Abstract
Limitless reproductive potential is one of the hallmarks of cancer cells. This ability is due to the maintenance of telomeres, erosion of which causes cellular senescence or death. While most cancer cells activate telomerase, a telomere-elongating enzyme, it remains elusive as to why cancer cells often maintain shorter telomeres than the cells in the surrounding normal tissues. Here, we show that forced telomere elongation in cancer cells promotes their differentiation in vivo. We elongated the telomeres of human prostate cancer cells that possess short telomeres by enhancing their telomerase activity. The resulting cells had long telomeres and retained the ability to form tumors in nude mice. Strikingly, these tumors exhibited many duct-like structures and reduced N-cadherin expression, reminiscent of well-differentiated adenocarcinoma. These changes were caused by telomere elongation and not by enhanced telomerase activity. Gene expression profiling revealed that tumor formation was accompanied by the expression of innate immune system-related genes, which have been implicated in maintaining tumor cells in an undifferentiated state and poor-prognosis cancers. In tumors derived from the telomere-elongated cells, upregulation of such gene sets is not observed. Our observations suggest a functional contribution of short telomeres to tumor malignancy by regulation of cancer cell differentiation.
INTRODUCTION
Telomeres are specialized structures that protect the ends of eukaryotic linear chromosomes. Because of the end replication problem regarding linear chromosomal DNA, telomeres shorten with each cell division. Eventually, critically shortened telomeres fail to protect the chromosome ends against the DNA damage response, resulting in cellular senescence or apoptosis (1). Accordingly, telomeres have been referred to as “a mitotic clock,” and indeed, rodents that retain very long telomeres rarely undergo telomere-dependent replicative senescence (2).
In humans, most cancer cells (∼80 to 90%) activate telomerase and elongate their telomeres to overcome the end replication problem (1, 3). In this respect, longer telomeres would be more advantageous for conferring immortality to cancer cells. However, even after telomerase reactivation solves the end replication problem, cancer cells often maintain shortened telomeres (4–6).
Human telomeric DNA is associated with specific protein complexes called shelterin (7). Shelterin consists of six proteins and regulates the length and integrity of telomeres (7). In general, telomere length in telomerase-positive cells is regulated by the “protein-counting” mechanism, in which longer telomeres contain higher levels of shelterin and therefore block the access of telomerase more intensely (8). This means that the telomere length of telomerase-positive cells is stabilized by the balance between telomerase-mediated telomere extension and its blockade by shelterin.
While telomere shortening suppresses cancer formation (9–12), it sometimes promotes genomic instability and enhances carcinogenesis and the development of malignancy (13). This oncogenic effect of telomere shortening would be derived from enhanced clonal evolution under catastrophic conditions of genomic instability. The telomere position effect influences chromatin status and gene expression (14), suggesting that differences in telomere length may directly affect the behavior of cancer cells.
In the present study, we investigated whether telomere length is more important than previously thought with regard to cancer, in addition to the promotion of cellular immortality.
MATERIALS AND METHODS
Cell culture and plasmids.
Cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% heat-inactivated fetal bovine serum. DAT-hTERT (N125A+T126A) was generated using PCR-based site-directed mutagenesis. LhTERTL was created by PCR amplification using primers that contain an overhanging loxP sequence (5′-ATAACTTCGTATAGCATACATTATACGAAGTTAT-3′) and was cloned into the XhoI and NcoI sites of pLNCX2 (BD Biosciences, Franklin Lakes, NJ).
Retroviral transduction and viral infection.
