Abstract
There is a need for a point-of-care serodiagnostic test for women and men for sexually transmitted infections (STIs) caused by Trichomonas vaginalis. Sera from women with this STI and sera from men that were analyzed in studies showing a relationship between serostatus and prostate cancer are highly seropositive in response to trichomonad α-actinin and its truncated protein (ACT-P2) (positive control sera). Epitope mapping experiments showed that positive control sera from women had antibodies to 13 distinct epitopes, 5 of which were detected by positive control sera from men. Sera from women and men that were unreactive with α-actinin (negative control sera) failed to detect any of the epitopes or other α-actinin amino acid sequences. The T. vaginalis α-actinin amino acid sequence and the sequences of the epitopes showed little or no identity with those of other proteins of microbial pathogens or the human α-actinin 1 (HuACTN1) homolog. Immunoassays such as dot blot, immunoblot, and enzyme-linked immunosorbent assays were used. Positive control sera did not detect HuACTN1 in immunoassays, and the range of levels of identity of α-actinin epitopes with HuACTN1 was 0% to 50%. Comparison of the T. vaginalis α-actinin epitopes with proteins in data banks, such as Tritrichomonas suis, Candida albicans, and Saccharomyces cerevisiae proteins, gave a range of identity levels of 0% to 22%. Specific 15-mer peptide epitopes of α-actinin with low to no identity with other proteins were synthesized and were reactive with positive control sera only. These findings identify epitopes of α-actinin as candidate serodiagnostic targets and suggest strongly that a highly seropositive reaction to α-actinin suggests exposure to T. vaginalis.
INTRODUCTION
Trichomonas vaginalis is the most common nonviral causative agent for sexually transmitted infections (STIs). The adverse consequences for the health of women have been well documented (1, 2). Although in the literature it is routinely reported that men infected by their partners resolve their infections, there is neither experimental evidence nor clinical studies proving this. Reports have shown the expression of numerous virulence factors that permit this ancient protist to survive in the changing host environment of the female urogenital region (3–18). Iron is an important modulator of upregulation and downregulation of the expression of virulence genes (8, 19–25). Key steps for host colonization result from overcoming the urogenital mucous layer (18) in preparation for specific cytoadherence to vaginal epithelial cells (7, 9, 11, 22, 24). The intimacy of the host-parasite relationship during colonization and infection is exceedingly complex, as evidenced by the large number of proteinases (4, 11, 15, 26) and the secretion by trichomonads of large amounts of putrescine for spermine uptake and back-conversion to spermidine (13, 26). This coating of T. vaginalis by polyamines and host macromolecules affects levels of cytoadherence and cytotoxicity (3, 13).
More recently, epidemiological evidence indicated a relationship between serostatus with respect to T. vaginalis and the possibility of lethal prostate cancer (27–29), and a new study found detectable T. vaginalis DNA in benign hyperplastic prostatic tissue (30). Testable hypotheses have been proposed to elucidate the molecular mechanisms for prostate cancer development and progression (31). The serodiagnostic targets for analyses of serum antibodies include one of the most immunogenic trichomonad proteins, α-actinin (32, 33), and a truncated protein called ACT-P2 (27–29). These diagnostic targets are different from that used in the initial immunochromatographic lateral flow diagnostic test for this STI (OSOM Trichomonas rapid test; Sekisui Diagnostics, San Diego, CA) (34). Although the OSOM test has excellent sensitivity and specificity and has been shown to be reliable worldwide in clinics and in self-sampling kits (35), the test does not detect antigen in male fluids such as urine.
In this study, we wanted to characterize the highly immunogenic α-actinin protein to establish further its utility as a target for the serodiagnosis of trichomonosis for both women and men. It is known that the sera of women with trichomonosis possess antibodies reactive with numerous trichomonad proteins, including α-actinin (referred to as positive control sera from women) (32, 36–39). Epitope mapping identified 13 peptide epitopes within α-actinin that were reactive with positive control sera from women. Interestingly, sera from men that were highly seropositive with respect to the trichomonad parent α-actinin and the truncated version called ACT-P2 (positive control sera from men) (27, 28) identified 5 epitopes that were a subset of those detected by positive control sera from women. The amino acid sequences of the epitopes had little or no sequence identity with the human α-actinin 1 (HuACTN1) homolog and proteins of other microbial pathogens, including a related Tritrichomonas suis and the yeasts Candida albicans and Saccharomyces cerevisiae. Further, all immobilized 15-mer peptides of representative epitopes were found to be reactive with positive control sera from both women and men. We discuss the significance of our results with respect to detection of serum antibodies among women and men exposed to T. vaginalis.
MATERIALS AND METHODS
ACT-P2 expression and purification.
The natural T. vaginalis α-actinin protein consists of 931 amino acids and is 106.2 kDa (32, 33). As mentioned earlier, this full-length highly immunogenic protein (32) was used to examine the relationship between seropositivity in men and prostate cancer (29). We then made subclones of the trichomonad α-actinin gene to determine the region of the protein most reactive with sera from men (27, 28). A subclone encoded a 558-amino acid protein from the amino terminus that was called ACT-P2. The coding region of ACT-P2, corresponding to amino acids 375 to 932, was PCR amplified and cloned in the pET23b expression vector with the kanamycin resistance gene (kan) for transformation of Escherichia coli BL21(DE3) cells. The resulting recombinant 558-amino acid sequence yielded a C-terminal His6-tagged fusion protein of 63.5 kDa. Bacteria were grown on Luria broth agar plates containing 25 μg/ml kanamycin; a suspension of recombinant E. coli was grown at 37°C in a shaker incubator at 220 rpm for 3 h prior to the addition of 1 mM isopropylthiogalactoside and then was incubated for an additional 3 h. The recombinant E. coli was centrifuged using a Sorvall SLA-1500 rotor at 8,000 rpm for 15 min at 4°C, and pellets were stored at −80°C until used. Immunoblot analysis confirmed the synthesis of ACT-P2 by using as a probe murine monoclonal antibody (MAb) HA423 (27–29) to trichomonad α-actinin or a MAb to His6 (Advanced Targeting Systems, San Diego, CA).
