giemsa and in part for DNA extraction. Between analyses, all samples were stored at −20°C.
Serology.
Serological tests included the high-sensitivity direct agglutination (HSDA) test (11) and commercial assays, including TXG- and TXM-Vidas (bioMérieux, Marcy l'Etoile, France) for specific IgG and IgM antibodies, respectively, and an immunosorbent agglutination assay (ISAgA) (bioMérieux) for specific IgM antibodies. Specific IgG avidity was measured by TXGA-Vidas (bioMérieux). Commercial assays were carried out according to the manufacturer's recommendations, and the results expressed in IU/ml (TXG-Vidas) or as indices (all others). HSDA results were expressed as the highest serum dilution at which the reaction was positive (titer).
Molecular detection of T. gondii DNA.
Complete DNA from blood and BAL fluid samples was extracted with a QIAmp DNA minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions, resuspended in 150 μl of nuclease-free water, and stored at −20°C. The T. gondii AF146527 gene (529-bp repetitive element), occurring up to 200 to 300 times in the T. gondii genome, was detected with a TaqMan probe (10 pmol/μl) (6-carboxyfluorescein [FAM]–ACG CTT TCC TCG TGG TGA TGG CG–6-carboxytetramethylrhodamine [TAMRA]) (12, 13). PCR was performed in a final volume of a 20-μl mixture containing 10 μl Maxima Probe/ROX quantitative PCR (qPCR) Master Mix (Fermentas [Thermo Fisher Scientific], Waltham, MA) (2×), 0.25 mM (each) primer, 0.10 mM TaqMan probe (Invitrogen, Life Technologies, Carlsbad, CA), 0.015 U/μl of uracil N-glycosylase (UNG), 25 mM MgCl2, and nuclease-free water plus 3 μl of extracted DNA. Amplification was performed over 40 cycles in an Eppendorf RT-PCR device (Eppendorf, Hamburg, Germany), using the following cycling conditions: 2 min at 50°C for UDG pretreatment and 10 min at 94°C for initial denaturation followed by 40 cycles of 15 s at 95°C for denaturation and 60 s at 60°C for annealing/extension.
Genotyping analysis.
Genotyping of T. gondii DNA in the blood and BAL fluid samples was initially performed at the NRLToxo by the PCR-restricted fragment length polymorphism (RFLP) method based on four markers, including SAG1, SAG2, GRA6, and GRA7. For each marker, the PCR mixture consisted of 12.5 μl PCR Master Mix (Fermentas) (2×), 1 μl 10 μM (each) forward and reverse primer, 7.5 μl nuclease-free water, and 3 μl DNA extracted from the sample in a 25-μl reaction volume. Positive controls consisted of T. gondii type I (RH), type II (Me49), and type III (NED) strains, while nuclease-free water was used as a negative control. PCR products were digested with appropriate restriction enzymes for different markers. The PCR mixture for digestion consisted of 12 μl of nuclease-free water, 2.5 μl of buffer, and 0.5 μl of the appropriate restriction enzyme. Restriction products were visualized by electrophoresis using a 3% agarose gel stained with ethidium bromide. Estimation of fragment size was based on comparison to a 50-bp DNA ladder (Fermentas).
T. gondii DNA in the blood sample was further genotyped at the French National Reference Center for toxoplasmosis with 15 microsatellite markers distributed over 10 of 14 chromosomes, as described previously (14). Briefly, for each primer pair, the forward primer was 5′ end labeled with fluorescein to allow sizing of PCR products electrophoresed in an automatic sequencer. PCR was carried out in a 25-μl reaction mixture consisting of 12.5 μl of 2× Qiagen Multiplex PCR Master Mix (Qiagen, Courtaboeuf, France), 5 pmol of each primer, and 1 μl of DNA. Cycling conditions were 15 min at 95°C; 30 s at 94°C, 3 min at 61°C, and 30 s at 72°C (35 cycles); and 30 min at 60°C. One μl of the PCR product—diluted 1:20 in deionized formamide—was mixed with 0.5 μl of a dye-labeled size standard (ROX 500; Applied Biosystems, Courtabœuf, France) and 23.5 μl of deionized formamide (Applied Biosystems). This mixture was denatured at 95°C for 5 min and then electrophoresed using an automatic sequencer (ABI Prism 3130xl; Applied Biosystems). The sizes of the alleles in bp were estimated using GeneMapper analysis software (version 4.0, Applied Biosystems).