Retroviral infection was performed essentially as previously described (15) with a slight modification. Briefly, retroviral supernatants were prepared by transient transfection of GP2-293 cells with control pLNCX2 or a series of human telomerase reverse transcriptase (hTERT) vectors with a pVSV-G packaging vector encoding the viral envelope protein. Cancer cells were infected with the retroviral supernatant in the presence of 8 μg/ml Polybrene. Infected cells were selected with 400 μg/ml of G418. To excise the exogenous hTERT, PC-3/mock and PC-3/LhTERTL cells (at population doubling [PD] 40) were infected with a recombinant adenovirus, AxCANCre (TaKaRa, Kyoto, Japan) (16), at a multiplicity of infection (MOI) of 40. After viral adsorption for 60 min, cells were extensively washed with DMEM containing 10% fetal bovine serum. At 7 to 10 days postinfection, excision of the exogenous hTERT was verified using Western blot analysis and the telomeric repeat amplification protocol (TRAP) assay as detailed below.
Western blot analysis.
Protein extraction from tumor xenografts was performed using radioimmunoprecipitation assay (RIPA) buffer and TissueLyser (Qiagen) according to the manufacturer's protocol. Cell lysates were prepared, and Western blot analysis was performed as previously described (15) with the following primary antibodies: rabbit anti-hTERT (1531-1, 1:1,000; Epitomics, Inc.), mouse monoclonal anti-human N-cadherin (M3613, 1:1,000; Dako), rabbit anti-phospho-histone H2A.X (9718S, 1:500; Cell Signaling Technology, Inc.), or mouse anti-GAPDH (anti-glyceraldehyde-3-phosphate dehydrogenase) (10R-G109a, 1.0 μg/ml; Fitzgerald Industries).
Telomere Southern blot analysis and telomerase assay.
Terminal restriction fragments were detected using Southern blot analysis with a 32P-labeled (CCCTAA)n probe, as previously described (15). Telomerase activity was detected using the TRAP assay (3). The telomeric products were separated using Tris-borate-EDTA (TBE)-PAGE and visualized by means of staining with SYBR green (TaKaRa).
Combined telomere FISH and immunofluorescence staining.
Cells were fixed with 2% paraformaldehyde–phosphate-buffered saline (PBS) for 10 min and permeabilized with 0.5% Nonidet P-40–PBS. Immunofluorescence staining with the primary antibody rabbit anti-hTERT (1531-1, 1:100) and fluorescence in situ hybridization (FISH) analysis with a Cy3-labeled telomere-specific (CCCTAA)3 peptide nucleic acid probe (Greiner Bio-One) were performed essentially as previously described (17).
Generation of subcutaneous xenografts in nude mice.
Animal experiments were approved by the Japanese Foundation for Cancer Research, Institutional Animal Care and Use Committee and conducted in accordance with institutional guidelines. Cells were mechanically dissociated to obtain single-cell suspensions and were diluted in Hanks' balanced salt solution (Gibco). Then, 1 × 106 cells were subcutaneously injected into 5-week-old female nude mice with a BALB/c genetic background. When the tumor reached at least 5 mm in diameter, mice were euthanized and tumor tissue was collected.
Cytochemistry and immunohistochemistry.
Tumor samples were fixed in Mildform10N (133-10311; Wako Pure Chemical Industries, Ltd.). All tissues were embedded in paraffin and cut at 4 to 5 μm. The sections were deparaffinized through xylene and a graduated alcohol series to water and incubated for 5 min in aqueous 3% hydrogen peroxide to block endogenous peroxidase. Histological sections were stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) stain. Immunohistochemical studies were performed on formalin-fixed, paraffin-embedded sections. After deparaffinization and appropriate epitope retrieval, the sections were incubated with mouse monoclonal anti-human N-cadherin (M3613, 1:500; Dako), rabbit anti-hTERT (1531-1, 1:100; Epitomics, Inc.), rabbit antivimentin (sc-5565, 1:50; Santa Cruz), mouse anti-cytokeratin 18 antibody (ab668, 1:50; Abcam), rabbit anti-DDX58 antibody (ab65588, 1:100; Abcam), rabbit anti-IRF7 antibody (ab115352, 1:100; Abcam), rabbit anti-IRF9 antibody (ab118189, 1:100; Abcam), rabbit anti-ISG15 antibody (ab14374, 1:100; Abcam), rabbit anti-OAS3 antibody (ab64163, 1:100; Abcam), rabbit anti-STAT1 antibody (ab118638, 1:100; Abcam), rabbit anti-MyD88 antibody (ab94527, 1:100; Abcam), or mouse monoclonal anti-phospho-histone H2A.X antibody (05-636, 1:400; Upstate). The sections were further incubated with biotinylated goat anti-mouse or anti-rabbit antibodies. The specific signals were then detected with streptavidin-conjugated horseradish peroxidase and with the use of diaminobenzidine as the chromogen.
