Abstract
A method for extracting poliovirus (PV) from stool extracts was developed. Magnetic nanoparticles sensitized with soluble PV receptor efficiently extracted PV pseudovirus (>99% extraction) or endogenous infectious PVs (>90% extraction) from stool extracts. This method would be useful for extraction of PV from crude biological samples.
TEXT
Laboratory diagnosis plays a critical role in the Global Polio Eradication Initiative by isolating and identifying poliovirus (PV) from the stool samples of acute flaccid paralysis (AFP) cases, which include polio and other paralytic cases. In the World Health Organization (WHO) Global Polio Laboratory Network, PV isolation has been performed based on the cell culture system using a human rhabdomyosarcoma cell line (RD cells) and a mouse L cell line expressing PV receptor (PVR; L20B cells) (1, 2). The advantages of cell culture-based procedure are (i) no requirement for equipment for molecular diagnosis and (ii) high sensitivity (detection limit of 1 infectious dose that contains 50 to 1,000 virions in picornavirus infection) (3). The disadvantages are the requirement for expertise of responsible personnel for the cell culture system and cytopathic effect (CPE) observation and speed of reporting: it takes 10 days to confirm the sample as PV negative (2). To improve efficiency of detection in terms of speed, the development of direct detection methods for PV strains, especially for vaccine-derived PV (VDPV), from stool extracts and environmental samples has been encouraged by WHO (http://www.polioeradication.org/Research/Grantsandcollaboration.aspx).
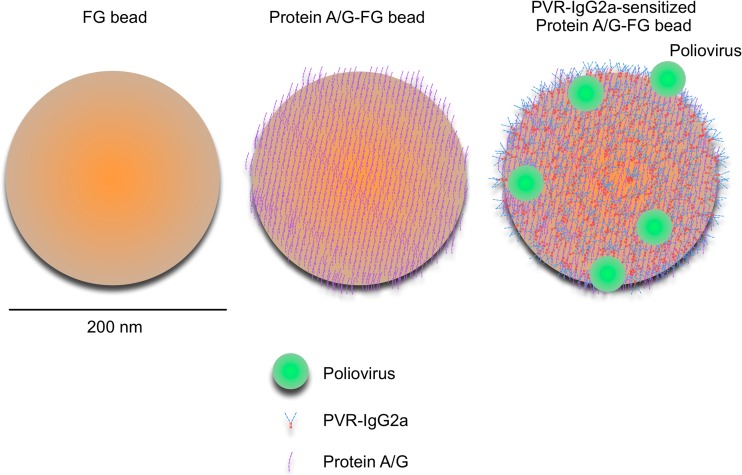
The major challenge in current detection methods for PV is the low sensitivity (e.g., 25,000 copies for detection of the Sabin 1 strain in the real-time reverse transcription-PCR [RT-PCR] system) (4–6). To increase the amount of PV available for the detection assays, I attempted to develop a method for extraction of PV from stool extracts by using soluble PV receptor (PVR) with magnetic nanoparticles. The utility of soluble PV receptor in a form of immunoadhesin (PVR-IgG2a), which is an immunoadhesin molecule consisting of extracellular domains of PVR and Fc domains of mouse IgG2a (7) (Fig. 1), for identification of PV isolates has been shown in a particle agglutination assay (8). The magnetic nanoparticles (FG beads; Tamagawa Seiki Co., Ltd.) have a diameter of 200 nm, and their utility has been established for efficient capture of ligands of small molecules (9, 10). Approximately 1,600 molecules of protein A/G (Thermo Scientific) were immobilized per bead via activated N-hydroxysuccinimide ester on the beads (visualized in Fig. 1, middle). The beads were sensitized with PVR-IgG2a by noncovalent binding via protein A/G. The concentration of the beads was 10 mg/ml (1.8 × 1012 beads per ml). The activities of PVR-IgG2a and protein A/G-immobilized beads were stable for >6 months and >4 months at 4°C, respectively. Experiments were performed with 3 different lots of the beads.
Fig 1.
Schematic view of magnetic nanobead sensitized with soluble PV receptor. Original magnetic nanobead (FG bead; left), magnetic nanobead coated with protein A/G (middle), and PVR-IgG2a-sensitized magnetic nanobead (right) are shown with protein A/G, PVR-IgG2a, and PV of actual size. Protein A/G is a recombinant chimeric protein that consists of 4 Fc-binding domains of protein A and 2 Fc-binding domains of protein G. Bar, 200 nm. Approximately 1,600 molecules of protein A/G were immobilized on a magnetic nanobead. The concentration of the magnetic beads used in this study was 1.8 × 1012 beads per ml.