For purification of ACT-P2, pellets of recombinant E. coli were thawed for 15 min on ice and suspended in 10 ml lysis buffer (50 mM Tris [pH 8.0], 300 mM NaCl, 10 mM β-mercaptoethanol [β-ME], and 0.1% Triton X-100), and lysates were sonicated 10 times (30 s each) at room temperature (RT). Sonicates were centrifuged using a Sorvall SS-34 rotor at 8,000 rpm for 20 min at 4°C, and the supernatant was applied to a Ni2+-nitrilotriacetic acid Superflow affinity column according to the manufacturer's instructions (Qiagen Inc., Valencia, CA). Purified ACT-P2 protein was confirmed by SDS-PAGE and immunoblotting using MAb HA423 as a probe, as described above.
HuACTN1 homolog.
The purified full-length HuACTN1 homolog used in this study was the isoform B protein of 892 amino acids (∼103 kDa) (Novus Biologicals, Littleton, CO). The soluble protein was used in 74 mM Tris-HCl (pH 8.0) containing 10 mM reduced glutathione. For enzyme-linked immunosorbent assays (ELISAs) and immunoblot assays, 1 μg of ACT-P2 or HuACTN1 was used. ELISAs were performed using wells of microtiter plates coated with ACT-P2 or HuACTN1, as detailed below. SDS-PAGE for immunoblotting onto nitrocellulose membranes for both ACT-P2 and HuACTN1 was carried out using 7.5% acrylamide gels, as described previously (36, 37).
Positive control sera from women and men and detection of antibodies to ACT-P2.
During the course of our research on T. vaginalis, we examined ∼1,000 serum samples from female patients with trichomonosis and, more recently, up to 20,000 serum samples from men (27–29) for seropositivity for trichomonad proteins and particularly α-actinin. Therefore, we were able to determine the levels of serum antibodies to total T. vaginalis proteins (3, 8), α-actinin, and ACT-P2 by ELISAs (27–29). Individual α-actinin-seropositive sera from women and men had identical or very similar reactivities to trichomonad proteins and α-actinin. This permitted us to pool the sera to obtain sufficient amounts for conducting epitope mapping experiments, as outlined below; the sera were considered positive control sera. Similarly, pooled seronegative sera from both women and men were considered negative control sera for parallel experiments conducted throughout.
Trichomonad natural α-actinin SPOTs membrane synthesis for epitope mapping.
Oligopeptides derived from the sequences of T. vaginalis α-actinin (GenBank accession number AAC72899) were synthesized on activated membranes using the SPOTs system (Sigma-Genosys, The Woodlands, TX). Five to 10 nmol of each peptide was covalently bound to a Whatman 50 cellulose support (Whatman, Maidstone, England) by the C terminus using 9-fluorenylmethoxycarbonyl-l-amino acid chemistry, with an acetylated N terminus. The oligopeptides were 11 amino acids in length and had sequential overlaps of eight amino acids. The oligopeptides spanned the entire sequence of the protein.
Probing of the α-actinin SPOTs membrane with positive and negative control sera and MAb HA423.
The membrane was first washed with a small volume of 100% methanol for 5 min, to avoid precipitation of hydrophobic peptides during the subsequent procedure. After three washes (10 min each) with 25 ml of Tris-buffered saline (TBS) (50 mM Tris-HCl [pH 8.0], 137 mM NaCl, and 2.7 mM KCl), the SPOTs membrane was incubated in blocking buffer (TBS containing 5% bovine serum albumin [BSA]) at RT for 2 h. The membrane was incubated with a 1:10 dilution of negative or positive control sera from women and men and incubated overnight at 4°C. The membrane was also probed with MAb HA423, which detects α-actinin. After three washes (5 min each) in TBS, the membrane was incubated with a 1:1,500 dilution of secondary anti-human IgG antibody as described above or anti-mouse IgG Fab (IgG fraction) prepared in blocking buffer. After three washes (5 min each) in TBS at RT, bound antibodies were detected using a color development reagent.
Immediately following color development and SPOTs analysis, the membrane was regenerated by three washes (10 min each) with water at RT with agitation. Bound antibody was stripped from the membrane by at least four washes (30 min each) with regeneration buffer I (62.5 mM Tris-HCl [pH 6.7], 2% SDS, and 100 mM β-ME) at 50°C with agitation. The membrane was washed three times (20 min each) with 10× phosphate-buffered saline (PBS) at RT with agitation, after which the membrane was washed three times (20 min each) with T-TBS (TBS [pH 8.0] containing 0.05% Tween 20) at RT with agitation. This was followed by three washes (10 min each) with TBS at RT with agitation. The presence of any visible spots resulted in the regeneration steps being repeated. As a control to show that the primary antibody had been completely removed, the membrane was reincubated with the appropriate secondary antibody and substrate solution and color was developed. Regeneration was continued until no reactivity was seen with the secondary antibody.
The epitope amino acid sequences were determined based on the reactivities of overlapping peptides (Table 1). Epitope sequences were compared with other proteins by using the protein-protein Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.nih.gov/BLAST). Amino acid sequence alignments of the proteins were performed with CLC Protein Workbench (CLC bio, Muhltal, Germany). Hydrophobicity plots (40) and antigenicity plots (41) were constructed using Lasergene MegAlign (DNASTAR, Madison, WI).
Table 1.
T. vaginalis α-actinin epitope mapping using MAb HA423 and positive control sera from women and men
| Spot no. | Amino acids | Epitope sequence | Epitope designation for reaction with: |
||
|---|---|---|---|---|---|
| Positive control sera from womena | Positive control sera from mena | MAbb | |||
| 2 | 4–14 | RREGLLDDAWE | W1 | ||
| 11–12 | 34–41 | IQFETIET | W2 | ||
| 21–23 | 67–71 | KQPKM | W3 | ||
| 50 | 148–158 | YEHVAVNNFTT | W4 | ||
| 69 | 205–215 | YVYLDPEDVID | W5 | ||
| 80–82 | 244–248 | ADKIK | W6 | ||
| 97–99 | 295–302 | RGKLASVI | W7 | ||
| 111–112 | 334–341 | NRPIPEIP | W8 | ||
| 152–153 | 457–464 | HHSQLITY | W9 | M1 | |
| 165–166 | 496–503 | YDEAIAFK | W10 | M2 | |
| 214–216 | 646–650 | KLNYK | W11 | M3 | |
| 215–217 | 649–653 | YKVTY | W12 | M4 | HA423 |
| 268–270 | 808–812 | KYFDK | W13 | M5 | |
Positive control sera from female patients with trichomonosis and positive control sera from men were highly reactive by ELISA with purified recombinant ACT-P2 immobilized on microtiter plates, by whole-cell ELISA with organisms on microtiter wells, as described previously (27, 29, 39), and by immunoblotting (Fig. 1).