Microarray and bioinformatic analyses.
RNA extraction from tumor xenografts was performed using the RNeasy kit and TissueLyser (Qiagen) according to the manufacturer's protocol. Total RNA was quantified using the Agilent 2100 Bioanalyzer (Agilent Technologies Inc.). Extracted RNA was labeled and hybridized onto the GeneChip Human Genome U133 Plus 2.0 array (Affymetrix Inc.). Data normalization, statistical analysis, Gene Ontology analysis, and genome browsing were performed using GeneSpring GX software (Agilent Technologies).
Quantitative real-time PCR.
Transcripts of immune response-related genes were detected by real-time PCR with a LightCycler 480 real-time PCR system by monitoring amplification with the Universal ProbeLibrary probes, which were labeled with fluorescein (FAM), as described in the manufacturer's protocol (Roche Applied Science).
Microarray data accession numbers.
The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo) under accession numbers GSE36649 and GSE41559.
RESULTS
hTERT overexpression in PC-3 cells induces duct-like structures in PC-3 tumors.
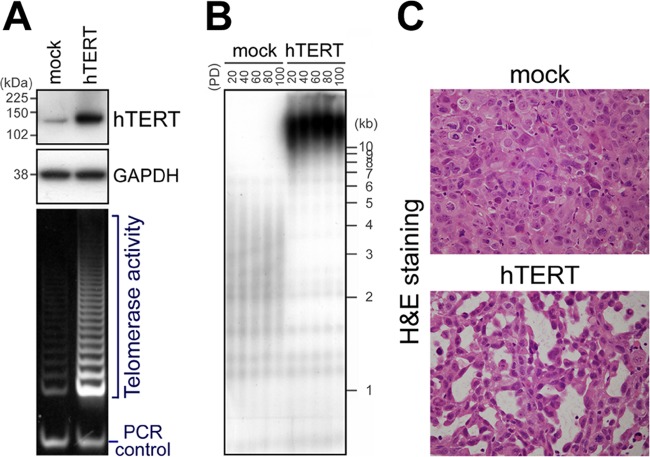
If short telomeres are advantageous to cancer cells, enforced elongation of telomeres will negatively affect cancer cell behavior. Because such an effect would be apparent in cancer cells with very short telomeres, we used PC-3 prostate cancer cells, which retain rather short telomeres (average telomere restriction fragment length = 3.95 kb) (18). First, we enhanced telomerase activity and telomere elongation in PC-3 cells. Human telomerase is a ribonucleoprotein that consists of two essential components, namely, human telomerase reverse transcriptase (hTERT) and human telomerase RNA (hTR or hTERC) (19). The expression of hTR is ubiquitous in human cells, and regulation of telomerase activity is controlled at the level of hTERT transcription (19). Therefore, we established a PC-3 subline that overexpressed exogenous hTERT (PC-3/hTERT). Upregulation of telomerase activity (Fig. 1A) and substantial telomere elongation (Fig. 1B) in PC-3/hTERT cells, compared with control cells (PC-3/mock), were confirmed.
Fig 1.
Induction of duct-like structures in tumors as a result of hTERT overexpression. (A) Retroviral overexpression of hTERT (upper and middle panels) and upregulation of telomerase activity (bottom panel) in PC-3 cells at PD 10. Size markers are indicated on the left. (B) Telomere elongation in PC-3/hTERT cells demonstrated using Southern blot analysis. (C) Hematoxylin and eosin staining of tissue sections of PC-3/mock and PC-3/hTERT cells in tumor xenografts. Duct-like structures were frequently observed in PC-3/hTERT tumors. Original magnification, ×400.
