Abstract
Maternal vaginal colonization with group B streptococcus (GBS) is a major risk factor for invasive GBS infection in newborns. The CDC-recommended method for detecting GBS colonization is to culture vaginal and rectal swabs in a selective broth followed by subculture on blood agar or a selective medium. A high incidence of antimicrobial resistance in the fecal microflora can compromise the recovery of GBS from the selective broth. Here, we compared CHROMagar StrepB (CA), Columbia colistin-nalidixic agar (CNA), and Trans-Vag selective broth enrichment for the isolation of GBS from 130 vaginal and 130 rectal swabs from pregnant women. The swabs were randomized for plating first on either CA or CNA, and they then were inoculated in Trans-Vag broth. GBS was cultured from 37.7% of the vaginal swabs and 33.1% of the rectal swabs. There were no differences in the detection rates for the vaginal swabs between CA (31.5%), CNA (26.2%), and the selective broth (30.0%). The sensitivities in relation to a composite score were 83.7%, 69.4%, and 79.6%, respectively. However, recovery of GBS from the rectal swabs was significantly higher from CA (29.2%; P < 0.0001) and CNA (23.8%; P = 0.002) than from the selective broth (9.2%). The sensitivities were 88.4%, 72.1%, and 27.9%, respectively. The order of plating on the solid medium was significant (P = 0.003), with GBS detection rates of 30.8% and 24.6% when swabs were plated first and second, respectively. These findings show that a selective broth is not suitable for the recovery of GBS from rectal swabs in settings such as ours, due to masking of the GBS colonies by persistent microflora.
INTRODUCTION
Infection by group B streptococcus (GBS) is one of the most common infections in newborns (1). Maternal colonization has been found to be a major risk factor for invasive GBS disease within 6 days of birth. Approximately 10 to 40% of pregnant women are colonized with GBS in either the vagina, the rectum, or both areas (2). The rate of peripartum transmission of GBS to newborns of colonized women is approximately 50%, after which 1 to 2% of these newborns develop invasive GBS infection in the first week of life (3, 4). The Centers for Disease Control and Prevention (CDC) has recommended that all pregnant women be screened for rectovaginal carriage of GBS at 35 to 37 weeks' gestation to identify women who should receive intrapartum antimicrobial prophylaxis (IAP). When successfully implemented, IAP targeted at GBS-colonized pregnant women has reduced the incidence of invasive GBS disease by 86 to 89% (5–7). The sensitivities of screening methods for the identification of maternal carriage of GBS depends on the timing of specimen collection, the source of the specimen, and the culture technique used. Optimally, specimens should be collected as close to delivery as possible. The use of vaginal and rectal swab specimens has been shown to yield higher GBS culture-positivity rates than vaginal swabs alone or cervical specimens (8–10). The current CDC recommendation for the isolation of GBS from vaginal and rectal or rectovaginal swabs is growth in a selective broth medium (Todd-Hewitt broth with gentamicin and colistin or nalidixic acid), followed by subculture on blood agar or a selective medium (11). The reported sensitivity of selective broth for the culture of GBS is 82% to 99% (12–15). There are, however, limitations to this approach. The procedure requires at least an additional 24 h of culture time compared to that for direct plating on selective agar, and isolated GBS-like colonies require further identification with ancillary tests, e.g., the CAMP factor or B antigen test (11). Furthermore, identification of GBS-like colonies on blood agar requires laboratory expertise, particularly when they are mixed with other microflora (16). Considering methods to decrease the GBS detection time, studies have shown that direct plating on colistin-nalidixic agar (CNA) is a low-cost alternative for GBS recovery, albeit with a lower sensitivity that has ranged from 59% to 83% (17–19). In recent years, several commercial chromogenic media, such as Granada medium, CHROMagar StrepB (CA), and chromID Strepto B agar, have been tested for their suitability for detecting GBS (18, 20). CA is a commercially available selective chromogenic medium that inhibits most saprophytic bacteria and yeasts and produces mauve-colored GBS colonies under aerobic conditions irrespective of their hemolytic properties, allowing direct visual identification. The use of chromogenic media may improve the yield of GBS while reducing labor costs and turnaround time. The clinical sensitivities of these media vary from study to study, with most studies reporting 93% to 98% for CA (16, 21), 40% to 91% for Granada medium (12, 18, 22), and 88% to 95% for chromID Strepto B agar (13, 18). The aim of this study was to evaluate direct plating on CA and CNA and selective broth enrichment for the isolation of GBS in swabs from pregnant women.
