Abstract
To determine the long-term carriage patterns, strain relatedness, and incidence of subsequent infections among methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) carriers, we screened 154 high school students for nasal carriage of S. aureus on 8 occasions over 11 months. Persistent carriage was defined as a positive culture on ≥7 occasions. Two consecutive isolates from the same subject comprised a pair, and strain relatedness was determined for each pair by molecular typing. Of 1,232 nasal swab cultures obtained on 8 occasions, 323 (26.2%) were positive for S. aureus. Forty-five isolates (3.7%) were MRSA and 278 isolates (22.6%) were MSSA from 12 and 63 subjects, respectively. Thirty-five (77.8%) MRSA isolates harbored a type IV or VT staphylococcal chromosomal cassette mec element. Among the 154 subjects, 52 (33.8%) were intermittent (1 to 6 positive swabs) carriers. Persistent carriage was identified in 23 (14.9%) subjects, and the incidence was not significantly different for MRSA and MSSA carriers (3/12 [25%] versus 20/63 [31.7%]; P = 0.7449). The MRSA and MSSA isolates were composed of 33 and 215 strain pairs, respectively. Of them, an indistinguishable genotype was identified in 33 (100%) MRSA pairs and 173 (80.5%) MSSA pairs (P = 0.0053). Five subjects developed cellulitis, and the incidence of this was higher for MRSA carriers (2/12 [16.7%]) than for MSSA carriers (1/63 [1.58%]; P = 0.0632) and noncarriers (2/79 [2.56%]; P = 0.0828). In conclusion, the long-term carriage patterns for MRSA and MSSA in healthy individuals were similar. MRSA carriers were more likely to carry a single strain, with a trend toward a higher chance of developing cellulitis than for MSSA carriers.
INTRODUCTION
Staphylococcus aureus is an extraordinarily versatile pathogen that can survive under hostile external environmental conditions, colonize mucous membranes and skin, and cause severe nonpurulent toxin-mediated disease or severe invasive purulent infections in humans (1, 2). Carriage of S. aureus in the nose is a known risk factor for the development of subsequent staphylococcal infection in selected populations (3, 4). Data obtained from longitudinal studies, mostly on methicillin-susceptible S. aureus (MSSA), suggest that there are 3 major patterns of S. aureus colonization in the general population, which are persistent carriage (20%), intermittent carriage (30%), and noncarriage (50%) (3, 5). Persistent carriers appear to have higher rates of subsequent infections and lower levels of immunoglobulin to staphylococcal antigens than those in the other 2 groups (4, 5).
In the past decade, infections caused by methicillin-resistant S. aureus (MRSA) strains have been increasingly identified in previously healthy hosts. MRSA has been the dominant cause of community-associated (CA) skin and soft tissue infections in many regions of the world (6, 7). The long-term carriage pattern of CA-MRSA in healthy hosts is less commonly reported and may be distinct from currently known patterns, which are mostly derived from observations on MSSA. In Taiwan, MRSA has accounted for >50% of childhood CA infections since 2005 (8). Carriage of MRSA in the nose was identified in 7.8% and 3.7% of the pediatric and adult populations in Taiwan, respectively (7, 9). The individuals with an increased risk of MRSA carriage included very young children (2 to 6 months old), those living in a crowded environment (i.e., with greater number of siblings, attending day care), adults with household members <7 years old, or those who received antibiotic treatment in the preceding year.
To better understand the carriage pattern of MRSA, the strain relatedness of consecutive isolates, and the incidence of subsequent infection in healthy individuals, we conducted an 11-month study investigating the status of S. aureus colonization in healthy individuals from a region in which MRSA is endemic.
MATERIALS AND METHODS
Ethical statements.
All 10th-grade students registered in a high school in the suburban areas of northern Taiwan were invited to participate in this study. Students were enrolled if informed consent was obtained from both the participants and their parents or legal guardians. This study was approved by the institutional review board of Chang Gung Memorial Hospital in May 2010.
Study design.
This 11-month longitudinal study was conducted from August 2010 to July 2011. A standardized questionnaire-based interview was used to collect the epidemiological information of each enrolled subject. The collected information included demographics, underlying conditions, lifestyle and health conditions, and conditions of the household family members (Table 1). These variables were hypothesized as being potential factors that are associated with S. aureus and/or MRSA colonization. The data from the written questionnaires were digitized and cleaned before being processed by statistical analysis.
Table 1.