Synthesis and reactivity of individual α-actinin epitopes.
Three 15-mer peptide epitopes identified from SPOTs membrane epitope mapping, with low levels of identity with respect to other human pathogens as well as the human α-actinin homolog, were synthesized in PEPscreen format (Sigma-Genosys). The reactivity of each peptide was tested with representative negative and positive control sera, either individually or in combination. Approximately 10 μg of peptide was blotted onto nitrocellulose membranes and air-dried overnight at RT. The epitope blots were then blocked with 2% ELISA-grade BSA (Sigma Chemical Co., St. Louis, MO) in PBS at 37°C for 2 h, followed by incubation overnight at RT with a 1:25 dilution in PBS of negative or positive control sera from women and men for ACT-P2. This was followed by secondary antibody incubation and color development as described above. All assays were performed in duplicate and repeated at least three times.
RESULTS
Positive control sera from women and men do not detect the human α-actinin homolog protein.
Negative and positive control sera from women and men were used to probe immunoblots for ACT-P2. As can be seen in Fig. 1, positive control sera from women (Fig. 1, lanes 3a and 3b) and from men (Fig. 1, lanes 5a and 5b) detected ACT-P2 in two separate experiments done at different times under identical experimental conditions. Negative control sera from women and from men gave no detectable bands (Fig. 1, lanes 2 and 4, respectively). The IgG1 MAb HA423 to the trichomonad α-actinin used as a probe gave strong reactivity in separate experiments (Fig. 1, lanes 1 and 6); not unexpectedly, irrelevant IgG1 MAb B44 reactive with trichomonad α-enolase, which was used as a negative control, detected no protein band (Fig. 1, lane 7).
Fig 1.
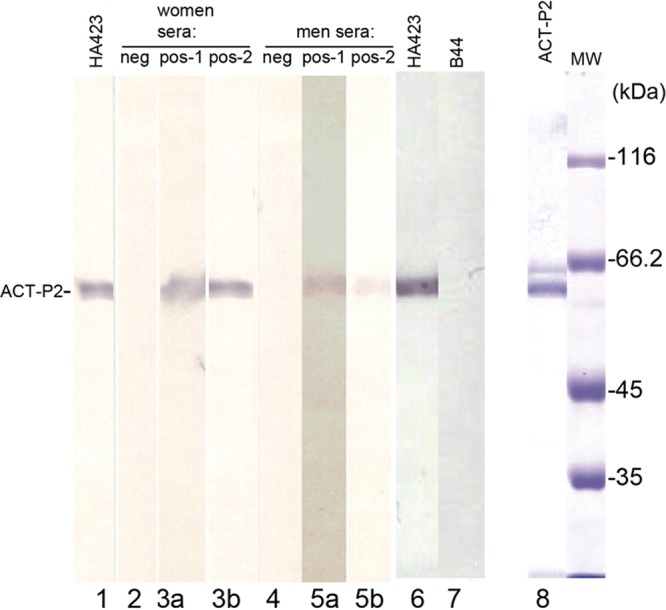
Immunoblot detection of ACT-P2 using IgG1 MAb HA423 and positive control sera from women and men (see Materials and Methods). Negative control sera from women (lane 2) and men (lane 4) were unreactive, compared with MAb HA423 (lanes 1 and 6). The IgG1 MAb B44 to trichomonad α-enolase was an additional negative control, to show the specificity of MAb HA423 (lane 7). Two separate experiments were performed using positive control sera from women (lanes 3a and 3b) and men (lanes 5a and 5b), to show reactivity with nitrocellulose blots with ACT-P2. A stained gel with purified ACT-P2 used for the immunoblots is also shown (lane 8). MW, molecular weight markers after SDS-PAGE and staining with Coomassie brilliant blue; neg, negative; pos, positive.
We next tested for immuno-cross-reactivity purified, commercially available human α-actinin (HuACTN1) with the pooled positive control sera from women and men used in Fig. 1. Figure 2A, lane 5, shows the intensely stained band of 1 μg HuACTN1. Gels with the same amounts of HuACTN1 were transferred to nitrocellulose membranes for immunoblotting. A duplicate blot with HuACTN1 was stained to confirm transfer of the protein (not shown). HuACTN1 was detected by neither the positive control sera (Fig. 2A, lane 4) nor HA423 (not shown), consistent with an earlier report (32). As described above, 1 μg of ACT-P2 (Fig. 2A, lane 3) was readily detected by MAb HA423 (Fig. 2A, lane 1) and rabbit antiserum to total T. vaginalis proteins (immune rabbit serum [IRS]) (Fig. 2A, lane 2). We then tested by immunoblotting using total proteins of T. vaginalis (Fig. 2B, lane 10), as described previously (3, 36, 37), whether the pooled positive control sera from both women and men detected numerous other trichomonad proteins. Figure 2B shows that pooled negative control sera did not detect any trichomonad proteins on immunoblots (Fig. 2B, lane 7), whereas pooled positive control sera recognized numerous proteins (Fig. 2B, lane 8). As a control and not surprisingly, many trichomonad proteins in the total-protein preparation were evident when blots were probed with IRS (Fig. 2B, lane 9) (36, 37). As with negative control sera from women and men (Fig. 2B, lane 7), no proteins were detected by control (prebleeding) normal rabbit serum. Finally, we tested for detection of nondenatured HuACTN1 coated onto wells of microtiter plates. Neither positive control sera from women and men nor MAb HA423 reacted to the HuACTN1-coated wells (not shown).
Fig 2.