RESULTS
Microscopic examination of Giemsa-stained BAL fluid smears and visualization of tachyzoites on day 20 post-HSCT indicated further testing in the NRLToxo. Serological analysis showed high-avidity specific IgG antibodies without specific IgM (trace amounts, however, were detected by the highly sensitive ISAgA assay) (Table 1). RT-PCR revealed parasite DNA, and the parasite burden was estimated, according to a standard curve, at nearly 15,000 parasites/ml in the blood and at a staggering 100,000 parasites/ml in the BAL fluid.
Control serology on day 30 post-HSCT showed a 2-fold rise in specific IgG antibodies (in both the commercial and HSDA tests) without the appearance of specific IgM. A favorable response to combination TMP-SMX and CLI therapy was reflected in transitory clinical improvement, while RT-PCR revealed a dramatically reduced parasite load of approximately 200 parasites/ml.
T. gondii DNA characterization.
Direct genotyping of T. gondii DNA from both the blood and BAL fluid samples with PCR-RFLP revealed that the parasite had type II alleles with the SAG1, SAG2, and GRA6 markers but alleles of both type I and type II with the GRA7 marker. This result triggered additional genotyping analysis with microsatellite markers which showed that the T. gondii DNA from the blood sample—designated TgH106019—was atypical and genetically divergent from the clonal type I, II, and III strains commonly found in western Europe or North America (Table 2). Furthermore, the allelic combination of this genotype with microsatellite markers was very uncommon, as it was also divergent from the pattern seen with genotypes of African, South American, and Asian strains available at the Toxoplasma Biological Resource Center collection in Limoges, France.
Table 2.
Genotyping results of T. gondii DNA with 15 microsatellite markers from a blood sample of the present case (TgH106019) and from 7 reference strains collected in North America, South America, Africa, Asia, and western Europe
| Type | Isolatea | Origin | Host | Microsatellite marker |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TUB2 | W35 | TgM-A | B18 | B17 | M33 | IV.1 | XI.1 | M48 | M102 | N60 | N82 | AA | N61 | N83 | ||||
| Atypical | TgH106019 | Serbia | Human | 291 | 242 | 203 | 156 | 336 | 165 | 274 | 354 | 223 | 174 | 130 | 109 | NAb | 101 | 310 |
| I | CT1 | United States | Cow | 291 | 248 | 209 | 160 | 342 | 169 | 274 | 358 | 209 | 168 | 145 | 119 | 265 | 87 | 306 |
| II | TgH32006 | France | Human | 289 | 242 | 207 | 158 | 336 | 169 | 274 | 356 | 215 | 174 | 142 | 111 | 281 | 91 | 310 |
| II | TgA32132 | France | Sheep | 289 | 242 | 207 | 158 | 336 | 169 | 274 | 356 | 221 | 174 | 138 | 111 | 277 | 91 | 312 |
| III | NED | France | Human | 289 | 242 | 205 | 160 | 336 | 165 | 278 | 356 | 209 | 190 | 147 | 111 | 267 | 91 | 312 |
| South American 1 | TgH24001 | Brazil | Human | 289 | 242 | 205 | 160 | 342 | 165 | 278 | 358 | 237 | 164 | 145 | 111 | 316 | 89 | 308 |
| African 1 | TgH32052 | Benin | Human | 291 | 248 | 205 | 160 | 342 | 165 | 274 | 354 | 231 | 166 | 147 | 111 | 273 | 89 | 306 |
| Chinese 1 | TgCtPRC04 | China | Cat | 293 | 242 | 211 | 160 | 336 | 169 | 274 | 354 | 215 | 172 | 145 | 123 | 281 | 93 | 308 |
TgA32132 is also known as strain FR-OVI-ARI061; all reference strains, except TgCtPRC04, are available at the Toxoplasma Biological Resource Center, Limoges, France.
NA, not amplified.
DISCUSSION
The presented case of fulminant clinical reactivation of an atypical T. gondii strain in a HSCT recipient with underlying NBS, a rare DNA repair disorder and immunodeficiency, is apparently the first such case ever described. Reactivation of T. gondii developed within days after HSCT and significantly contributed to the fatal outcome.
In seropositive allogeneic HSCT recipients, the hazard of T. gondii reactivation is generally high. The donor's seronegative status seems to contribute to this risk, as approximately 80% of all reported events of toxoplasmosis are observed in cases of donor/recipient serological mismatch (3, 15). Clinical symptoms of reactivation are usually observed within 2 to 4 months post-HSCT (1), with a median onset at 64 days (2) and with fewer than 10% of cases occurring before day 30 post-HSCT (1). While toxoplasmic encephalitis is the most frequent presentation, the lungs are the most common site of extracerebral toxoplasmosis (16, 17). Pneumonitis, followed by ARDS and rapid dissemination resulting in multiple organ failure, is not rare in HSCT recipients, a setting in which it is usually manifested early, during the first month post-HSCT, and is often fatal (10, 18).