Univariate analysis of epidemiologic factors associated with MRSA and MSSA nasal carriage in 154 high school students in northern Taiwan during 2010 to 2011
| Epidemiological factor | Total (n = 154) | MRSA carriers (n = 12) | MSSA carriers (n = 63) | Non-carriers (n = 79) | MRSA carrier vs noncarrier (P)a | MSSA carrier vs noncarrier (P)a |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Female gender (no. [%]) | 72 (46.8) | 7 (58.3) | 22 (34.9) | 43 (54.4) | NS | 0.0204 |
| Weight (mean ± SD) (kg) | 57.9 ± 11.7 | 56.4 ± 6.4 | 59.5 ± 11.7 | 56.6 ± 12.2 | NS | NS |
| Height (mean ± SD) (cm) | 164.9 ± 7.4 | 164.9 ± 5.0 | 165.8 ± 6.7 | 164.2 ± 8.1 | NS | NS |
| Lifestyle or health condition (no. [%]) | ||||||
| Regular exercise | 73 (47.4) | 7 (58.3) | 31 (49.2) | 35 (44.3) | NS | NS |
| Play sports with vigorous body contact | 40 (26.0) | 2 (16.7) | 20 (31.8) | 18 (22.8) | NS | NS |
| Frequent exercise (>10 h per week) | 8 (5.2) | 1 (8.3) | 5 (7.9) | 2 (2.5) | NS | NS |
| Frequent alcohol consumption (>12 episodes in past year) | 5 (3.3) | 0 (0) | 2 (3.2) | 3 (3.8) | NS | NS |
| Smoking | 2 (1.3) | 0 (0) | 0 (0) | 2 (2.5) | NS | NS |
| Hepatitis | 1 (0.7) | 0 (0) | 1 (1.6) | 0 (0) | NS | |
| Diabetes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Resident of dormitory | 5 (3.3) | 0 (0) | 2 (3.2) | 3 (3.8) | NS | NS |
| Shower every day | 152 (98.7) | 12 (100) | 62 (98.4) | 78 (98.7) | NS | NS |
| Tattoo(s) or piercing(s) | 15 (9.7) | 2 (16.7) | 3 (4.8) | 10 (12.7) | NS | NS |
| Regular visits to health care facility | 13 (8.4) | 3 (25) | 7 (11.1) | 3 (3.8) | 0.0261b | 0.0659 |
| Recent cutaneous infection (≤3 mo) | 8 (5.2) | 0 (0) | 2 (3.2) | 6 (7.6) | NS | NS |
| Atopic dermatitis | 8 (5.2) | 0 (0) | 1 (1.6) | 7 (8.9) | NS | 0.0618 |
| Recent clinic visit (<3 mo) | 82 (53.3) | 8 (66.7) | 37 (58.7) | 37 (46.8) | NS | NS |
| Hospitalization in the past year | 6 (3.9) | 0 (0) | 2 (3.2) | 4 (5.1) | NS | NS |
| Recent antibiotic use (<3 mo) | 12 (7.8) | 2 (16.7) | 3 (4.8) | 7 (8.9) | NS | NS |
| Condition of household members | ||||||
| No. in household (mean ± SD) | 4.4 ± 1.2 | 4.5 ± 1.2 | 4.4 ± 1.3 | 4.3 ± 1.1 | NS | NS |
| Works in medical facility (no. [%]) | 13 (8.4) | 3 (25) | 5 (7.9) | 5 (6.3) | 0.0583b | NS |
| Long-term bedridden for any member (no. [%]) | 8 (5.2) | 2 (16.7) | 2 (3.2) | 4 (5.1) | NS | NS |
| Lives with younger sibling (no. [%]) | 82 (53.3) | 9 (75) | 37 (58.7) | 36 (45.6) | 0.0426b | NS |
| No. of younger siblings (mean ± SD) | 1.3 ± 0.7 | 1.3 ± 0.5 | 1.4 ± 0.9 | 1.2 ± 0.4 | NS | NS |
| Poor economic conditions (no. [%]) | 26 (16.9) | 2 (16.7) | 9 (14.3) | 15 (19.0) | NS | NS |
NS, nonsignificant.
Fisher's exact test.
A cotton swab sample from both anterior nares was obtained from each participant for S. aureus isolation by 2 investigators (S.-C.W. and H.-Y.C.) and a study assistant. The nasal swabs were immediately placed in transport medium (Venturi Transystem; Copan Innovation Ltd., Limerick, Ireland) and transported to the microbiology laboratory at Chang Gung Memorial Hospital for the detection of S. aureus by standard methods. The nasal swab was performed at monthly intervals during the first 5 months and at 3-month intervals during the last 6 months of the study. A total of 8 nasal swabs were thus obtained from each participant during the 11 months of the study. Persistent and intermittent carriages were defined as 7 or 8 positive cultures and 1 to 6 positive cultures, respectively, on 8 nasal swab-sampling occasions.
Active surveillance of staphylococcal diseases.
All participants were asked to report any illness during the study period. In addition, at the time of obtaining nasal swabs, acute cutaneous lesions were investigated by the 2 investigators (S.-C.W. and H.-Y.C.). The health condition of each subject was also surveyed by phone calls every two weeks. Subjects with putative staphylococcal diseases were transferred to clinics of the Chang Gung Memorial Hospital and were reassessed by the primary investigator (C.-J.C.). Specimens from the site of the acute cutaneous lesions, if available, were collected for the culture of S. aureus. Such subjects were followed until the total resolution of the symptoms and signs of infections was confirmed.