We expected that long telomeres may exert a negative effect on cancer cell behavior in vitro and/or in vivo. Under normal growth conditions in culture dishes, no difference was found in the growth rates of PC-3/hTERT and control cells (see Fig. S1A in the supplemental material). Moreover, long telomeres in PC-3/hTERT cells were maintained through at least 100 population doublings (PDs) (over ∼3 months) (Fig. 1B). Thus, we next examined whether the role of the elongated telomeres emerges under tumor microenvironmental conditions. To investigate the behavior of PC-3 cells with long telomeres in vivo, we subcutaneously injected these cells into nude mice and monitored subsequent tumor formation. Between the cell lines, there was no considerable difference in tumor formation (see Fig. S1C in the supplemental material). Interestingly, the histology of the xenograft tumors revealed that PC-3/hTERT cells clearly formed duct-like structures but that control cells did not (Fig. 1C). Similar results were obtained with HBC4 human breast cancer cells (see Fig. S2 in the supplemental material). These results suggest that long telomeres induce a phenotypic change in PC-3 cells, which is reminiscent of glandular differentiation in vivo.
Telomere elongation and not enhanced hTERT expression induces the duct-like structures in PC-3 tumors.
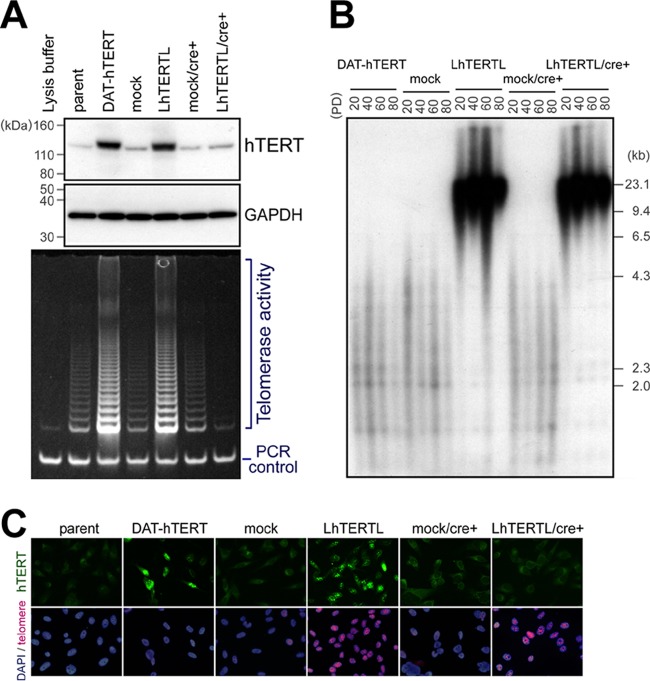
Next, we examined whether the formation of the duct-like structures resulted from telomere elongation and not from increased levels of hTERT protein. hTERT has a nontelomeric function through a mechanism that is independent of telomerase activity (20, 21). To distinguish the effect of elongated telomeres from that of hTERT overexpression, we further employed two constructs for exogenous hTERT. N125A+T126A-hTERT is catalytically active in vitro but fails to elongate telomeres in intact cells (22). The N125A+T126A mutations are located in the so-called “dissociates activities of telomerase” (DAT) region, which is necessary for telomere elongation (22). We established a PC-3/DAT-hTERT cell line which overexpressed this hTERT mutant and had enhanced telomerase activity (Fig. 2A). As expected, these cells did not elongate their telomeres even after prolonged cultivation (Fig. 2B). The second strategy was the removal of the hTERT transgene after telomere elongation using the Cre/loxP system (23). We added the loxP sequence at both the 5′ and 3′ ends of the wild-type hTERT cDNA and established the stable PC-3/LhTERTL (loxP-hTERT-loxP) cell line. After confirming telomere elongation in the cells, we then excluded exogenous hTERT by the introduction of Cre recombinase at PD 40 using an adenovirus. In these cell lines, hTERT overexpression and telomere elongation were further confirmed by immunofluorescence staining and telomere fluorescence in situ hybridization (FISH), respectively (Fig. 2C). No difference was found in the growth rates of these cell lines in culture dishes (see Fig. S1B in the supplemental material). The resulting PC-3/LhTERTL/cre+ cells maintained long telomeres through at least 40 PDs (PDs 40 to 80, ∼1 month) (Fig. 2B). We estimated that this time period is long enough to maintain elongated telomeres during tumor development in nude mice.