Although serology remains the mainstay in the diagnosis of toxoplasmosis, its value in immunosuppressed patients is limited by the inability to mount an adequate humoral immune response. Indeed, the concentration of specific IgG is, in most cases, including the one presented here, at levels below the ones registered pretransplantation (15). Hence, the definitive diagnosis of reactivated toxoplasmosis in HSCT recipients is based on the isolation of the parasite or on the detection of parasitic DNA in blood, body fluids, or tissues. Although direct microscopy is generally neglected due to its low sensitivity, it is the simplest and most rapid means to diagnose disseminated toxoplasmosis (1, 15). In the presented case, toxoplasmosis was suggested by visualization of tachyzoites on microscopic examination of Giemsa-stained BAL fluid smears. However, only PCR-based methods, particularly the quantitative ones, allow both timely introduction of specific treatment and monitoring of the therapeutic response (3, 18, 19). Indeed, in our patient, the extremely high initial parasite loads, as revealed by RT-PCR in both blood and BAL fluid, significantly decreased only after several days of specific treatment, indicating a good therapeutic response.
The patient's genetic immunodeficiency (both cellular and humoral), aggravated by immunosuppressive therapy both pre- and posttransplantation, likely triggered an early toxoplasmosis reactivation (2, 15). Additional factors that may have contributed to early and heavy T. gondii dissemination and rapid clinical deterioration include the absence of posttransplantation prophylaxis in an immunosuppressed seropositive HSCT recipient, who, moreover, received a graft from a seronegative donor, and the atypical genotype of the T. gondii strain involved.
For prophylaxis, there is no common ground with respect to either the specific drugs used for the prevention of T. gondii dissemination or the time of initiation and duration of prophylaxis. The most widely used prophylactic regimen is a combination of TMP and SMX, while a positive PCR result from blood (indicating reactivation) requires a switch to pyrimethamine-sulfadiazine (PYR-SDZ) or PYR-CLI or raising the doses of TMP-SMX (3). In the presented case, however, post-HSCT prophylaxis was withheld to avoid jeopardizing engraftment (10) based on reports indicating that >90% of the cases of reactivation occur later (beyond 30 days post-HSCT) (1). However, PCR-based preemptive therapy may protect against death due to toxoplasmosis in approximately 80% of patients (3). Thus, a decision to withhold post-HSCT prophylaxis should entail a weekly PCR follow-up, and based on previous studies (3, 19) and this report, RT-PCR (from blood) should be used for routine monitoring in all seropositive patients from day 1 post-HSCT.
The T. gondii population structure consists of three major clonal lineages designated types I, II, and III which are, with an emphasis on type II, predominant in Europe and North America (20, 21), whereas the strains isolated in Africa and South America are highly polymorphic and referred to as atypical (22). Limited experience with T. gondii genotypes circulating in southeastern Europe indicates the presence of only clonal types, with the predominance of type II (23, 24, 25). However, the infecting strain in the reported patient was genetically divergent from strains commonly isolated in Europe. Epidemiological data indicated no explanation as to its origin, since the patient was born and raised in Montenegro (a southeastern Europe country neighboring Serbia) and never traveled outside Europe and his dietary habits were described as nonhazardous. So, the atypical genotype suggests either that foodstuffs from areas with atypical strains had somehow found their way to the local market or that there is a larger genetic diversity of T. gondii in southeastern Europe, an option that needs to be further explored.
Atypical T. gondii strains have been shown to correlate with increased clinical severity of the infection in immunocompetent patients (26) and congenital toxoplasmosis (27), but this is the first report of such an association in an immunocompromized patient previously not evidenced in the setting of immunosuppression (22). In contrast, Patrat-Delon et al. recently reported a favorable outcome in a heart transplant patient with fulminant T. gondii reactivation (although at much lower parasite loads than described here), in which the infecting strain was a typical clonal (type II) one (19).
In conclusion, for HSCT to prove the beneficial treatment option in NBS patients with lymphoid malignancies that it promises to be in terms of survival rate improvement, absence of relapses, and correction of underlying immunodeficiency (9), centers performing such a delicate procedure must be aware of T. gondii-related risks. In cases of presumed reactivation risk such as in seropositive HSCT patients, routine monitoring of toxoplasmosis by PCR-based quantitative methods (at present, RT-PCR) should be part of the standard follow-up protocol.