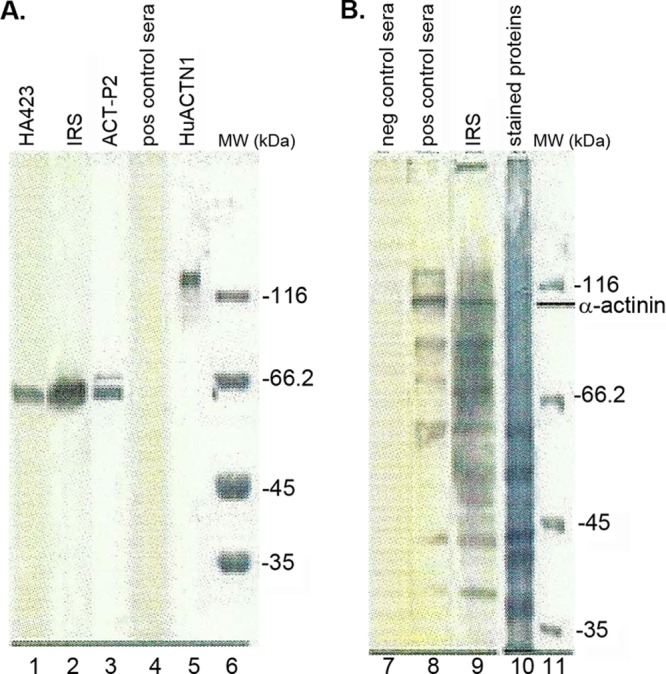
Gels showing that pooled positive control sera from both women and men are unreactive with HuACTN1 and have antibodies to numerous trichomonad proteins. (A) Lanes 1 and 2, immunoblots showing strong reactivity for ACT-P2 with MAb HA423 (lane 1) and rabbit antiserum to total T. vaginalis proteins (IRS) (lane 2), as in Fig. 1. Lane 3, stained gel showing the protein band of 1 μg ACT-P2 used for blotting onto nitrocellulose membranes (lanes 1 and 2). Lane 4, immunoblot showing no evident band for HuACTN1 using pooled positive control sera from women and men as a probe. Lane 5, stained gel after SDS-PAGE of 1 μg of HuACTN1. Lane 6, stained gel showing molecular weight (MW) standards. (B) Lane 7, immunoblot with negative control sera. No detection of any bands was seen with negative control sera (lane 7) or with control (prebleeding) normal rabbit serum (not shown) (36, 37). Lanes 8 and 9, duplicate nitrocellulose blots of immobilized total proteins probed with pooled positive control sera from both women and men (lane 8) and IRS (lane 9). Lane 10, Coomassie brilliant blue-stained gel of proteins after SDS-PAGE of total trichloroacetic acid-precipitated trichomonad proteins, as described previously (36, 37). Lane 11, stained gel showing molecular weight (MW) markers. The electrophoretic mobility of the natural trichomonad α-actinin protein is indicated. All experiments were repeated at least three times. For this experiment, corresponding peroxidase-conjugated goat anti-IgG antibodies were used for detection of the murine (HA423) (lane 1), rabbit (IRS) (lanes 2 and 9), and human (positive and negative control sera) (lanes 4, 7, and 8) antibodies.
α-Actinin epitopes react with positive control sera from women and men and MAb HA423.
We next tested the positive control sera from women and men that were strongly reactive with ACT-P2 for IgG antibodies to overlapping 11-mer peptides on a custom SPOTs membrane (see Materials and Methods). Table 1 lists the 13 epitopes and corresponding amino acid sequences (labeled W1 through W13) recognized by sera from women. M1 through M5 represent the subset of epitopes detected by sera from men. The IgG1 MAb HA423 detected the same epitope as W12/M4. Negative control sera from women and men and the MAbs B43 and B44 to trichomonad glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5) and α-enolase (19), respectively, which were the same isotype as HA423, were unreactive with the SPOTs membrane.
Figure 3A shows a representative reaction for epitope detection of overlapping 11-mer peptides for spots numbered 214 through 217. The highly reactive spots 214 through 217 for positive control sera from women indicate the epitope sequence of 643-VEFKLNYKVTYS-656 (Fig. 3B). Likewise, the spot 216 and 217 peptides reactive with positive control sera from men suggest the sequence 649-YKVTYS-656 as the epitope. No reactivity was seen with negative control sera from either women or men. The MAb HA423 epitope is 643-VEFKLNYKVTY-653. Figure 3C1 shows the strong dot blot reaction of positive control sera from women and men to ACT-P2 immobilized on nitrocellulose membranes. We then synthesized 15-mer peptides overlapping W10/M2 (AQPLYDEAIAFKEEV [the epitope is underlined]) and W13/M5 (FKDTFKYFDKDKSNS) (Table 1), immobilized 1 μg of each peptide together, and probed with positive control sera. The sera of both women and men reacted with the combined 15-mer peptide epitopes (Fig. 3C2). Figure 3C3 presents densitometric scans of the reactive spots and shows the greater level of detection by sera from men than by sera from women. Finally, Fig. 3D1 shows the reactivity of individual 15-mer peptides containing the W9/M1 (SVNRHHSQLITYIKH), W10/M2, and W13/M5 epitopes with positive control sera. Figure 3D2 illustrates densitometric scans showing sera from men giving greater intensities for W9/M1 and W10/M2 than sera from women. The levels of detection for the epitope W13/M5 were identical for sera from women and sera from men. Negative control sera from both women and men showed no reactions in these dot blots of epitopes.
Fig 3.
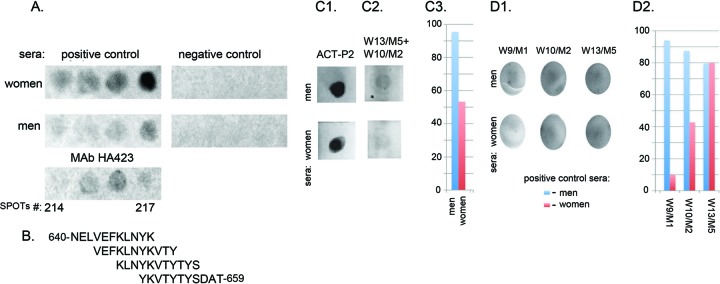
SPOTs analysis with positive control sera from women and men, detecting overlapping peptides on SPOTs membranes of a representative epitope and reactions with immobilized ACT-P2 and synthetic 15-mer peptides used in combination or singly. (A) IgG antibody detection of overlapping peptides from spots 214 through 217 using positive control sera from women and men and MAb HA423. Negative control sera from women and men were unreactive. Positive control sera from women detected all four overlapping 11-mer peptides, with peptide 217 having the strongest signal. The sera from men recognized peptides 216 and 217, with the strongest signal for peptide 217. (B) Corresponding amino acid sequences of the individual oligopeptides. Peptide 214 (top) includes amino acids 640 to 650, and peptide 217 (bottom) includes amino acids 649 to 659. (C1) Signal intensities obtained for 1 μg of ACT-P2 immobilized on nitrocellulose membranes and detected by IgG of positive control sera from both women and men. (C2) Same serum reactivities with the combination of 1 μg each of the synthetic 15-mer peptides for W13/M5 and W10/M2, possessing the epitopes presented in Table 1. (C3) Densitometric scans using ImageJ software to determine the relative intensities of the reactions shown in C2. (D1) Relative reactions of 1 μg of the 15-mer peptides containing the epitopes indicated in Table 1, immobilized on nitrocellulose membranes, with positive control sera from women and men. (D2) Densitometric scans using ImageJ software, indicating the relative intensities of the reactions shown in D1. The 15-mer peptides containing the W9/M1 and W10/M2 epitopes gave higher intensities with sera from men than with sera from women.