MATERIALS AND METHODS
Study design.
The study was approved by the Human Research Ethics Committee of the University of the Witwatersrand (IRB/Protocol-M090937) and conducted at prenatal community clinics in Soweto, Johannesburg. Included were HIV-uninfected pregnant women at more than 20 weeks of gestation who were able to provide informed consent for participation. Exclusion criteria were concurrent antibiotic use, an acute illness, a symptomatic vaginal discharge, and a known or suspected condition in which clinical vaginal examinations were contraindicated. A convenience sampling of lower vaginal and rectal swabs was collected from 130 pregnant women between January 2011 and April 2011 by trained study nurses.
Sample collection.
Samples were collected with rayon-tipped swabs that were placed into 5 ml of Amies transport medium without charcoal (catalog no. MW170, Transwab Amies; Medical Wire, UK). All samples were transported to the laboratory within 4 h of collection, where they were processed within 2 h.
Randomization and processing of samples.
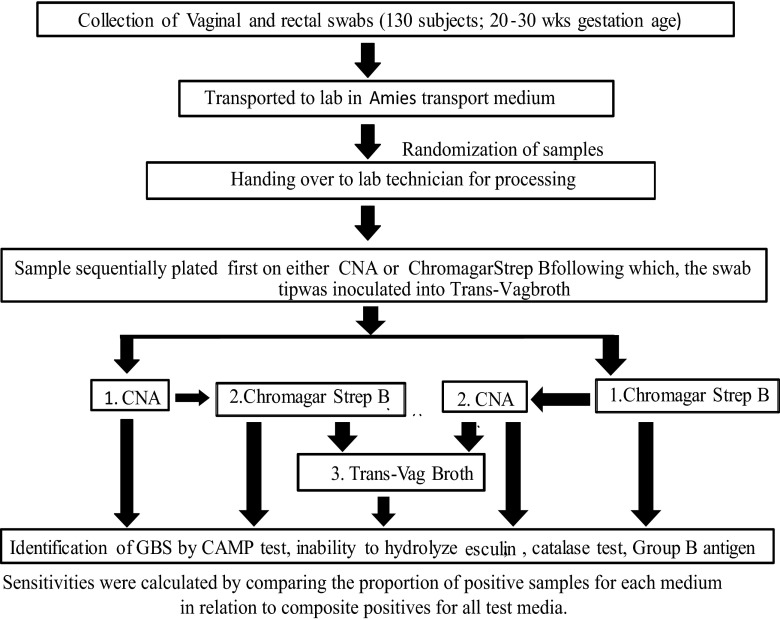
All vaginal and rectal swabs were randomized using a randomization log. The swab number was linked to three different randomly numbered labels, one for each culture medium type. Each sample was randomized to be plated first on either CNA or CA, after which the swab tip was inoculated into the selective (Trans-Vag) broth (Todd-Hewitt broth with 8 μg/ml gentamicin and 15 μg/ml nalidixic acid) and incubated for 24 h before being plated on 5% sheep blood agar (SBA) (Fig. 1). The randomization process ensured that the technicians reading the plates and entering the data were blinded with respect to the participant, the type of swab (rectal or vaginal), the order of plating, and the swab/plate pairs. The CNA and SBA plates were incubated at 37°C in 5% CO2 for 18 to 24 h, and the CA plates were incubated at 37°C for 18 to 24 h under aerobic conditions. If the expected colonies were not visible after 24 h of incubation, the plates were reincubated for an additional 24 h and reexamined for growth. Up to four GBS-like colonies were isolated from the solid culture medium on the basis of the colony morphology and mauve color on CA and the colony morphology and beta-hemolysis on CNA and blood agar. Isolates were confirmed to be GBS by testing for CAMP factor, an inability to hydrolyze esculin, catalase negativity, and group B antigen latex agglutination positivity (Omega Diagnostics, Scotland, UK). The CA and CNA prepoured plates were purchased from Media Mage (Johannesburg, South Africa).