Characterization of S. aureus isolates.
Identification of S. aureus was performed according to the standard procedure described elsewhere (7), and MRSA status was confirmed according to Clinical and Laboratory Standards Institute 2011 guidelines. The isolates were further characterized using molecular methods. Protein A gene (spa) typing was performed for each S. aureus isolate. The polymorphic X region of spa was amplified by PCR with the primers 1095F (5′-AGACGATCCTTCGGTGAGC-3′) and 1517R (5′-GCTTTTGCAATGTCATTTACTG-3′) (10). PCR products were sequenced and spa types were assigned by analysis of the nucleotide sequences using BioNumerics version 6.5 (Applied Maths NV). The isolates that we could not spa type were determined by multilocus sequencing typing (MLST) (http://www.mlst.net) (11). Allelic profiles were assigned through a comparison of the sequences from each locus with the sequences of the known alleles in the S. aureus MLST database, and these were defined accordingly as sequence types. Pulsed-field gel electrophoresis (PFGE) was performed with strains for which the spa types or MLST could not be determined. The PFGE typing and analysis of PFGE patterns are described elsewhere (12).
The presence of Panton-Valentine leukocidin (PVL) genes was determined in all S. aureus isolates by a PCR technique described by Lina et al. (13). Staphylococcal cassette chromosome mec element (SCCmec) typing of MRSA isolates was performed using a multiplex PCR strategy described in a previous study (14). The control strains for SCCmec types I, II, III, and IVa, kindly provided by Keiichi Hiramatsu, were S. aureus NCTC10442, N315, 85/2082, and JCSC4744, respectively. SCCmec typing for type VT was determined by using a particular primer described elsewhere (15), and S. aureus strain TSGH-17, kindly provided by Chi-Chien Wang, was used as a control.
Determination of genetic relatedness among consecutive isolates.
Two consecutive isolates from the same participant comprised a strain pair. Strain relatedness was investigated in isolates accounting for each pair. Isolates in a pair were interpreted as indistinguishable if they had the same profile with regard to spa typing, MLST or PFGE pattern, and PVL gene carriage. Carriage of the same SCCmec type was required for defining indistinguishable strains in MRSA pairs.
Statistics.
A comparison of categorical variables between participants with different carriage patterns (persistent versus noncarriage) and different strains (MRSA versus MSSA) was performed using a chi-square test, or with Fisher's exact test, where appropriate, while differences in numerical variables were tested by a two-sample t test or nonparametric Wilcoxon two-sample test, where appropriate. Multiple logistic regression analysis was applied to explore the factors associated with MRSA and MSSA carriage. Only significant factors (P < 0.1) from the univariate comparison were included in the final model of multivariate analysis. Statistical significance was defined as a P value of <0.05 in these tests. The data were analyzed with SAS software version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Epidemiological features and yield rate of nasal swab cultures.
A total of 163 high school students were recruited to this study. Nine subjects who withdrew from the study or did not complete all nasal swabs for any reason were excluded from the final analysis. Of the remaining 154 participants, 72 (46.8%) were female. The participants were distributed among 15 classes with a median of 9 participants in each class (range, 1 to 20). The demographics and epidemiological features of the participants are displayed in Table 1.
Across the 8 nasal swab-sampling occasions, a total of 1,232 nasal swab samples were obtained from the 154 participants. Cultures from these swabs yielded 323 (26.2%) S. aureus strains, including 45 MRSA (3.7%) and 278 (22.6%) MSSA strains. The detailed carriage rates of MRSA and MSSA from each sampling occasion in the survey are shown in Fig. 1. Among 45 MRSA isolates, 7 genotypes were identified by a combination of spa types, SCCmec types, and the presence of PVL genes. spa type t437 (equivalent to sequence type 59 [ST59]) and SCCmec type IV/VT were predominant and were identified in 31 (68.9%) and 35 (77.8%) MRSA isolates, respectively. PVL genes were identified in 18 (40%) MRSA isolates, which is significantly higher than the number identified in MSSA isolates (9 [3.2%]; P < 0.0001).
Fig 1.
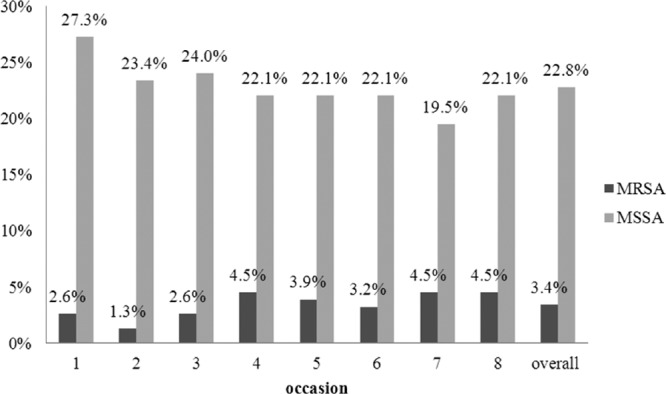
Carriage rates of MRSA and MSSA in the nares of 154 adolescents as documented from nasal swab cultures sampled on 8 occasions during an 11-month survey.