The specificity and efficiency of extraction from cell culture medium (Dulbecco's modified Eagle's medium) were evaluated with PV pseudoviruses (PVpv) prepared in HEK293 cells and titrated in HEp-2c cells, which encapsidated luciferase-encoding PV replicon in capsid proteins derived from type 1 PV(Mahoney), type 2 PV(MEF-1), or type 3 PV(Saukett A) (11, 12). Type 1 PVpv (PV1pv, 1,000 infectious units [IU] in 100 μl cell culture medium) was efficiently extracted by PVR-IgG2a-sensitized beads with 0.13 μl (2.3 × 105 beads, >90% extraction) or 0.25 μl (4.5 × 105 beads) of the bead solution (>99% extraction) (Fig. 2A, left). Viral genomes were associated with the beads, indicating that PV was extracted by the beads rather than inactivated in the supernatant (Fig. 2A, right). Mock-sensitized beads showed nonspecific extraction of PV1pv (11% to 23% extraction) in a dose-independent manner. Extraction of PV3pv was less efficient than those of PV1pv and PV2pv. To achieve >90% extraction for 1,000 IU of PV3pv, 0.25 μl of the bead solution was required. For 10 IU of PVpv, efficient extraction (>95% extraction) was observed with only 0.13 μl of the bead solution.
Fig 2.
Extraction of PVpv from cell culture medium by magnetic nanobeads. (A) Extraction of PV1pv or PV1(Sabin) from the cell culture medium by mock-sensitized or PVR-IgG2a-sensitized magnetic nanobeads. (Left) The indicated amounts of the beads were added to the cell culture medium containing PV1pv (1,000 IU in 100 μl) and were then incubated at 4°C for 1 h. The beads were collected with a magnetic separator, and then the titer of PV1pv remaining in the supernatant was measured. The efficiency of extraction (%) by the beads is shown. (Right) The indicated amounts of the PVR-IgG2a-sensitized magnetic beads were added to the cell culture medium containing PV1(Sabin) (1.0 × 105 50% cell culture infective doses [CCID50] in 100 μl) and were then incubated at 4°C for 1 h. The beads were collected with a magnetic separator, and then the numbers of copies of viral genome on the beads and in the supernatant were determined by real-time RT-PCR. The numbers of copies of viral genome in supernatant without the beads were taken as 100%. (B) The indicated amounts of PVR-IgG2a-sensitized magnetic nanobeads were added to the cell culture medium containing PVpv (1,000, 100, and 10 IU in 100 μl) and were then incubated at 4°C for 1 h. The beads were collected with a magnetic separator, and then the titer of PVpv remaining in the supernatant was measured. The efficiency of extraction (%) by the beads is shown.
The efficiency of extraction of PV1pv exogenously added to the stool extracts (approximately 2% [wt/vol] extract in phosphate-buffered saline, derived from polio cases; total of 7 samples, including 5 PV-positive samples) was evaluated (Fig. 3A). Extraction of PV1pv from the stool extracts was less efficient than that from cell culture medium, and 4.0 μl of the bead solution was required for >90% extraction of PV1pv from the stool extracts (100 μl). Next, the efficiency of extraction of endogenous PV strains (all of them were previously identified as Sabin-like strains) from the stool extracts that were positive for PV by the cell culture method (samples 2, 3, 4, 5, and 6) was examined (Fig. 3B). Extraction efficiency was evaluated by the appearance of CPE of L20B cells after inoculation of the stool extract with or without treatment by PVR-IgG2a-sensitized beads. Ninety to 99% reduction of the titer of PV1(Sabin) (control sample) caused delay in the appearance of CPE (4+) of the inoculated cells by 1 day. Appearance of CPE of L20B cells inoculated with the stool extracts (100 μl) was delayed 1 day by the treatment with 1 or 5 μl of PVR-IgG2a-sensitized beads, suggesting >90% extraction of endogenous infectious PV strains. The efficiency of extraction of endogenous PV strains by PVR-IgG2a-sensitized beads was also evaluated by recently developed Sabin strain-specific real-time reverse transcription-PCR (RT-PCR) (13), with modifications (Table 1). Endogenous PV was efficiently extracted from samples 3 and 4 (>60% to almost 100%). However, for samples 1, 2, and 3, the extraction efficiency was low (11% to 22%). These results suggested that PVR-IgG2a-sensitized beads efficiently extract infectious virus from the stool extracts and also that there are substantial amounts of noninfectious virus in some stool extracts.
Fig 3.