Fig 1.
Procedure for the collection, randomization, and laboratory handling of the vaginal and rectal swabs for the isolation of group B streptococcus.
Statistical methods.
Descriptive statistics included statements of frequency with percentages, means ± standard deviations (SDs), and medians with interquartile ranges (IQRs). Sensitivities (detection rates) were calculated by comparing the proportion of positive samples for each medium in relation to a gold standard, which was a composite positive for all test media. The McNemar test for correlated percentages and the chi-square test with Yates' correction were used to compare the culture methods, with P values of <0.05 being considered statistically significant.
RESULTS
Study population.
The mean age (SD) of the women at swabbing was 25.7 years (±5.9 years), and the mean gestational age (SD) was 23.8 weeks (±3.2 weeks). The median parity and gravidity were 1 (IQR, 0 to 1) and 2 (IQR, 1 to 2), respectively.
CA and CNA versus Trans-Vag selective broth enrichment.
Overall, 37.7% of the vaginal swabs and 33.1% of the rectal swabs tested positive for GBS (Table 1). There were no statistically significant differences in the detection rates for the vaginal swabs between CA (31.5%), CNA (26.2%), and the selective broth (30.0%). The sensitivities with respect to the composite scores were 83.7%, 69.4%, and 79.6%, respectively. The recovery of GBS from the rectal swabs, however, was significantly higher from CA (29.2%; P < 0.0001) and CNA (23.8%; P = 0.002) than from the selective broth medium (9.2%). The sensitivities were 88.4%, 72.1%, and 27.9%, respectively. Despite the higher sensitivity reported for CA than for CNA for the vaginal and rectal swabs, these differences were not statistically significant (P > 0.05).
Table 1.
Performance characteristics of CA, CNA, and the selective broth for detection of GBS from vaginal and rectal swabs
| Medium | Vaginal swabs (n = 130) |
Rectal swabs (n = 130) |
||||
|---|---|---|---|---|---|---|
| No. (%) positive for GBS | Sensitivity (%)a | Accuracy (%)b | No. (%) positive for GBS | Sensitivity (%)a | Accuracy (%)b | |
| CA | 41 (31.5) | 83.7 | 155/167 (92.8) | 38 (29.2) | 88.4 | 132/159 (83.0) |
| CNA | 34 (26.2) | 69.4 | 129/153 (84.3) | 31 (23.8) | 72.1 | 116/150 (77.3) |
| Selective broth | 39 (30.0) | 79.6 | 155/171 (90.6) | 12 (9.2) | 27.9 | 48/88 (54.5) |
| Total GBS isolated | 49 (37.7) | 43 (33.1) | ||||
Sensitivity was calculated in comparison to composite scores of all media.
Accuracy was calculated as the number of confirmed GBS colonies isolated/total number of GBS-like colonies that were confirmed as either positive or negative for GBS.
Accuracy.
The accuracy of a medium was defined as the percentage of isolated colonies that were identified as GBS by confirmatory testing divided by the total number of GBS-like colonies that were isolated from a plate and subjected to confirmatory tests. The denominator includes both confirmed GBS colonies (true positives) and colonies having appearances similar to those of GBS but failing the confirmatory tests (false positives). For the vaginal swabs, CA was the most accurate for discriminating between true-positive and false-positive isolates (92.8%), followed by selective broth enrichment (90.6%) and CNA (84.3%). There were no significant differences in the accuracies of primary identification between CA and the selective broth or between CNA agar and the selective broth. However, after direct plating, colonies were significantly more likely to be GBS following isolation with CA than with CNA (P = 0.026).