Factors associated with MRSA and MSSA carriage.
Of 154 participants, MRSA carriage and MSSA carriage at any sampling occasion in the survey were identified in 12 (7.8%) and 63 (40.9%) subjects, respectively. The epidemiological factors associated with MRSA and MSSA carriage are displayed in Table 1. Multiple logistic regression analysis identified having a family member who works at a health care facility (25% versus 6.3%; adjusted odds ratio [aOR], 8.98; P = 0.0184), having young children at home (75% versus 45.6%; aOR, 6.46; P = 0.0318), and making regular visits to health care facilities (25% versus 3.8%; aOR, 23.8; P = 0.0042) as independent factors for MRSA carriage (Table 2). There was no significant factor associated with increased risk of MSSA carriage. Female gender appeared to be a protective factor against MSSA carriage and accounted for 34.9% of MSSA carriers and 54.4% of noncarriers (aOR, 0.45; P = 0.0256) (Table 2).
Table 2.
Multivariate analysis of risk factors associated with MRSA and MSSA carriage in adolescentsa
| Risk factor | MRSA carrier vs noncarrier |
MSSA carrier vs noncarrier |
||
|---|---|---|---|---|
| aORb (95% CI) | P | aORb (95% CI) | P | |
| Female gender | 0.45 (0.22–0.91) | 0.0256 | ||
| Household member working in health care facility | 8.98 (1.4–55.63) | 0.0184 | ||
| Younger sibling at home | 6.46 (1.18–35.46) | 0.0318 | ||
| Regular visits to health care facility | 23.83 (2.72–209.01) | 0.0042 | 4.34 (0.96–19.58) | 0.0565 |
| Atopic dermatitis | 0.18 (0.02–1.57) | 0.1191 | ||
Only significant factors in univariate analysis (P < 0.1) were included in the final model of the multiple logistic regression analysis.
aOR, adjusted odds ratio; CI, confidence interval.
Carriage patterns and epidemiological factors of persistent carriage.
The incidences of persistent, intermittent, and noncarriage stratified by MRSA, MSSA, and S. aureus are shown in Table 3. Seventy-nine (51.3%) subjects did not carry S. aureus at any sampling occasion. Persistent carriage was identified in 23 (14.9%) subjects, including 3 MRSA and 20 MSSA carriers. The incidences of persistent carriage were not significantly different between MRSA carriers and MSSA carriers (3/12 versus 20/64; P = 0.7449, Fisher's exact test) (Table 3). Frequent visits to medical facilities for any reason was the only significant epidemiological factor associated with persistent carriage of S. aureus, and it was more frequently identified in persistent carriers than in noncarriers (4/23 [17.4%] versus 3/79 [3.8%]; P = 0.0440, Fisher's exact test).
Table 3.
Incidences of subjects with persistent, intermittent, and noncarriage of nasal S. aureus among 154 high school students
| Organism carried by subjects | Carriage pattern (no. [%]) |
||
|---|---|---|---|
| Persistent | Intermittent | Noncarriage | |
| MRSA | 3 (1.9) | 9 (5.8) | 142 (92.2) |
| MSSA | 20 (13.0) | 43 (28.1) | 91 (59.1) |
| S. aureus | 23 (14.9) | 52 (33.8) | 79 (51.3) |
The incidence of persistent carriage did not differ significantly between MRSA carriers and MSSA carriers (P = 0.7449, Fisher's exact test).
Strain relatedness of consecutive isolates in MRSA and MSSA carriers.
The strain relatedness of 45 MRSA isolates in 12 subjects and 20 MSSA persistent carriers is displayed in Tables 4 and 5. The MRSA isolates comprised 33 strain pairs, and all (100%) of them were of indistinguishable genotypes. For MSSA carriers, a total of 215 strain pairs were identified, and 173 (80.5%) pairs of these were of indistinguishable genotypes (Table 6). The overall incidence of carrying indistinguishable strains was significantly lower in MSSA carriers than in MRSA carriers (P = 0.0053). The difference remained significant when the comparison was made between the 2 groups of persistent carriers (80.0% versus 100%; P = 0.0263) (Table 6). The MSSA intermittent carriers also had a lower incidence of carrying indistinguishable strains than did MRSA intermittent carriers (81.3% versus 100%, respectively), though the difference did not reach statistical significance due to the relatively small number of strain pairs (P = 0.1155) (Table 6).
Table 4.