ACKNOWLEDGMENTS
The work was supported by a grant (project no. III41019) from the Ministry of Education, Science and Technological Development of Serbia.
We are grateful to Isabelle Villena (Reims, France) for kindly supplying the antigen for HSDA.
Footnotes
Published ahead of print 12 June 2013
REFERENCES
- 1. Derouin F, Pelloux H, ESCMID Study Group on Clinical Parasitology 2008. Prevention of toxoplasmosis in transplant patients. Clin. Microbiol. Infect. 14:1089–1101 [DOI] [PubMed] [Google Scholar]
- 2. Martino R, Maertens J, Bretagne S, Rovira M, Deconinck E, Ullmann AJ, Held T, Cordonnier C, European Group for Blood and Marrow Transplantation Infectious Diseases Working Party 2000. Toxoplasmosis after hematopoietic stem cell transplantation. Clin. Infect. Dis. 31:1188–1194 [DOI] [PubMed] [Google Scholar]
- 3. Martino R, Bretagne S, Einsele H, Maertens J, Ullmann AJ, Parody R, Schumacher U, Pautas C, Theunissen K, Schindel C, Muñoz C, Margall N, Cordonnier C, Infectious Disease Working Party of the European Group for Blood and Marrow Transplantation 2005. Early detection of Toxoplasma gondii infection by molecular monitoring of Toxoplasma gondii in peripheral blood samples after allogeneic stem cell transplantation. Clin. Infect. Dis. 40:67–78 [DOI] [PubMed] [Google Scholar]
- 4. Weemaes CMR, Hustinx TWJ, Scheres JMJC, van Munster PJ, Bakkeren JA, Taalman RD. 1981. A new chromosomal instability disorder: Nijmegen breakage syndrome. Acta Paediatr. Scand. 70:557–564 [DOI] [PubMed] [Google Scholar]
- 5. Hiel JA, Weemaes CM, van den Heuvel LP, van Engelen BG, Gabreëls FJ, Smeets DF, van der Burgt I, Chrzanovska KH, Bernatowska E, Krajewska-Walasek M, Bialecka M, Abramczuk D, Gregorek H, Michalkiewicz J, Perek D, Midro AT, Seemanová E, Belohradsky BH, Sölder B, Barbi G, Wegner RD, Sperling K, Dixon J, Maraschio P, Marseglia GL, Green A, Taylor AM, Der Kaloustian VM, Komatsu K, Matsuura S, Conley ME, Concannon P, Gatti RA, the International Nijmegen Breakage Syndrome Study Group 2000. Nijmegen breakage syndrome. Arch. Dis. Child. 82:400–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasic S, Vujic D, Fiorini M, Notarangelo LD. 2004. T-cell lymphoblastic leukemia/lymphoma in Nijmegen breakage syndrome. Haematologica 89:ECR27. [PubMed] [Google Scholar]
- 7. Jovanovic A, Minic P, Scekic-Guc M, Djuricic S, Cirkovic S, Weemaes C, Pasic S. 2009. Successful treatment of Hodgkin's lymphoma in Nijmegen breakage syndrome. J. Pediatr. Hematol. Oncol. 31:49–52 [DOI] [PubMed] [Google Scholar]
- 8. Dembowska-Baginska B, Perek D, Brozyna A, Wakulinska A, Olczak-Kowalczyk D, Gladkowska-Dura M, Grajkowska W, Chrzanowska KH. 2009. Non-Hodgkin lymphoma (NHL) in children with Nijmegen-breakage syndrome (NBS). Pediatr. Blood Cancer 52:186–190 [DOI] [PubMed] [Google Scholar]
- 9. Albert MH, Gennery AR, Greil J, Cale CM, Kalwak K, Kondratenko I, Mlynarski W, Notheis G, Führer M, Schmid I, Belohradsky BH. 2010. Successful SCT for Nijmegen breakage syndrome. Bone Marrow Transplant. 45:622–626 [DOI] [PubMed] [Google Scholar]
- 10. Chandrasekar PH, Momin F, The Bone Marrow Transplant Team 1997. Disseminated toxoplasmosis in marrow recipients: a report of three cases and a review of the literature. Bone Marrow Transplant. 19:685–689 [DOI] [PubMed] [Google Scholar]
- 11. Desmonts G, Remington J. 1980. Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J. Clin. Microbiol. 11:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Homan W, Vercammen M, De Braekeleer J, Verschueren H. 2000. Identification of a 200–300 fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 30:69–75 [DOI] [PubMed] [Google Scholar]