The accuracy of GBS identification from the rectal swabs was significantly better with CA (83.0%; P < 0.0001) and CNA (77.3%; P = 0.0004) than with selective broth enrichment (54.5%). CA was found to be more accurate than CNA, but these differences were not statistically significant (P = 0.26). The numbers of GBS-like colonies that were identified from the cultured vaginal and rectal swabs were similar between CA (167 vaginal, 159 rectal) and CNA (153 vaginal, 150 rectal). However, about half the numbers of GBS-like colonies were identified among the other flora following selective broth enrichment from the rectal swabs (171 vaginal, 88 rectal). A higher percentage of false-positive GBS isolates was reported from the rectal swabs than from the vaginal swabs with CA (17.0% versus 7.2%; P = 0.01), CNA (22.7% versus 15.7%; P = 0.16), and the selective broth (45.5% versus 9.4%; P < 0.0001) (Table 1).
Impact of randomization and the order of plating.
The order in which the swabs were plated onto the two selective media had a significant effect on the culture positivity rate. Out of 260 swabs tested directly on CNA and CA, a total of 86 (33.1%) swabs were positive for GBS. Direct culture on CNA and CA yielded GBS from 80 (30.8%) swabs when plated first compared to 64 (24.6%) when plated second (P = 0.003). GBS was isolated from both media in 58 (22.3%) of the swabs. The numbers of swabs from which GBS was isolated from a single medium were 22 (8.5%) and 6 (2.3%) from the first and second platings, respectively. When CNA and CA were plated first, the sensitivities were 86.4% and 87.5%, respectively, and when CNA and CA were plated second, the sensitivities were 57.4% and 82.2%, respectively (Table 2).
Table 2.
Performance characteristics of CNA and CA with respect to the order of plating
| Specimen | CNA |
CA |
Trans-Vaga |
Total no. (%) positive for GBS | |||
|---|---|---|---|---|---|---|---|
| No. (%) positive for GBS | Sensitivity (%) | No. (%) positive for GBS | Sensitivity (%) | No. (%) positive for GBS | Sensitivity (%) | ||
| Plated first on CAN | |||||||
| Vaginal (n = 65) | 20 (30.8) | 80 | 21 (32.3) | 84 | 23 (35.4) | 92 | 25 (38.5) |
| Rectal (n = 65) | 18 (27.7) | 94.7 | 16 (24.6) | 84.2 | 8 (12.3) | 42.1 | 19 (29.2) |
| Total (n = 130) | 38 (29.2) | 86.4 | 37 (28.5) | 84.1 | 31 (23.8) | 70.4 | 44 (33.8) |
| Plated first on CA | |||||||
| Vaginal (n = 65) | 14 (21.5) | 58.3 | 20 (30.8) | 83.3 | 16 (24.6) | 66.7 | 24 (36.9) |
| Rectal (n = 65) | 13 (20) | 54.2 | 22 (33.8) | 91.7 | 4 (6.2) | 16.7 | 24 (36.9) |
| Total (n = 130) | 27 (20.8) | 56.2 | 42 (32.3) | 87.5 | 20 (15.4) | 41.7 | 48 (36.9) |
After direct plating, the swab was placed in Trans-Vag broth.
DISCUSSION
Current CDC guidelines for prenatal GBS screening recommend the collection of a single rectovaginal swab or separate vaginal and rectal swabs that are enriched overnight in Todd-Hewitt broth with antibiotics, followed by culture on blood agar or a selective medium (11). While these guidelines were developed within the context of high-income countries, there are few data on the validity of this method in low- to middle-income countries where there are differences in the distributions of saprophytic organisms and antimicrobial resistance is common (23–26). In this study, we compared the recovery rates of GBS by standard methods with direct plating onto either CNA or CA from either the vaginal or rectal swabs from pregnant South African women to determine whether these alternatives would provide better recovery rates. One of the main reasons for this comparison was the presence of vaginal flora that are resistant to antimicrobial agents, particularly Proteus spp., which occur in a small percentage of swabs and are able to persist in Trans-Vag broth and swarm the subcultured blood agar plate, rendering it unreadable (P. V. Adrian, personal communication). This necessitated the use of both Trans-Vag broth and direct plating on CNA in a previous study of vaginal swabs from South Africa (27).