Genotypes of MRSA isolates from the nasal swabs obtained from 12 subjects on 8 sampling occasionsa
| Subject no. | Genotype identified on indicated nasal swab sampling occasion |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| 4 | t437+VT | t437+VT | t437+VT | t437+VT | t437+VT | t437+VT | t437+VT | t437+VT |
| 13 | t3520−II | t3520−II | t3520−II | t3520−II | t3520−II | t3520−II | t3520−II | |
| 14 | ST59−IV | ST59−IV | ST59−IV | ST59−IV | ST59−IV | ST59−IV | ST59−IV | |
| 26 | t437+VT | |||||||
| 29 | t8924−NTb | t8924−NTb | ||||||
| 41 | t015−IV | |||||||
| 49 | t3525−NTb | |||||||
| 97 | t437−IV | t437−IV | t437−IV | t437−IV | ||||
| 119 | t026−IV | t026−IV | t026−IV | |||||
| 123 | t437+VT | t437+VT | t437+VT | t437+VT | t437+VT | |||
| 142 | t437+VT | t437+VT | t437+VT | t437+VT | ||||
| 158 | t437−IV | t437−IV | ||||||
The genotype of each strain is presented as spa type (or sequence type [ST]), carriage of PVL genes is presented in superscript, and the SCCmec type is listed. For instance, t437+VT indicates spa type t437, carriage of PVL genes, and SCCmec type VT. Shaded cells indicate that genotypes in 2 consecutive isolates (pairs) were indistinguishable.
NT, nontypeable.
Table 5.
Genotypes of S. aureus isolates among 20 persistent MSSA carriersa
| Subject no. | Genotype identified on indicated nasal swab sampling occasion |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| 8 | t306− | t306− | t306− | t306− | t267− | t306− | t306− | t306− |
| 17 | t267− | t267− | t267− | t267− | t267− | t267− | t267− | t267− |
| 37 | t5355− | t5355− | t1445+ | t5355− | t5355− | t5355− | t5355− | t5355− |
| 46 | ST96− | ST96− | ST96− | t306− | ST96− | ST96− | ST96− | t437− |
| 60 | t144− | t144− | t144− | t144− | t144− | t144− | t144− | t144− |
| 61b | BU− | BU− | BU− | ST2261− | t1509− | BU− | BU− | BU− |
| 66 | t2868− | t2868− | t2868− | t2868− | t2868− | t2868− | t2868− | t2868− |
| 67 | t3099− | t338− | t338− | t338− | t338− | t338− | t3099− | t521− |
| 80 | t091− | t091− | t091− | t091− | t091− | t091− | t091− | t148− |
| 85 | t437+ | t437+ | t437+ | t437+ | t437+ | t437+ | t437+ | t189− |
| 124 | t2868− | t2868− | t2868− | t2868− | t2868− | t2868− | t2868− | t2868− |
| 125 | t189− | t189− | t189− | t189− | t189− | t189− | t189− | t189− |
| 137 | t189− | t189− | t189− | t189− | t189− | t189− | t8914− | t2859− |
| 144 | t1492− | t1492− | t1492− | t1492− | t267− | ST15− | t081− | t081− |
| 154 | t8917− | t8917− | t8917− | t8917− | t8917− | t8917− | t8917− | t8917− |
| 52b | ST2258− | AV1− | ST2259− | t1509− | t1509− | t1509− | t189− | |
| 107 | t164− | t164− | t164− | t164− | t164− | t164− | t164− | |
| 134 | t8917− | t8917− | t8917− | t8917− | t8917− | t8917− | t8917− | |
| 143 | t437− | t437− | t437− | t437− | t437− | t437− | t437− | |
| 159 | t2859− | t1492− | t2859− | t2859− | t2859− | t2859− | t8914− | |
The genotype of each strain is presented as spa type (or sequence type [ST]), and carriage of PVL genes is presented in superscript. Shaded cells indicate that genotypes of 2 consecutive isolates (pairs) were indistinguishable.
Some strains from subjects 52 and 61 were genotyped by pulsed-field gel electrophoresis (types AV1 and BU, respectively), because they could not be determined by either MLST or spa typing.
Table 6.
Incidences of indistinguishable strain pairs among MRSA and MSSA carriers
| Type of carrier | Indistinguishable genotype incidence |
P | |||
|---|---|---|---|---|---|
| MRSA pairs |
MSSA pairs |
||||
| Total no. | No. (%) | Total no. | No. (%) | ||
| Persistent | 19 | 19 (100) | 135 | 108 (80.0) | 0.0263a |
| Intermittent | 14 | 14 (100) | 80 | 65 (81.3) | 0.1155a |
| Total | 33 | 33 (100) | 215 | 173 (80.5) | 0.0053 |
Fisher's exact text.
Subsequent infections.