Extraction of PVpv and endogenous PV from the stool extracts by PVR-IgG2a-sensitized magnetic nanobeads. (A) Extraction of PV1pv exogenously added to the stool extracts derived from polio cases. The indicated amounts of the beads were added to the stool extracts containing PV1pv (10,000 IU in 100 μl) and were then incubated at 4°C for 1 h. The beads were collected with a magnetic separator, and then the titer of PV1pv remaining in the supernatant was measured. Infectivity of PV1pv in the stool extracts without the beads is also shown (black bars). The efficiency of extraction (%) by the beads and PV1pv infection (%) in the stool extracts (the final concentration of the stool extracts was 5% [vol/vol]) are shown. The infectivity of PV1pv in the cell culture medium without the stool extracts was taken as 100%. (B) Extraction of endogenous PVs from the stool extracts derived from polio cases. PV-positive stool extracts were treated with or without the indicated amounts of the beads, and then the residual infectivity of the extracts was analyzed in L20B cells. The cells were observed for CPE for 5 days after inoculation. CPE scores of +1, +2, +3, and +4 indicate the appearance of CPE in approximately 25, 50, 75, and 100% of the cells, respectively. PV1(Sabin) of the indicated titer was inoculated into the cells as a control. p.i., postinfection. (C) Extraction of PV3pv exogenously added to the stool extracts derived from AFP cases. Four microliters of the beads was added to the stool extracts containing PV3pv (20,000 IU in 100 μl) and was then incubated at 4°C for 1 h. The beads were collected with a magnetic separator, and then the titer of PV3pv remaining in the supernatant was measured. Infectivity of PV3pv in the stool extracts without the beads is also shown. The efficiency of extraction (%) by the beads and PV3pv infection (%) in the stool extracts without the beads (the final concentration of the stool extracts was 5% [vol/vol]) are shown. The infectivity of PV3pv in the cell culture medium without the stool extracts was taken as 100%. The numbers of the samples above or below 90% extraction are shown.
Table 1.
Extraction of endogenous PV from the stool extractsa
| Patient | Viral genomic RNA source | Real-time PCR with primers for: |
PV detected by real-time PCR | PV isolated by cell culture system | ||
|---|---|---|---|---|---|---|
| Sabin 1 | Sabin 2 | Sabin 3 | ||||
| 1 | Sample 1 | (11) | 260 | ND | Sabin 2 | Negative |
| Sample 2 | (12) | 560 | ND | Sabin 2 | PV2-Sabin-like | |
| 2 | Sample 3 | 80 | 8.6E+05 | 1.9E+05 | Sabin 1, 2, and 3 | PV2,3-Sabin-like |
| 3 | Sample 4 | 6.6E+04 | 5.3E+04 | 850 | Sabin 1, 2, and 3 | PV1,2-Sabin-like |
| Sample 5 | 5.3E+05 | 8.2E+05 | 3.5E+03 | Sabin 1, 2, and 3 | PV1,2-Sabin-like | |
| 4 | Sample 6 | 170 | 46 | (10) | Sabin 1 and 2 | PV1-Sabin-like |
| Sample 7 | 150 | 38 | ND | Sabin 1 and 2 | NPV | |
| 1 | Beads from sample 1 | ND | 37 | ND | Sabin 2 | Negative |
| Beads from sample 2 | ND | 62 | ND | Sabin 2 | PV2-Sabin-like | |
| 2 | Beads from sample 3 | ND | 1.9E+05 | 3.0E+04 | Sabin 2 and 3 | PV2,3-Sabin-like |
| 3 | Beads from sample 4 | 4.3E+04 | 6.7E+04 | 410 | Sabin 1, 2, and 3 | PV1,2-Sabin-like |
| Beads from sample 5 | 3.1E+05 | 9.3E+05 | 2.3E+03 | Sabin 1, 2, and 3 | PV1,2-Sabin-like | |
| 4 | Beads from sample 6 | 76 | ND | ND | Sabin 1 | PV1-Sabin-like |
| Beads from sample 7 | (37) | ND | ND | Negative | NPV | |
Viral genomic RNAs were purified from 100 μl of the stool extracts (samples 1 to 7) or from PVR-IgG2a-sensitized beads (5 μl) after incubation with 100 μl of the stool extracts (beads from samples 1 to 7) and were subjected to real-time RT-PCR. Real-time RT-PCR was performed with redesigned primers for Sabin 1 (5′-CTCAGCTTCCACCAAGAATAAGG-3′ and 5′-TAAAAGATTGATGGATTTGATGAGG-3′) and Sabin 3 (sense primer, 5′-GGGAAAATTTTACTCCCAATTCAAC-3′) along with the original primers for Sabin 2 and Sabin 3 (antisense primer) by using the One Step SYBR PrimeScript Plus RT-PCR kit (TaKaRa) with an Applied Biosystems 7500 Fast real-time PCR system (Applied Biosystems), a reverse transcription step at 42°C for 30 min, and 40 cycles of thermal cycling of 95°C for 3 s and 60°C for 60 s. The numbers of copies of viral genome are shown. The numbers of copies below the quantification limit of the assay are shown in parentheses. ND, not detected; NPV, nonpoliovirus.