In our study, there were no statistically significant differences in the recoveries of GBS from vaginal swabs between the different media, suggesting that any of these three options is valid in our clinical setting and the choice can be made based on cost, availability, and desired turnaround time. In contrast, the recovery of GBS from rectal swabs following selective broth enrichment was inferior to that with either of the direct plating methods. The main reasons for the poor recovery of GBS were survival and overgrowth of non-GBS organisms in the Trans-Vag broth, which masked the presence of GBS on the subculture plates. The results of our study differ from those of several previously published reports, in which it was shown that the selective broth method is more sensitive than direct plating on a selective medium for recovering GBS from rectal or rectovaginal swabs (12–15). While these results may be valid in settings such as those where the studies were conducted, other studies from Brazil, Denmark, and Spain demonstrated better recoveries of GBS from rectal swabs with a solid selective medium than with a selective broth (28–30). Pigmented enrichment broth, such as StrepB carrot broth, has been shown to increase the sensitivity of GBS detection by culture over that with LIM broth (Todd-Hewitt broth with colistin and nalidixic acid) (31) when used as an enrichment step prior to PCR screening (32); however, GBS growth and pigment development can still be suppressed by the overgrowth of fecal bacteria and mask the presence of GBS on culture (32). Since the mechanism behind the poor recovery of GBS from rectal swabs is related to persistence and overgrowth of resistant fecal bacteria (30), it is likely that the collection of a single rectovaginal swab or the coprocessing of vaginal and rectal swabs may be severely compromised by the use of a selective broth in our setting.
Overall, CA produced the highest recovery rate of GBS from the vaginal and rectal swabs. One advantage of the chromogenic medium was that it was significantly easier to discriminate between GBS and group D streptococcus (GDS) in cocolonized samples due to differences in the color development of the colonies. GBS turns mauve, whereas GDS turns blue. This is particularly important in comparisons with samples plated on blood agar, where the GBS colonies are in the minority and single GBS colonies can be overlooked amid large numbers of morphologically similar GDS. Despite CA being more expensive than CNA or a selective broth-blood agar combination, CA offers cost savings in terms of reduced processing time and the ease with which GBS colonies can be identified on the plates. Moreover, due to the increased accuracy of colony identification through pigment formation, the number of confirmatory tests required is reduced, as the need for additional tests to rule out the morphologically similar colonies of group D streptococci is eliminated. While the calculated values of accuracy will vary with the skill and experience of the users, CA offers an easier learning curve compared to that for a blood-based isolation medium. In a comparison of the two selective solid agars, CA had higher sensitivity and accuracy than CNA for the vaginal and rectal swabs, although these values were not statistically significant, most likely due to a limited sample size.
From a review of the literature, no study in a clinical setting had considered the order of plating when comparing and reporting the sensitivities of culture media to isolate GBS. In this study, the order of plating had a significant effect on the recovery of GBS, which suggests that future studies where solid media are compared should follow randomized protocols to prevent detection bias based on plating order. Possible reasons for the lower recovery rate of GBS from the swabs plated for a second time include removal of the organisms from the surface of the swab in the first plating and blockage of the swab surface by sterile agar from the previous plating, preventing transfer of organisms. The sensitivity of direct plating on CNA when plated first in this study was similar to that described by Louie et al. (19), who also inoculated swabs on CNA first.
In conclusion, selective broth enrichment showed significantly lower sensitivity for the recovery of GBS from rectal swabs. This method is not suitable for the recovery of GBS from rectal swabs or rectovaginal swabs in regions with a high prevalence of antimicrobial-resistant flora. The recovery of GBS from the vaginal swabs plated on CA appears to have sensitivity equal to that of the selective broth and offers a less time-consuming and less labor-intensive process. For these reasons, our laboratory has started to use CA for current and future studies.