During the 11 months of study, 5 subjects developed cellulitis involving the feet (2 cases), an arm (1 case), buttocks (1 case), and periorbital area (1 case). Unfortunately, no specimens were available for culture in these cases. Of the 5 subjects with infections, 2 subjects were MRSA intermittent carriers (see Table 4, subjects 119 and 142), 1 subject was an MSSA intermittent carrier who had 5 consecutive positive cultures from the 4th to 8th sampling occasions, and the other 2 subjects were S. aureus noncarriers. The infection incidence was higher for MRSA carriers (16.6%) than for MSSA carriers (1.58%) and noncarriers (2.53%), but this was not statistically significant (P = 0.0632 and P = 0.0828, respectively, by Fisher's exact test).
DISCUSSION
The results from the current study demonstrate that half of the Taiwanese adolescents had persistent (14.9%) or intermittent (33.8%) nasal carriage of S. aureus. These data are in agreement with those of previous investigations (3, 5). It is accepted that persistent carriers differ from intermittent carriers and noncarriers in many ways, including having higher bacterial loads in the nose, lower levels of immunoglobulins G and A against staphylococcal antigens, and a greater incidence of subsequent infections (16, 17). Studies have further demonstrated that persistent S. aureus carriers tend to carry the same strain, whereas intermittent carriers may carry different strains over time (18, 19). The identification of persistent carriers, particularly those carrying MRSA, followed by the implementation of effective measures to eliminate the carried strains, may help control the spread of MRSA in the community and lessen the burden of staphylococcal diseases (20).
However, after analyzing the genotypes of 2 consecutive isolates from the same subjects, we did not observe the above-mentioned differences between persistent carriers and intermittent carriers (see Table 6, indistinguishable genotypes). Rather, the most significant finding from the current study was the different behaviors of MRSA and MSSA strains in long-term nasal colonization. MRSA carriers tended to carry identical strains either persistently or intermittently, whereas approximately 20% of the MSSA carriers demonstrated changes in the strain carried during the study period, irrespective of whether they were persistent or intermittent carriers. This finding was unexpected and can be misleading due to the relatively small number of MRSA carriers in the current study and the fact that the MRSA strains were frequently predominantly limited clones in a defined environment (21, 22).
Indeed, previous molecular epidemiology studies of CA-MRSA indicated that a prevalent clone, ST59, was circulating in Taiwan and accounted for >80% of MRSA carriage isolates in both pediatric and adult populations (7, 9). The majority of nasal MRSA isolates in the current study were also of the ST59 clone (equivalent to spa type t437). Nevertheless, despite the predominance of ST59 in the environment of the study site, we observed intermittent non-ST59 MRSA carriers who were recolonized by their original strains after a 6- to 8-month interval (e.g., see Table 4, subjects 29 and 119).
Furthermore, MRSA ST59 consists of several subclones that can be distinguished by simultaneously applying multiple typing methods. The indistinguishable genotypes defined in the current study were determined by identical molecular characteristics of the strains, including spa type and SCCmec type, along with the presence of PVL genes. This classification method should largely minimize the misclassification of strain relatedness due to the colonization by a predominant clone.
MRSA strains appear to be capable of colonizing humans for an extended period of time. In a follow-up study involving patients with clinical culture or surveillance tests that were positive for MRSA, approximately 50% and 20% of patients remained colonized by MRSA after 1 year and 4 years later, respectively, although the strain relatedness was not defined in that study (23). We believe that the different behaviors of MRSA and MSSA colonization observed in the current study are unlikely to be markedly biased. It appears that MRSA differs from MSSA not only because it harbors resistance to antimicrobial agents but also because it may involve interaction with hosts during nasal colonization.
Risk factors for MRSA colonization were usually evaluated at the time of hospital admission but were less commonly reported in healthy individuals. In 1 large-scale surveillance study, we investigated the epidemiological factors associated with MRSA colonization among 6,057 healthy children (7). Young children (i.e., age 2 to 6 months old) and children who spent much time in a crowded environment (i.e., with a greater number of children in the family, or attending day care) had a significantly higher risk of MRSA colonization. This finding was supported by the observation in the current study that the adolescents with younger siblings were more likely to carry MRSA than those without. Although a majority of the MRSA nasal isolates were of the CA genotype (i.e., SCCmec type IV or V), it was intriguing to note that exposures to health care facilities either directly (regular visits to health care facilities) or indirectly (household members who are medical staff) increased the risk of colonization. Hosts who had underlying conditions that required regular medical attention may partly explain this association. Alternatively, this observation may be due to the spread of CA-MRSA to hospitals. Indeed, we have noted an increasing trend of strains with CA genotypes being found in nosocomial MRSA bloodstream infections in the past few years (24, 25).
Data from the present study reflect that frequent visits to health care facilities was the only epidemiological factor associated with persistent carriage of S. aureus. A host factor may be implicated in the long-term carriage of S. aureus; unfortunately, we were unable to characterize these factors, such as the genetic differences between persistent and other types of carriers. Similar studies investigating the mechanisms of persistent carriage revealed several persistent carriage-associated genetic factors, including polymorphisms in the inflammatory response genes (i.e., C-reactive proteins, complement factor H, interleukin-4) and the gene encoding the glucocorticoid receptor (26, 27).