Fig 2.
Telomere-elongated PC-3 cells without hTERT overexpression. (A) Western blot analysis (upper panel) and TRAP assay (bottom panel) at population doubling (PD) 50. DAT-hTERT and wild-type hTERT with loxP (LhTERTL) elevated telomerase activity. After the introduction of Cre at PD 40, only endogenous hTERT and telomerase activities were detected in PC-3/LhTERTL/cre+ cells. (B) Telomere Southern blot analysis. PC-3/DAT-hTERT cells did not have elongate telomeres despite enhanced telomerase activity. PC-3/LhTERTL cells had elongated telomeres, which were maintained even after shutdown of the transgene until at least PD 80. (C) Combined immunofluorescence and FISH at PD 50, just before subcutaneous injection. Most cells showed the expected patterns of hTERT and telomere signal intensities.
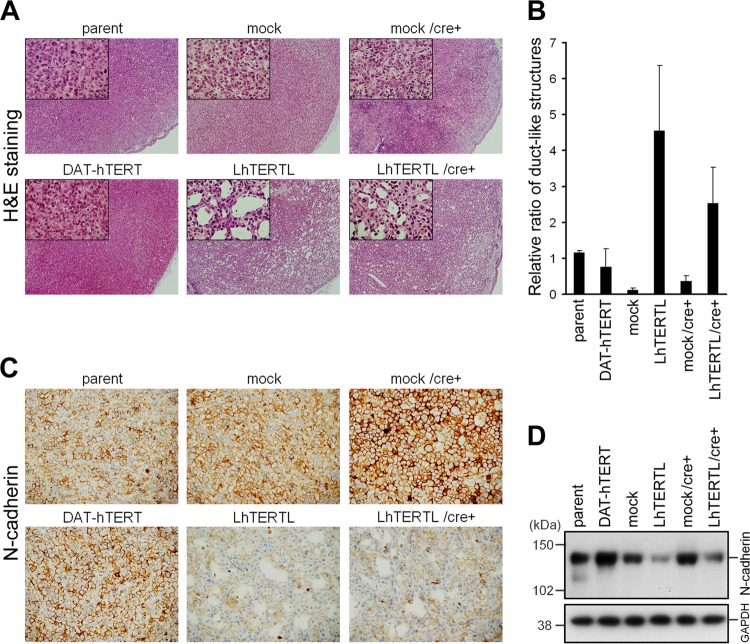
We transplanted these cell lines into nude mice by subcutaneous injection. Again, PC-3/LhTERTL tumors, which retained long telomeres and exogenous hTERT expression, exhibited duct-like structures (Fig. 3A, lower center panel). Strikingly, similar results were obtained using PC-3/LhTERTL/cre+ tumors, in which the hTERT transgene had been excluded but the telomeres remained elongated (Fig. 3A, lower right panel). In contrast, PC-3/DAT-hTERT cells, in which telomerase activity was upregulated but the telomeres were short, scarcely exhibited duct-like structures (Fig. 3A, lower left panel), as did the parental, mock and mock/cre+ cell lines (Fig. 3A, upper three panels). Quantification of the number of duct-like structures indicated that this morphological alteration is associated with elongated telomeres rather than forced expression of hTERT (Fig. 3B). Immunohistochemical staining demonstrated that most of the cells in the tumors derived from hTERT-overexpressing cells retained hTERT overexpression, irrespective of ductal structure formation (see Fig. S3, lower left and center panels, in the supplemental material). Meanwhile, duct-like structures in PC-3/LhTERTL/cre+ tumors were not derived from the residual cells that had escaped from Cre-mediated shutdown of hTERT overexpression (see Fig. S3, lower right panel, in the supplemental material). We further found that tumors with elongated telomeres expressed less N-cadherin, which is a marker of mesenchymal cell lineage and undifferentiated carcinoma (24) (Fig. 3C). N-cadherin expression of two telomere-elongated PC-3 cells was also reduced in culture dishes before subcutaneous injection (Fig. 3D). Collectively, while the PC-3 cells with elongated telomeres maintained an ability to form tumors in vivo, they formed the ductal structures and reduced the expression of an undifferentiated/malignant marker protein, reminiscent of normal glandular epithelial cells. These observations indicate that telomere length influences cancer cell differentiation in a tumor microenvironment.