ACKNOWLEDGMENTS
This project was funded by the South African National Research Foundation (Vaccine Preventable Diseases Research Chair Grant). The laboratory has received funding from Novartis for clinical trials of GBS vaccines. The funders had no role in study design, data collection or analysis, the decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 22 May 2013
REFERENCES
- 1. Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, Mohle-Boetani J, Gershman K, Schaffner W, Petit S, Zansky SM, Morin CA, Spina NL, Wymore K, Harrison LH, Shutt KA, Bareta J, Bulens SN, Zell ER, Schuchat A, Schrag SJ. 2009. Evaluation of universal antenatal screening for group B streptococcus. N. Engl. J. Med. 360:2626–2636 [DOI] [PubMed] [Google Scholar]
- 2. Picard FJ, Bergeron MG. 2004. Laboratory detection of group B streptococcus for prevention of perinatal disease. Eur. J. Clin. Microbiol. Infect. Dis. 23:665–671 [DOI] [PubMed] [Google Scholar]
- 3. Beal S, Dancer S. 2006. Antenatal prevention of neonatal group B streptococcal infection. Rev. Gynaecol. Perinat. Pract. 6:218–225 [Google Scholar]
- 4. Heath PT, Feldman RG. 2005. Vaccination against group B streptococcus. Expert Rev. Vaccines 4:207–218 [DOI] [PubMed] [Google Scholar]
- 5. Brozanski BS, Jones JG, Krohn MA, Sweet RL. 2000. Effect of a screening-based prevention policy on prevalence of early-onset group B streptococcal sepsis. Obstet. Gynecol. 95:496–501 [DOI] [PubMed] [Google Scholar]
- 6. Lin FY, Brenner RA, Johnson YR, Azimi PH, Philips JH, III, Regan JA, Clark P, Weisman LE, Rhoads GG, Kong F, Clemens JD. 2001. The effectiveness of risk-based intrapartum chemoprophylaxis for the prevention of early-onset neonatal group B streptococcal disease. Am. J. Obstet. Gynecol. 184:1204–1210 [DOI] [PubMed] [Google Scholar]
- 7. Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold KE, Craig AS, Harrison LH, Reingold A, Stefonek K, Smith G, Gamble M, Schuchat A. 2002. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N. Engl. J. Med. 347:233–239 [DOI] [PubMed] [Google Scholar]
- 8. Platt MW, McLaughlin JC, Gilson GJ, Wellhoner MF, Nims LJ. 1995. Increased recovery of group B streptococcus by the inclusion of rectal culturing and enrichment. Diagn. Microbiol. Infect. Dis. 21:65–68 [DOI] [PubMed] [Google Scholar]
- 9. Chan SHS, Wan K, Lee WH. 2000. Review on group B streptococcal infection. Hong Kong J. Paediatr. (New Ser.) 5:166–174 [Google Scholar]
- 10. Quinlan JD, Hill DA, Maxwell BD, Boone S, Hoover F, Lense JJ. 2000. The necessity of both anorectal and vaginal cultures for group B streptococcus screening during pregnancy. J. Fam. Pract. 49:447–448 [PubMed] [Google Scholar]
- 11. Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC) 2010. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm. Rep. 59:1–36 [PubMed] [Google Scholar]
- 12. Gupta C, Briski LE. 2004. Comparison of two culture media and three sampling techniques for sensitive and rapid screening of vaginal colonization by group B streptococcus in pregnant women. J. Clin. Microbiol. 42:3975–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Craven RR, Weber CJ, Jennemann RA, Dunne WM., Jr 2010. Evaluation of a chromogenic agar for detection of group B streptococcus in pregnant women. J. Clin. Microbiol. 48:3370–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones N, Oliver K, Jones Y, Haines A, Crook D. 2006. Carriage of group B streptococcus in pregnant women from Oxford, UK. J. Clin. Pathol. 59:363–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Busetti M, D'Agaro P, Campello C. 2007. Group B streptococcus prevalence in pregnant women from North-Eastern Italy: advantages of a screening strategy based on direct plating plus broth enrichment. J. Clin. Pathol. 60:1140–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poisson DM, Chandemerle M, Guinard J, Evrard ML, Naydenova D, Mesnard L. 2010. Evaluation of CHROMagar StrepB: a new chromogenic agar medium for aerobic detection of group B streptococci in perinatal samples. J. Microbiol. Methods 82:238–242 [DOI] [PubMed] [Google Scholar]