Gender appeared to be a significant factor affecting MSSA colonization in this adolescent population. The higher incidence of S. aureus colonization in males than in females has been identified in several studies, including a population-based study involving 9,622 persons aged ≥1 year old in the United States (28). Intriguingly, we did not recognize such an association in the surveillance study in Taiwanese children aged 2 to 60 months (7). The female sex hormones, particularly estrogen, which have a potent immune-modulating effect, may modify the host innate immunity and have an impact on S. aureus colonization. This speculation is supported by a recent study conducted in Germany, which demonstrated a substantial impact of hormonal contraceptives on S. aureus carriage in young women (29). Alternatively, gender may be a covariate to other significant epidemiological factors, such as personal hygiene habits; however, these data were not collected and analyzed in the current study.
Colonization with S. aureus has been identified as an important risk factor for the development of S. aureus infections in both community and hospital settings (4, 30). Evidence further suggests that colonization with MRSA or strains harboring virulence genes imposes a significantly greater risk for the development of subsequent infections than does colonization with MSSA (31). The enhanced virulence of CA-MRSA has been well recognized, and the underlying mechanism has gradually been revealed over the past few years (32–35). Although our data also suggest a greater virulence potential for MRSA strains, we were unable to draw any definite conclusion on autoinfections of the carried S. aureus strains due to the limited cases with subsequent infections, and because no responsible pathogen was isolated from the diseased subjects. Further study with a greater number of subjects is warranted to address this question.
To our knowledge, there has been no long-term regular follow-up study on MRSA carriage in healthy individuals. Our data suggest that healthy individuals with certain risks were more likely to be colonized with MRSA and to develop subsequent infections. The MRSA strains from the same individuals were usually of indistinguishable genotypes, whereas in 20% of MSSA carriers, irrespective of persistent or intermittent carriage, the carried strain changed during the period of carriage. Screening healthy individuals who are at risk for MRSA carriage, followed by effective decolonization measures, may help interrupt the spread of this pathogen and ease the burden of CA-MRSA disease.
ACKNOWLEDGMENTS
The study was supported by grants from Chang Gung Memorial Hospital (CMRPG490101 and CMRPG490102) and partly supported by grants from the National Science Council (NSC 100-2314-B-182A-025-MY2).
Footnotes
Published ahead of print 15 May 2013
REFERENCES
- 1. Archer GL. 1998. Staphylococcus aureus: a well-armed pathogen. Clin. Infect. Dis. 26:1179–1181 [DOI] [PubMed] [Google Scholar]
- 2. Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 [DOI] [PubMed] [Google Scholar]
- 3. Kluytmans J, van Belkum A, Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11–16 [DOI] [PubMed] [Google Scholar]
- 5. Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5:751–762 [DOI] [PubMed] [Google Scholar]
- 6. Creech CB, Jr, Kernodle DS, Alsentzer A, Wilson C, Edwards KM. 2005. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr. Infect. Dis. J. 24:617–621 [DOI] [PubMed] [Google Scholar]
- 7. Chen C-J, Hsu K-H, Lin T-Y, Hwang K-P, Chen P-Y, Huang Y-C. 2011. Factors associated with nasal colonization of methicillin-resistant Staphylococcus aureus among healthy children in Taiwan. J. Clin. Microbiol. 49:131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang Y-C, Ho C-F, Chen C-J, Su L-H, Lin T-Y. 2008. Comparative molecular analysis of community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus isolates from children in northern Taiwan. Clin. Microbiol. Infect. 14:1167–1172 [DOI] [PubMed] [Google Scholar]
- 9. Wang J-T, Liao C-H, Fang C-T, Chie W-C, Lai M-S, Lauderdale T-L, Lee W-S, Huang J-H, Chang S-C. 2009. Prevalence of and risk factors for colonization by methicillin-resistant Staphylococcus aureus among adults in community settings in Taiwan. J. Clin. Microbiol. 47:2957–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harmsen D, Claus H, Witte W, Rothgänger J, Claus H, Turnwald D, Vogel U. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41:5442–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen C-J, Su L-H, Lin T-Y, Huang Y-C. 2010. Molecular analysis of repeated methicillin-resistant Staphylococcus aureus infections in children. PLoS One 5:e14431. 10.1371/journal.pone.0014431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128–1132 [DOI] [PubMed] [Google Scholar]