Hydrophobicity and antigenicity profiles of the natural α-actinin sequence.
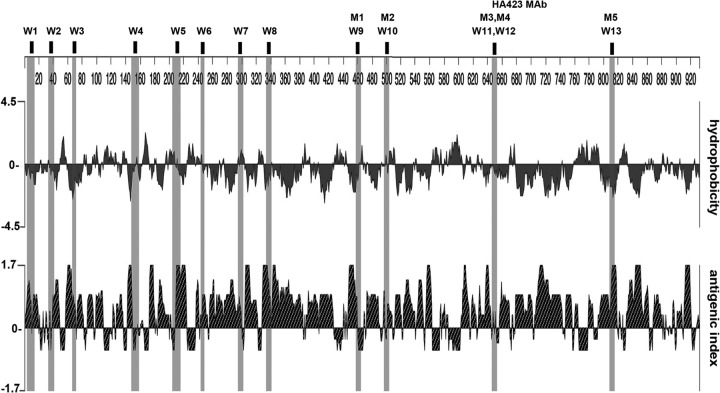
We then analyzed the immunoreactive epitopes (Table 1) for hydrophobicity and antigenicity. Figure 4 demonstrates the mapping of the epitopes with the respective profiles and shows that most epitopes were hydrophilic and corresponded to the predicted antigenicity. As representative examples, epitopes W3, W4, W8, HA423/M4/W12, and M5/W13 each demonstrated prominent hydrophilic and antigenic characteristics that were not inconsistent with serum antibody detection.
Fig 4.
Hydrophobicity and antigenicity plots of the T. vaginalis α-actinin amino acid sequence. Epitopes recognized by positive control sera from women and men and MAb HA423, as presented in Table 1, are listed at the top and highlighted in the corresponding regions of the plots.
Sequence alignment of T. vaginalis α-actinin with proteins of other representative organisms and HuACTN1.
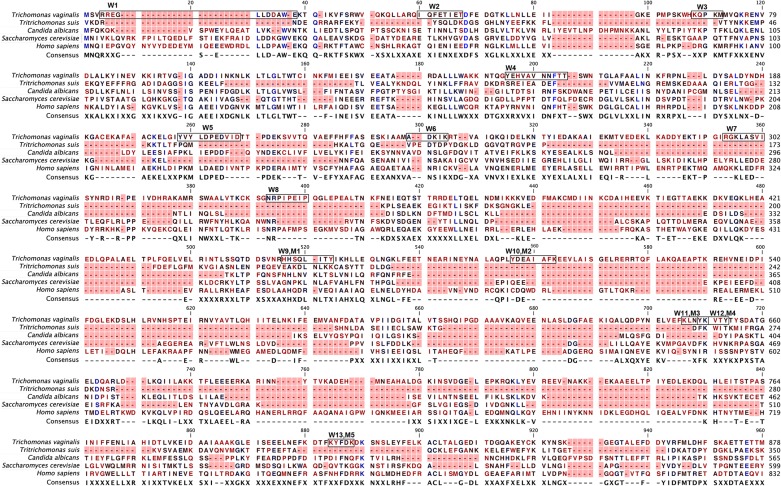
BLAST analysis of the T. vaginalis α-actinin amino acid sequence is presented in Fig. 5 and shows little amino acid identity with α-actinin-like proteins of different species. The low level of identity of amino acids is particularly noteworthy for the boxed epitope sequences indicated above the T. vaginalis amino acid sequence, which were detected by positive control sera from both women and men. Table 2 summarizes the amino acid sequence identity comparisons for the individual epitopes with sequences for Tritrichomonas suis, a related trichomonad, and the yeasts C. albicans and S. cerevisiae. These organisms were chosen because of their relationships to STIs and/or eukaryotic pathogens. The range of amino acid identity levels was 0% to 22%. The amino acid identity of T. vaginalis α-actinin with HuACTN1 was 0% to 50%. Not unexpectedly, the seemingly high level of sequence identity of any epitope with the corresponding region of HuACTN1 decreased when neighboring amino acids were analyzed. Specific synthesized peptide epitopes of α-actinin with low to no amino acid sequence identity with other proteins were reactive with positive control sera from both women and men, as shown for representative epitopes in Fig. 2.
Fig 5.
Amino acid sequence alignment of T. vaginalis α-actinin with the amino acid sequences of proteins from representative organisms and HuACTN1 (see Materials and Methods). The epitopes identified with pooled positive control sera from women and men, as indicated in Table 1, are boxed. Red foreground, gaps in the sequence; red lettering, 0 to 33% identity; black lettering, approximately 34 to 67% identity; blue lettering, 68 to 100% identity.
Table 2.
Epitope sequence identity comparisons between T. vaginalis α-actinin and T. suis, C. albicans, and S. cerevisiae proteins and HuACTN1
| Comparison protein | Epitope sequence identity (%)a |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9/M1 | W10/M2 | W11/M3 | W12/M4/HA423 | W13/M5 | |
| T. suis | 9 | 0 | 0 | 9 | 0 | 14 | 0 | 0 | 11 | 0 | 20 | 20 | 0 |
| C. albicans | 9 | 0 | 20 | 18 | 18 | 14 | 0 | 0 | 22 | 0 | 0 | 20 | 20 |
| S. cerevisiae | 11 | 0 | 20 | 9 | 0 | 20 | 13 | 25 | 11 | 13 | 20 | 20 | 0 |
| HuACTN1 | 21 | 50 | 40 | 46 | 36 | 40 | 25 | 38 | 13 | 13 | 20 | 0 | 40 |
The individual epitopes indicated by W1 through W13, M1 through M5, and HA4223 are as listed in Table 1.