Fig 3.
PC-3 cell differentiation due to telomere elongation. (A) H&E-stained tissue sections. PC-3/DAT-hTERT cells (lower left panel) remained in an undifferentiated state similar to that for control cells (upper panel), whereas PC-3/LhTERTL and PC-3/LhTERTL/cre+ cells showed duct-like structures (lower center and right panels). Original magnification, ×40. Insets, magnified views (×200). (B) Quantification of duct-like structures. Parameters are the average and standard deviation of the number of ducts per unit area in each tumor (n = 2 or 3). Reproducibility and significant differences were assessed using additional xenograft tumor samples for microarray experiments (see Fig. S3 in the supplemental material). (C) Immunohistochemistry of N-cadherin. Suppression of N-cadherin expression was observed in PC-3/LhTERTL and PC-3/LhTERTL/cre+ cells (lower center and right panels). Original magnification, ×200. (D) Western analysis demonstrated that the reduction of N-cadherin expression in telomere-elongated PC-3 cells before subcutaneous injection.
Transcriptional upregulation of innate immune/cellular defense systems is lost in PC-3 xenograft cells with elongated telomeres.
It is known that cell differentiation is often accompanied by dynamic changes in genome-wide transcriptional changes. To elucidate the molecular mechanism for the above-described phenotypic changes due to telomere elongation, we employed a microarray approach to monitor gene expression profiling that might be important for the differentiation of PC-3 cells in vivo. We subcutaneously injected four PC-3 cell lines, namely, mock, LhTERTL, mock/cre+, and LhTERTL/cre+, into nude mice and collected the resultant xenograft tumors. Formation of the duct-like structures was reproducibly observed only in PC-3 cells with elongated telomeres; significant differences (two-tailed t test) were found between each combination of control xenograft tumors and xenograft tumors with elongated telomeres in terms of the rate of duct-like structures per unit area (see Fig. S4A in the supplemental material). Immunohistochemistry for cytokeratin 18 (an epithelial marker) and vimentin (a mesenchymal marker) was also carried out on these four PC-3 xenograft tumors (see Fig. S4B in the supplemental material). The results showed that increased cytokeratin 18 expression and decreased vimentin expression of telomere-elongated xenograft tumors compared to that of control tumors. These observations support that telomere-elongated PC-3 tumors resemble normal epithelial tissues more than control tumors. We also confirmed the maintenance of long telomeres in PC-3/LhTERTL and PC-3/LhTERTL/cre+ xenograft tumors by FISH analysis (see Fig. S4C in the supplemental material).