- 14. Huang Y-C, Ho CF, Chen C-J, Su L-H, Lin T-Y. 2007. Nasal carriage of methicillin-resistant Staphylococcus aureus in household contacts of children with community-acquired diseases in Taiwan. Pediatr. Infect. Dis. J. 26:1066–1068 [DOI] [PubMed] [Google Scholar]
- 15. Huang Y-C, Su L-H, Wu T-L, Lin T-Y. 2006. Changing molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream isolates from a teaching hospital in Northern Taiwan. J. Clin. Microbiol. 44:2268–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Belkum A, Verkaik NJ, de Vogel CP, Boelens HA, Verveer J, Nouwen JL, Verbrugh HA, Wertheim HF. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J. Infect. Dis. 199:1820–1826 [DOI] [PubMed] [Google Scholar]
- 17. Verkaik NJ, de Vogel CP, Boelens HA, Grumann D, Hoogenboezem T, Vink C, Hooijkaas H, Foster TJ, Verbrugh HA, van Belkum A, van Wamel WJ. 2009. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J. Infect. Dis. 199:625–632 [DOI] [PubMed] [Google Scholar]
- 18. Eriksen NH, Espersen F, Rosdahl VT, Jensen K. 1995. Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol. Infect. 115:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. VandenBergh MF, Yzerman EP, van Belkum A, Boelens HA, Sijmons M, Verbrugh HA. 1999. Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. J. Clin. Microbiol. 37:3133–3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skov R, Christiansen K, Dancer SJ, Daum RS, Dryden M, Huang YC, Lowy FD. 2012. Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA). Int. J. Antimicrob. Agents. 39:193–200 [DOI] [PubMed] [Google Scholar]
- 21. Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. U. S. A. 99:7687–7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aires de Sousa M, de Lencastre H. 2004. Bridges from hospitals to the laboratory: genetic portraits of methicillin-resistant Staphylococcus aureus clones. FEMS Immunol. Med. Microbiol. 40:101–111 [DOI] [PubMed] [Google Scholar]
- 23. Robicsek A, Beaumont JL, Peterson LR. 2009. Duration of colonization with methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 48:910–913 [DOI] [PubMed] [Google Scholar]
- 24. Chen C-J, Hsueh P-R, Su L-H, Chiu C-H, Lin T-Y, Huang Y-C. 2009. Change in the molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream infections in Taiwan. Diagn. Microbiol. Infect. Dis. 65:199–201 [DOI] [PubMed] [Google Scholar]
- 25. Chen S-Y, Liao C-H, Wang J-L, Chiang W-C, Lai M-S, Chie W-C, Chen W-J, Chang S-C, Hsueh P-R. 2012. Methicillin-resistant Staphylococcus aureus (MRSA) staphylococcal cassette chromosome mec genotype effects outcomes of patients with healthcare-associated MRSA bacteremia independently of vancomycin minimum inhibitory concentration. Clin. Infect. Dis. 55:1329–1337 [DOI] [PubMed] [Google Scholar]
- 26. Emonts M, Uitterlinden AG, Nouwen JL, Kardys I, Maat MP, Melles DC, Witteman J, Jong PT, Verbrugh HA, Hofman A, Hermans PW, van Belkum A. 2008. Host polymorphisms in interleukin 4, complement factor H, and C-reactive protein associated with nasal carriage of Staphylococcus aureus and occurrence of boils. J. Infect. Dis. 197:1244–1253 [DOI] [PubMed] [Google Scholar]
- 27. van den Akker EL, Nouwen JL, Melles DC, van Rossum EF, Koper JW, Uitterlinden AG, Hofman A, Verbrugh HA, Pols HA, Lamberts SW, van Belkum A. 2006. Staphylococcus aureus nasal carriage is associated with glucocorticoid receptor gene polymorphisms. J. Infect. Dis. 194:814–818 [DOI] [PubMed] [Google Scholar]
- 28. Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, Killgore G, Tenover FC. 2006. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J. Infect. Dis. 193:172–179 [DOI] [PubMed] [Google Scholar]
- 29. Huang Y-C, Chou Y-H, Su L-H, Lien R-I, Lin T-Y. 2006. Methicillin-resistant Staphylococcus aureus colonization and its association with infection among infants hospitalized in neonatal intensive care units. Pediatrics 118:469–474 [DOI] [PubMed] [Google Scholar]
- 30. Zanger P, Nurjadi D, Gaile M, Gabrysch S, Kremsner PG. 2012. Hormonal contraceptive use and persistent Staphylococcus aureus nasal carriage. Clin. Infect. Dis. 55:1625–1632 [DOI] [PubMed] [Google Scholar]
- 31. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. 2004. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin. Infect. Dis. 39:971–979 [DOI] [PubMed] [Google Scholar]
- 32. Zanger P, Nurjadi D, Schleucher R, Scherbaum H, Wolz C, Kremsner PG, Schulte B. 2012. Import and spread of Panton-Valentine leukocidin-positive Staphylococcus aureus through nasal carriage and skin infections in travelers returning from the tropics and subtropics. Clin. Infect. Dis. 54:483–492 [DOI] [PubMed] [Google Scholar]
- 33. Otto M. 2011. A MRSA-terious enemy among us: end of the PVL controversy? Nat. Med. 17:169–170 [DOI] [PubMed] [Google Scholar]
- 34. Diep BA, Otto M. 2008. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 16:361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. 2009. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 106:5883–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]