DISCUSSION
Research in our laboratory led to the development of the lateral flow immunochromatographic diagnostic test for detection of trichomonad protein in female patients; this diagnostic test was commercialized (OSOM Trichomonas rapid test; Sekisui Diagnostics, San Diego, CA) and currently is in use in the United States and other countries (34, 35). This diagnostic test does not work with either urine or secretion samples obtained from male patients or urine samples spiked with lysates of total T. vaginalis proteins, based on our analysis in the laboratory. The recent reports suggesting a relationship between seropositivity for ACT-P2 of T. vaginalis and prostate cancer (27–29) reveal the need for a serum-based point-of-care diagnostic test that utilizes a highly specific target. Our data show that the 558-amino-acid ACT-P2 is a good target for detection of antibodies in both women and men seropositive for T. vaginalis. The region of ACT-P2 was found to possess less homology with other α-actinin-related proteins (33), further reinforcing its diagnostic value. The fact that the epitopes detected by the positive control sera from men are located toward the C terminus revealed why ACT-P2 was a good target for our earlier screening for serum antibodies (27–29). It is noteworthy that there is no or little identity of the peptide epitope sequences with other proteins in data banks and among T. suis, C. albicans, and S. cerevisiae proteins and HuACTN1 (Table 2 and Fig. 5). This suggests strongly that high levels of seropositivity are the result of exposure to T. vaginalis. Of particular interest, the 15-mer synthetic peptide epitopes found within ACT-P2 immobilized on nitrocellulose membranes were detected by positive control sera from both women and men that were reactive to α-actinin (Fig. 3). Perhaps not surprisingly, most of the peptide epitope amino acid sequences represented portions of the protein that were hydrophilic (41) and antigenic (40) (Fig. 4). The representative 15-mer synthetic peptides corresponding to W10/M2 and W13/M5 that were readily detected on immobilized surfaces were in fact highly hydrophilic (Fig. 3). This further suggests that the reactive 15-mer epitopes represent linear, readily detectable epitopes. It is conceivable that one or more recombinant proteins encoding a series of highly reactive epitopes might represent diagnostic targets in the future.
It is not surprising that α-actinin represents a target for serodiagnostic testing. This is one of the most immunogenic proteins of T. vaginalis (32, 33). Its function is to associate with actin, which is important because of the dramatic rapid morphological transformation that this organism undergoes immediately following contact with vaginal epithelial cells (11), prostate epithelial cells (J. F. Alderete, unpublished data), and extracellular matrix proteins such as laminin (6, 42) and fibronectin (10, 42, 43). Indeed, recent transcriptomic and proteomic analyses (7, 44) revealed the dramatically increased expression levels of α-actinin required for cytoskeletal rearrangements for morphological changes upon adherence to vaginal epithelial cells and binding to fibronectin (5, 11, 42, 43). Further, equally elevated amounts of mRNA coding for trichomonad GAPDH (5) and α-enolase (19) were found, and both of these proteins are surface ligands for binding fibronectin (5, 19). There are four human α-actinin homologs, none of which is cross-reactive with MAb HA423 to the trichomonad α-actinin (Table 2 and Fig. 2) and with positive control sera from women (32) and men (Fig. 2). These human α-actinin proteins are known to have a less conserved central region, as is the case for all actin-binding proteins of the spectrin family.
Equally noteworthy is that the epitopes detected by MAb HA423 and positive control sera from women and men are invariant. Laboratory-adapted T. vaginalis isolates grown in batch culture for >20 years possess MAb 423-immunoreactive α-actinin with the same Mr. Further, more than 50 fresh clinical isolates, one-half of which are type II P270 phenotypically varying isolates with the double-stranded RNA virus (45), all possess α-actinin detected by the MAb and positive control sera from women and men. We have seen no relationship between T. vaginalis with or without Mycoplasma and changes in α-actinin (46, 47). Thus, this invariant stable immunogenic protein appears suitable for a rapid serodiagnostic test for trichomonosis.
Of interest is the number of epitopes detected by positive control sera from female patients compared with sera from men. This may be attributable to different presentations of the proteins during immune surveillance resulting from the unique urogenital regions of women versus men. It is known that female patients with trichomonosis possess IgG antibodies to numerous trichomonad proteins in serum and the vagina (5, 8, 32, 36, 37, 48, 49), perhaps indicating a more vigorous antibody response during infection than that of men. Studies by others demonstrated the highly immunogenic nature of α-actinin and the serum antibody response to α-actinin in women (31). However, these data show that men respond to exposure to T. vaginalis by producing serum IgG antibodies, especially to the epitopes located toward the carboxy-terminal region with the least identity with other known proteins. Importantly, what remains unknown is the temporal relationship between seropositivity and initial exposure to this STI; this critical absence of clinical information might be corrected through future availability of a serodiagnostic test for women and men. What is known (albeit with only a small sample) is that, 1 week after treatment of women with trichomonosis, vaginal antibodies to proteinases were not detectable (17). Data regarding the temporal nature of antibody levels after both infection and treatment are needed.
The literature is replete with examples of peptide epitopes utilized for diagnostic tests for infectious diseases. For example, rapid diagnostic tests for Plasmodium falciparum employ epitopes of histidine-rich proteins (50). Diagnosis of visceral leishmaniasis is performed with rapid antigen-based tests (51), and specific epitopes of the proteins p120 and p140 are used for detection of Ehrlichia chaffeensis and Ehrlichia canis (52), respectively. This shows the value of characterization of immunogenic epitopes for development of specific targets for serodiagnosis.
In summary, our results present evidence for the validity of α-actinin and the truncated ACT-P2 as targets for serodiagnosis in both women and men exposed to T. vaginalis. This seems important for the future not only for screening men who possibly were exposed to this STI, in relation to the possibility of prostate cancer development, but also for developing a more rapid, noninvasive test for women. Alternatively, our approach highlights the methods by which peptide epitopes of immunogens might be identified as targets for antibody detection to determine exposure to and infection by this significant sexually transmitted disease pathogen.