Finally, the efficiency of extraction of PV3pv exogenously added to the stool extracts derived from nonpolio AFP cases was examined (total of 150 samples, including 35 samples with NPV) (Fig. 3C; see also Table S1 in the supplemental material). PV3pv infectivity in some stool extracts was severely impaired. Twenty-five stool extracts showed reduced PV3pv infectivity below 3× standard deviation from the average (>42% reduction of the infectivity) (25/150, 17% of samples), and there seems to be some correlation between the infectivity and the extraction efficiency. Nevertheless, PVR-IgG2a-sensitized beads efficiently extracted PV3pv from most of the stool extracts (>90% extraction for 144/150, 96% of samples).
These magnetic nanoparticles would be useful for concentrating PV from a large volume of stool extracts and environmental water and helpful for the development of direct detection and characterization of wild-type PV strains from biological samples.
Supplementary Material
ACKNOWLEDGMENTS
I am grateful to Junko Wada for her excellent technical assistance and to Hiroyuki Shimizu for kind and helpful discussions.
This report is based on research funded by the Bill & Melinda Gates Foundation and in part by grants-in-aid for the Promotion of Polio Eradication and Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor and Welfare, Japan.
The findings and conclusions contained within are those of the author and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation.
Footnotes
Published ahead of print 22 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00499-13.
REFERENCES
- 1. Wood DJ, Hull B. 1999. L20B cells simplify culture of polioviruses from clinical samples. J. Med. Virol. 58:188–192 [PubMed] [Google Scholar]
- 2. World Health Organization 2004. Polio laboratory manual (4th ed), WHO/IVB/04.10, and supplement to the WHO polio laboratory manual. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3. Rueckert RR. 1996. Picornaviridae: the viruses and their replication, p 609–654 In Fields BN, Knipe DM, Howley PM. (ed), Fields virology, 3rd ed, vol 1 Lippincott-Raven, Philadelphia, PA [Google Scholar]
- 4. Kilpatrick DR, Nottay B, Yang CF, Yang SJ, Mulders MN, Holloway BP, Pallansch MA, Kew OM. 1996. Group-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosine residue at positions of codon degeneracy. J. Clin. Microbiol. 34:2990–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kilpatrick DR, Nottay B, Yang CF, Yang SJ, Da Silva E, Penaranda S, Pallansch M, Kew O. 1998. Serotype-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosine residues at positions of codon degeneracy. J. Clin. Microbiol. 36:352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kilpatrick DR, Yang CF, Ching K, Vincent A, Iber J, Campagnoli R, Mandelbaum M, De L, Yang SJ, Nix A, Kew OM. 2009. Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. J. Clin. Microbiol. 47:1939–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arita M, Horie H, Arita M, Nomoto A. 1999. Interaction of poliovirus with its receptor affords a high level of infectivity to the virion in poliovirus infections mediated by the Fc receptor. J. Virol. 73:1066–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arita M, Masujima S, Wakita T, Shimizu H. 2010. Development of a particle agglutination method with soluble virus receptor for identification of poliovirus. J. Clin. Microbiol. 48:2698–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. 2010. Identification of a primary target of thalidomide teratogenicity. Science 327:1345–1350 [DOI] [PubMed] [Google Scholar]
- 10. Ito Y, Ito T, Karasawa S, Enomoto T, Nashimoto A, Hase Y, Sakamoto S, Mimori T, Matsumoto Y, Yamaguchi Y, Handa H. 2012. Identification of DNA-dependent protein kinase catalytic subunit (DNA-PKcs) as a novel target of bisphenol A. PLoS One 7:e50481. 10.1371/journal.pone.0050481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arita M, Nagata N, Sata T, Miyamura T, Shimizu H. 2006. Quantitative analysis of poliomyelitis-like paralysis in mice induced by a poliovirus replicon. J. Gen. Virol. 87:3317–3327 [DOI] [PubMed] [Google Scholar]
- 12. Arita M, Iwai M, Wakita T, Shimizu H. 2011. Development of a poliovirus neutralization test with poliovirus pseudovirus for measurement of neutralizing antibody titer in human serum. Clin. Vaccine Immunol. 18:1889–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laassri M, Dipiazza A, Bidzhieva B, Zagorodnyaya T, Chumakov K. 2013. Quantitative one-step RT-PCR assay for rapid and sensitive identification and titration of polioviruses in clinical specimens. J. Virol. Methods 189:7–14 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.