- 13. Vujanić M, Ivović V, Kataranovski M, Nikolić A, Bobić B, Klun I, Villena I, Kataranovski D, Djurković-Djaković O. 2011. Toxoplasmosis in naturally infected rodents in Belgrade, Serbia. Vector Borne Zoonotic Dis. 11:1209–1211 [DOI] [PubMed] [Google Scholar]
- 14. Ajzenberg D, Collinet F, Mercier A, Vignoles P, Dardé ML. 2010. Genotyping of T. gondii isolates with 15 microsatellite markers in a single multiplex PCR assay. J. Clin. Microbiol. 48:4641–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Derouin F, Devergie A, Auber F, Gluckman E, Beauvais B, Garin YJF, Lariviere M. 1992. Toxoplasmosis in bone marrow-transplant recipients: report of seven cases and review. Clin. Infect. Dis. 15:267–270 [DOI] [PubMed] [Google Scholar]
- 16. Catterall JR, Hofflin JM, Remington JS. 1986. Pulmonary toxoplasmosis. Am. Rev. Respir. Dis. 133:704–705 [DOI] [PubMed] [Google Scholar]
- 17. Remington JS, Desmonts G. 1995. Toxoplasmosis, p 349–364 In Remington JS, Klein JO. (ed), Infectious diseases of the fetus and the newborn infant, 4th ed Saunders, Philadelphia, PA [Google Scholar]
- 18. Mulanovich VE, Ahmed SI, Öztürk T, Khokhar FA, Kontoyiannis DP, de Lima M. 2011. Toxoplasmosis in allo-SCT patients: risk factors and outcomes at a transplantation center with a low incidence. Bone Marrow Transplant. 46:273–277 [DOI] [PubMed] [Google Scholar]
- 19. Patrat-Delon S, Gangneux JP, Lavoué S, Lelong B, Guiguen C, le Tulzo Y, Robert-Gangneux F. 2010. Correlation of parasite load determined by quantitative PCR to clinical outcome in a heart transplant patient with disseminated toxoplasmosis. J. Clin. Microbiol. 48:2541–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dardé ML, Bouteille B, Pestre-Alexandre M. 1992. Isoenzyme analysis of 35 Toxoplasma gondii isolates and the biological and epidemiological implications. J. Parasitol. 78:786–794 [PubMed] [Google Scholar]
- 21. Howe DK, Sibley LD. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 172:1561–1566 [DOI] [PubMed] [Google Scholar]
- 22. Ajzenberg D, Yera H, Marty P, Paris L, Dalle F, Menotti J, Aubert D, Franck J, Bessières MH, Quinio D, Pelloux H, Delhaes L, Desbois N, Thulliez P, Robert-Gangneux F, Kauffmann-Lacroix C, Pujol S, Rabodonirina M, Bougnoux ME, Cuisenier B, Duhamel C, Hai Duong T, Filisetti D, Flori P, Gay-Andrieu F, Pratlong F, Nevez G, Totet A, Carme B, Bonnabau H, Dardé ML, Villena I. 2009. Genotype of 88 T. gondii isolates associated with toxoplasmosis in immunocompromised patients and correlation with clinical findings. J. Infect. Dis. 199:1155–1167 [DOI] [PubMed] [Google Scholar]
- 23. Djurković-Djaković O, Klun I, Khan A, Nikolić A, Knežević-Usaj S, Bobić B, Sibley LD. 2006. A human origin type II strain of Toxoplasma gondii causing severe encephalitis in mice. Microbes Infect. 8:2206–2212 [DOI] [PubMed] [Google Scholar]
- 24. Ivović V, Vujanić M, Živković T, Klun I, Djurković-Djaković O. 2012. Molecular detection and genotyping of Toxoplasma gondii from clinical samples, p 103–119 In Djurković-Djaković O. (ed), Toxoplasmosis—recent advances. InTech, Rijeka, Croatia [Google Scholar]
- 25. Costache CA, Colosi HA, Blaga L, Györke A, Paştiu AI, Colosi IA, Ajzenberg D. 2013. First isolation and genetic characterization of a Toxoplasma gondii strain from a symptomatic human case of congenital toxoplasmosis in Romania. Parasite 20:11. 10.1051/parasite/2013011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carme B, Demar M, Ajzenberg D, Dardé ML. 2009. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg. Infect. Dis. 15:656–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Delhaes L, Ajzenberg D, Sicot B, Bourgeot P, Dardé ML, Dei-Cas E, Houfflin-Debarge V. 2010. Severe congenital toxoplasmosis due to a Toxoplasma gondii strain with an atypical genotype: case report and review. Prenat. Diagn. 30:902–905 [DOI] [PubMed] [Google Scholar]