ACKNOWLEDGMENTS
We thank Laurence N. Kolonel of the University of Hawai'i Cancer Center and Siobhan Sutcliffe of Washington University at St. Louis for their generous contribution of additional male sera that were highly seropositive for ACT-P2 and that permitted us to screen for epitopes of α-actinin. We also thank Patrick Joyce, Adan Medina, and Clare Tang, undergraduate student members of the Alderete laboratory, for their assistance during the course of these experiments and for their thoughtful discussions.
This work was supported in part by the Washington Research Foundation.
Footnotes
Published ahead of print 24 April 2013
REFERENCES
- 1. Swygard H, Sena A, Hobbs M, Cohen M. 2004. Trichomoniasis: clinical manifestations, diagnosis and management. Sex. Transm. Infect. 80:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lehker MW, Alderete JF. 2000. Biology of trichomonosis. Curr. Opin. Infect. Dis. 13:37–45 [DOI] [PubMed] [Google Scholar]
- 3. Peterson KM, Alderete JF. 1982. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect. Immun. 37:755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neale KA, Alderete JF. 1990. Analysis of the proteinases of representative Trichomonas vaginalis isolates. Infect. Immun. 58:157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lama A, Kucknoor A, Mundodi V, Alderete JF. 2009. Glyceraldehyde-3-phosphate dehydrogenase is a surface-associated, fibronectin-binding protein of Trichomonas vaginalis. Infect. Immun. 77:2703–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casta e Silva Filho F, de Souza W, Lopes JD. 1988. Presence of laminin-binding proteins in trichomonads and their role in adhesion. Proc. Natl. Acad. Sci. U. S. A. 85:8042–8046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kucknoor AS, Mundodi V, Alderete JF. 2005. Adherence to human vaginal epithelial cells signals for increased expression of Trichomonas vaginalis genes. Infect. Immun. 73:6472–6478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lehker MW, Alderete JF. 1992. Iron regulates growth of Trichomonas vaginalis and the expression of immunogenic trichomonad proteins. Mol. Microbiol. 6:123–132 [DOI] [PubMed] [Google Scholar]
- 9. Garcia AF, Chang TH, Benchimol M, Klumpp DJ, Lehker MW, Alderete JF. 2003. Iron and contact with host cells induce expression of adhesins on surface of Trichomonas vaginalis. Mol. Microbiol. 47:1207–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alderete JF, Benchimol M, Lehker MW, Crouch ML. 2002. The complex fibronectin-Trichomonas vaginalis interactions and trichomonosis. Parasitol. Int. 51:285–292 [DOI] [PubMed] [Google Scholar]
- 11. Arroyo R, González-Robles A, Martínez-Palomo A, Alderete JF. 1993. Signaling of Trichomonas vaginalis for ameboid transformation and adhesin synthesis follows cytoadherence. Mol. Microbiol. 7:299–309 [DOI] [PubMed] [Google Scholar]
- 12. Garcia AF, Alderete JF. 2007. Characterization of the Trichomonas vaginalis surface-associated AP65 and the binding domain interacting with trichomonads and host cells. BMC Microbiol. 7:116. 10.1186/1471-2180-7-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia AF, Benchimol M, Alderete JF. 2005. Trichomonas vaginalis polyamine metabolism is linked to host cell adherence and cytotoxicity. Infect. Immun. 73:2602–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kucknoor AS, Mundodi V, Alderete JF. 2007. The proteins secreted by Trichomonas vaginalis and vaginal epithelial cell response to secreted and episomally-expressed AP65. Cell. Microbiol. 9:2586–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Provenzano D, Alderete JF. 1995. Analysis of human immunoglobulin-degrading cysteine proteinases of Trichomonas vaginalis. Infect. Immun. 63:3388–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alderete JF, Provenzano D, Lehker MW. 1995. Iron mediates Trichomonas vaginalis resistance to complement lysis. Microb. Pathog. 19:93–103 [DOI] [PubMed] [Google Scholar]
- 17. Alderete JF, Newton E, Dennis C, Neale KA. 1991. The vagina of women infected with Trichomonas vaginalis has numerous proteinases and antibody to trichomonad proteinases. Genitourin. Med. 67:469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lehker MW, Sweeney D. 1999. Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex. Transm. Infect. 75:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mundodi V, Kucknoor AS, Alderete JF. 2008. α-Enolase is a surface associated plasminogen-binding protein of Trichomonas vaginalis. Infect. Immun. 76:523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsu HM, Ong SJ, Lee MC, Tai JH. 2009. Transcriptional regulation of an iron-inducible gene by differential and alternate promoter entries of multiple Myb proteins in the protozoan parasite Trichomonas vaginalis. Eukaryot. Cell 8:362–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsai CD, Liu HW, Tai JH. 2002. Characterization of an iron-responsive promoter in the protozoan pathogen Trichomonas vaginalis. J. Biol. Chem. 277:5153–5162 [DOI] [PubMed] [Google Scholar]
- 22. Lehker MW, Arroyo R, Alderete JF. 1991. The regulation by iron of the synthesis of adhesins and cytoadherence levels in the protozoan Trichomonas vaginalis. J. Exp. Med. 174:311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryu JS, Choi HK, Min DY, Ha SE, Ahn MH. 2001. Effect of iron on the virulence of Trichomonas vaginalis. J. Parasitol. 87:457–460 [DOI] [PubMed] [Google Scholar]
- 24. Alderete JF, Nguyen J, Mundodi V, Lehker MW. 2004. Heme-iron increases levels of AP65-mediated adherence by Trichomonas vaginalis. Microb. Pathog. 36:263–271 [DOI] [PubMed] [Google Scholar]
- 25. Ardalan S, Lee BC, Garber GE. 2009. Trichomonas vaginalis: the adhesins AP51 and AP65 bind heme and hemoglobin. Exp. Parasitol. 121:300–306 [DOI] [PubMed] [Google Scholar]
- 26. Alvarez-Sanchez ME, Carvajal-Gamez BI, Solano-Gonzalez E, Martinez-Benitez M, Garcia AF, Alderete JF, Arroyo R. 2008. Polyamine depletion down-regulates gene expression of the Trichomonas vaginalis cytotoxic CP65, a 65-kDa cysteine proteinase involved in cellular damage. Int. J. Biochem. Cell Biol. 40:2442–2451 [DOI] [PubMed] [Google Scholar]