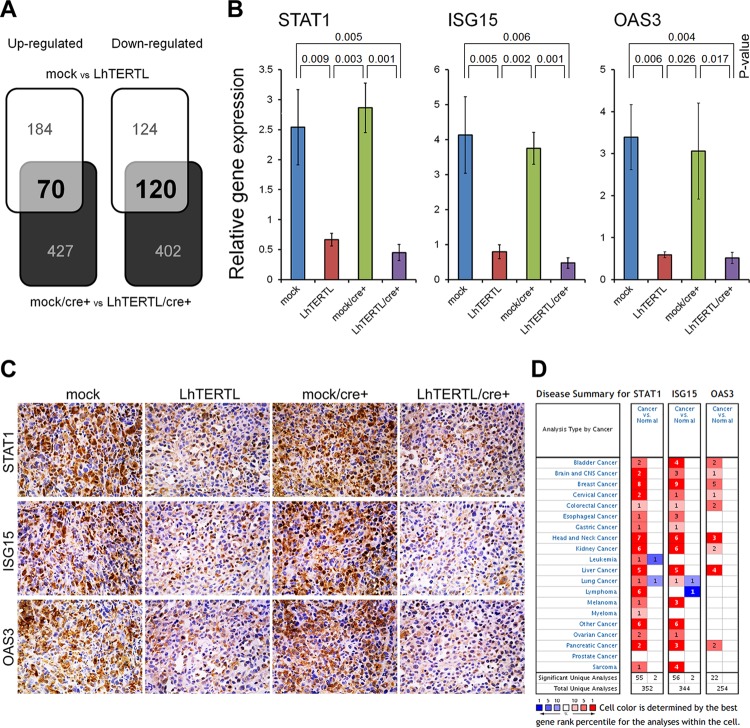
We extracted RNA from four independent xenograft tumors per original cell line and compared the gene expression profiles of control and telomere-elongated cells, in both the presence and absence of the exogenous hTERT protein. We identified 70 significantly upregulated probes (61 genes) (P < 0.05 by the two-tailed t test) and 120 significantly downregulated probes (90 genes) with changes in expression of >2.0-fold (Fig. 4A, shaded elements; see Data set S1 in the supplemental material). Gene ontology analysis of these 190 up- and downregulated probes (151 genes) revealed that the immune responses and cellular defenses against pathogen categories were significantly enriched (a total of 54,675 probe sets; P < 0.01 by Fisher's exact test) among the various biological functions and events (see Fig. S5A in the supplemental material). Expression of most genes that were classified into these categories was very low and was not affected by hTERT overexpression or telomere elongation in culture dishes (see below). However, during tumor formation in vivo, these genes were highly upregulated if the tumors were derived from mock or mock/cre+ cells. In contrast, the tumors derived from the PC-3 cells with elongated telomeres substantially lost such transcriptional responses (see Fig. S5B in the supplemental material). These observations suggest that PC-3 cells with short telomeres activate immune response-related genes in the tumor microenvironment, which may repress the formation of the duct-like structures. Suppression of the 10 genes for STAT1, ISG15, OAS3, DDX58, IRF7, IRF9, IFI44, IFITM2, MyD88, and XAF1 was confirmed using quantitative real-time PCR (Fig. 4B; see Fig. S6A in the supplemental material). We also confirmed suppression of protein expression in seven of these genes by means of immunohistochemistry (Fig. 4C; see Fig. S6B in the supplemental material). Similar results were obtained with HBC4 human breast cancer cells (see Fig. S6C and D in the supplemental material). Gene expression profiles of the four PC-3 cell lines in culture dishes revealed that the immune response and cellular defense mechanisms against pathogen categories were not enriched by gene ontology (GO) analysis (see Fig. S5A in the supplemental material).
Fig 4.