- 17. Bosch-Mestres J, Martin-Fernandez RM, Jimenez de Anta-Losada MT. 2003. Comparative study of three culture media for detecting group B streptococcus colonization in pregnant women. Enferm. Infecc. Microbiol. Clin. 21:346–349 (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 18. El Aila NA, Tency I, Claeys G, Saerens B, Cools P, Verstraelen H, Temmerman M, Verhelst R, Vaneechoutte M. 2010. Comparison of different sampling techniques and of different culture methods for detection of group B streptococcus carriage in pregnant women. BMC Infect. Dis. 10:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Louie L, Kotowich L, Meaney H, Vearncombe M, Simor AE. 2010. Evaluation of a new chromogenic medium (StrepB select) for detection of group B streptococcus from vaginal-rectal specimens. J. Clin. Microbiol. 48:4602–4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poisson DM, Evrard ML, Freneaux C, Vives MI, Mesnard L. 2011. Evaluation of CHROMagar StrepB agar, an aerobic chromogenic medium for prepartum vaginal/rectal group B streptococcus screening. J. Microbiol. Methods 84:490–491 [DOI] [PubMed] [Google Scholar]
- 21. Charron J, Demandion E, Laudat P. 2009. Détection rapide par culture de Streptococcus agalactiae dans les prélèvements génitaux sur un nouveau milieu chromogène CHROMagar StrepB, poster 5508. Abstr. Réunion Interdisciplinaire de Chimiothérapie Anti-Infectieuse, Paris [Google Scholar]
- 22. Overman SB, Eley DD, Jacobs BE, Ribes JA. 2002. Evaluation of methods to increase the sensitivity and timeliness of detection of Streptococcus agalactiae in pregnant women. J. Clin. Microbiol. 40:4329–4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nys S, Okeke IN, Kariuki S, Dinant GJ, Driessen C, Stobberingh EE. 2004. Antibiotic resistance of faecal Escherichia coli from healthy volunteers from eight developing countries. J. Antimicrob. Chemother. 54:952–955 [DOI] [PubMed] [Google Scholar]
- 24. Newman MJ, Seidu A. 2002. Carriage of antimicrobial resistant Escherichia coli in adult intestinal flora. West Afr. J. Med. 21:48–50 [PubMed] [Google Scholar]
- 25. Shanahan PM, Wylie BA, Adrian PV, Koornhof HJ, Thomson CJ, Amyes SG. 1993. The prevalence of antimicrobial resistance in human faecal flora in South Africa. Epidemiol. Infect. 111:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vila J, Pal T. 2010. Update on antibacterial resistance in low-income countries: factors favoring the emergence of resistance. Open Infect. Dis. J. 4:38–54 [Google Scholar]
- 27. Cutland CL, Madhi SA, Zell ER, Kuwanda L, Laque M, Groome M, Gorwitz R, Thigpen MC, Patel R, Velaphi SC, Adrian P, Klugman K, Schuchat A, Schrag SJ. 2009. Chlorhexidine maternal-vaginal and neonate body wipes in sepsis and vertical transmission of pathogenic bacteria in South Africa: a randomised, controlled trial. Lancet 374:1909–1916 [DOI] [PubMed] [Google Scholar]
- 28. Hansen SM, Sorensen UB. 2003. Method for quantitative detection and presumptive identification of group B streptococci on primary plating. J. Clin. Microbiol. 41:1399–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaves M, Jr, Padua RA, Campanerut PA, Pelloso SM, Carvalho MD, Siqueira VL, Scodro RB, Cardoso RF. 2010. Preliminary evaluation of Hitchens-Pike-Todd-Hewitt medium (HPTH) for detection of group B streptococci in pregnant women. J. Clin. Lab. Anal. 24:403–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gil EG, Rodriguez MC, Bartolome R, Berjano B, Cabero L, Andreu A. 1999. Evaluation of the Granada agar plate for detection of vaginal and rectal group B streptococci in pregnant women. J. Clin. Microbiol. 37:2648–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Church DL, Baxter H, Lloyd T, Miller B, Elsayed S. 2008. Evaluation of StrepB carrot broth versus Lim broth for detection of group B streptococcus colonization status of near-term pregnant women. J. Clin. Microbiol. 46:2780–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Munson E, Napierala M, Munson KL, Culver A, Hryciuk JE. 2010. Temporal characterization of carrot broth-enhanced real-time PCR as an alternative means for rapid detection of Streptococcus agalactiae from prenatal anorectal and vaginal screenings. J. Clin. Microbiol. 48:4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]