- 27. Stark JR, Judson G, Alderete JF, Mundodi V, Kucknoor AS, Giovannucci EL, Platz EA, Sutcliffe S, Fall K, Kurth T, Ma J, Stampfer MJ, Mucci LA. 2009. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians' Health Study. J. Natl. Cancer Inst. 101:1406–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sutcliffe S, Alderete JF, Till C, Goodman PJ, Hsing AW, Zenilman JM, De Marzo AM, Platz EA. 2009. Trichomonosis and subsequent risk of prostate cancer in the Prostate Cancer Prevention Trial. Int. J. Cancer 124:2082–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sutcliffe S, Giovannucci E, Alderete JF, Chang TH, Gaydos CA, Zenilman JM, De Marzo AM, Willett WC, Platz EA. 2006. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 15:939–945 [DOI] [PubMed] [Google Scholar]
- 30. Mitteregger D, Aberle SW, Makristathis A, Walochnik J, Brozek W, Marberger M, Kramer G. 2012. High detection rate of Trichomonas vaginalis in benign hyperplastic tissue. Med. Microbiol. Immunol. 201:113–116 [DOI] [PubMed] [Google Scholar]
- 31. Sutcliffe S, Neace C, Magnuson NS, Reeves R, Alderete JF. 2012. Trichomonosis, a common curable STI, and prostate carcinogenesis: a proposed molecular mechanism. PLoS Pathog. 8:e1002801. 10.1371/journal.ppat.1002801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Addis MF, Rappelli P, Pinto De Andrade AM, Rita FM, Colombo MM, Cappuccinelli P, Fiori PL. 1999. Identification of Trichomonas vaginalis alpha-actinin as the most common immunogen recognized by sera of women exposed to the parasite. J. Infect. Dis. 180:1727–1730 [DOI] [PubMed] [Google Scholar]
- 33. Addis MF, Rappelli P, Delogu G, Carta F, Cappuccinelli P, Fiori PL. 1998. Cloning and molecular characterization of a cDNA clone coding for Trichomonas vaginalis alpha-actinin and intracellular localization of the protein. Infect. Immun. 66:4924–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pillay A, Lewis J, Ballard RC. 2004. Evaluation of Xenostrip-Tv, a rapid diagnostic for Trichomonas vaginalis infection. J. Clin. Microbiol. 42:3853–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones HE, Lippman SA, Caiaffa-Filho HH, Young T, van de Wijgert JHHM. 2013. Performance of rapid self-test for detection of Trichomonas vaginalis in South Africa and Brazil. J. Clin. Microbiol. 51:1037–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alderete JF. 1983. Antigenic analysis of several pathogenic strains of Trichomonas vaginalis. Infect. Immun. 39:1041–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alderete JF. 1983. Identification of immunogenic and antibody-binding proteins on the membrane of pathogenic Trichomonas vaginalis. Infect. Immun. 40:284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee HY, Hyung S, Lee JW, Kim J, Shin MH, Ryu JS, Park SJ. 2011. Identification of antigenic proteins in Trichomonas vaginalis. Korean J. Parasitol. 49:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alderete JF. 1984. Enzyme-linked immunosorbent assay for detecting antibody to Trichomonas vaginalis: use of whole cells and aqueous extract as antigen. Br. J. Vener. Dis. 60:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kyte J, Doolittle RF. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105–132 [DOI] [PubMed] [Google Scholar]
- 41. Hopp TP, Woods KR. 1981. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. U. S. A. 78:3824–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crouch ML, Alderete JF. 1999. Trichomonas vaginalis interactions with fibronectin and laminin. Microbiology 145:2835–2843 [DOI] [PubMed] [Google Scholar]
- 43. Crouch ML, Benchimol M, Alderete JF. 2001. Binding of fibronectin by Trichomonas vaginalis is influenced by iron and calcium. Microb. Pathog. 31:131–144 [DOI] [PubMed] [Google Scholar]
- 44. Huang K, Huang P, Ku F, Lin R, Alderete JF, Tang P. 2012. Comparative transcriptomic and proteomic analyses of Trichomonas vaginalis following adherence to fibronectin. Infect. Immun. 80:3900–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dailey DC, Alderete JF. 1991. The phenotypically variable surface protein of Trichomonas vaginalis has a single, tandemly-repeated immunodominant epitope. Infect. Immun. 59:2083–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dessi D, Rappelli P, Diaz N, Cappuccinelli P, Fiori PL. 2006. Mycoplasma hominis and Trichomonas vaginalis: a unique case of symbiotic relationship between two obligate human parasites. Front. Biosci. 11:2028–2034 [DOI] [PubMed] [Google Scholar]
- 47. van der Schee C, Sluiters HJ, vander Meijden WI, van Beek P, Peerbooms P, Verbrugh H, van Belkum A. 2001. Host and pathogen interaction during vaginal infection by Trichomonas vaginalis and Mycoplasma hominis or Ureaplasma urealyticum. J. Microbiol. Methods 45:61–67 [DOI] [PubMed] [Google Scholar]
- 48. Alderete JF, Newton E, Dennis C, Neale KA. 1991. Antibody in sera of patients infected with Trichomonas vaginalis is to trichomonad proteinases. Genitourin. Med. 67:331–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alderete JF, Newton E, Dennis C, Engbring J, Neale KA. 1991. Vaginal antibody of patients with trichomoniasis is to a prominent surface immunogen of Trichomonas vaginalis. Genitourin. Med. 67:220–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee N, Gatton ML, Pelecanos A, Bubb M, Gonzalez I, Bell D, Cheng Q, McCarthy JS. 2012. Identification of optimal epitopes for Plasmodium falciparum rapid diagnostic tests that target histidine-rich proteins 2 and 3. J. Clin. Microbiol. 50:1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vaish M, Sharma S, Chakravarty J, Sundar S. 2012. Evaluation of two novel rapid rKE16 antigen-based tests for diagnosis of visceral leishmaniasis in India. J. Clin. Microbiol. 50:3091–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luo T, Zhang X, McBride JW. 2009. Major species-specific antibody epitopes of the Ehrlichia chaffeensis p120 and E. canis p140 orthologs in surface-exposed tandem repeat regions. Clin. Vaccine Immunol. 16:982–990 [DOI] [PMC free article] [PubMed] [Google Scholar]