Innate immune-related genes overexpressed in cancer are suppressed in telomere-elongated xenograft tumors. (A) Altered gene expression profiles due to telomere elongation in PC-3 xenografts, with (upper white segments) or without (lower black segments) hTERT overexpression. With changes of >2.0-fold, expression of 190 probes (151 genes) was changed irrespective of hTERT overexpression/excision. For each probe, at least one xenograft had a signal value of >100. Detailed information is provided in Data set S1 in the supplemental material. (B) The RNA expression of three genes was quantified using real-time PCR. Error bars indicate the standard deviations (n = 3). The expression value for each gene was normalized to that of β-actin. There were significant differences in each combination of control (mock or mock/cre+) and telomere-elongated (LhTERTL or LhTERTL/cre+) xenograft tumors (P < 0.05 by the two-tailed t test). (C) Immunohistochemical staining of STAT1, ISG15, and OAS3. Original magnification, ×200. (D) Summary of disease analyzed using the Oncomine database, which indicates the number of significant unique microarray results with changes of >2.0-fold (P < 0.0001 by the two-tailed t test) with increased (red) or decreased (blue) expression in tumor relative to normal tissue.
According to the Oncomine database (www.oncomine.org), most of the genes that were upregulated in control xenograft tumors, but not in PC-3 xenograft tumors with elongated telomeres, exhibited overexpression in the various tumors but not in the respective normal tissues (Fig. 4D; see Fig. S5C in the supplemental material). This observation suggests that the short telomeres in cancer cells retain their malignancy in vivo by means of the transcriptional activation of innate immune/cellular defense related-genes in some way.
In yeast and even in human cancer cells, long telomeres have the so-called “telomere position effect” (TPE), which results in reversible silencing of genes near telomeres (14). Accordingly, one may speculate that telomere elongation in PC-3 cells preferentially causes transcriptional repression of the genes that are located near telomeres. However, in the present study, the chromosomal loci of the immune response-related genes affected by telomere elongation were randomly distributed along chromosome arms, and there was no preference to the distal ends of the chromosomes (see Fig. S7A in the supplemental material). Thus, TPE does not seem to account for the transcriptional repression of these genes.
The PC-3 cells used in our study retained rather short telomeres. Therefore, one may speculate that the DNA damage response elicited by the short telomeres induced senescence or crisis in the xenograft tumors and modulated the tumor behavior. We counted the number of cells positive for γH2AX, a hallmark indicator of DNA damage, per unit area in each tumor (n = 5). No significant differences (P < 0.05, two-tailed t test) were found between each combination of control xenograft tumors and those with elongated telomeres. Meanwhile, Western blot analysis demonstrated that γH2AX accumulation was slightly reduced in telomere-elongated xenografts (see Fig. S7B in the supplemental material). Indeed, while critically shortened telomeres have been shown to repress tumor development in many experimental settings (9–12), control cells and cells with elongated telomeres in the current study did not show a considerable difference in their growth rates or tumor sizes (see Fig. S1C in the supplemental material). Thus, it is possible that long telomeres regulate the innate immune related-gene transcriptions through unknown mechanisms, apart from the telomere position effect or DNA damage response.
DISCUSSION
In the present study, we demonstrated that forced elongation of telomeres in cancer cells affects differentiation and the expression of N-cadherin, cytokeratin 18, vimentin, and innate immune and cellular defense system-related genes in vivo. In a clinical setting, cancer malignancy and its pathological grade score often decrease in cancer cells with a highly differentiated status. Many genes whose expression was suppressed by telomere elongation in the present study seem to participate in tumor malignancy. For example, N-cadherin has been identified as a crucial mediator of prostate cancer metastasis and castration resistance (25). Again, our meta-analysis using the Oncomine database revealed that overexpression of innate immune system-related genes is common among various cancers relative to normal tissues (Fig. 4D; see Fig. S5C in the supplemental material). Interestingly, only the expression of the C5 (complement component 5) gene was increased in telomere-elongated xenografts compared with control xenografts (see Fig. S5B [lower graph, 17th gene from the right] in the supplemental material). Correspondingly, according to the Oncomine database, the expression of the C5 gene is significantly downregulated in the various tumors but not in the respective normal tissues (see Fig. S5C [third gene from the left] in the supplemental material). Moreover, it has been recently reported that the low-expression signature of IFN/STAT1 signaling genes, including the STAT1, IFI44, ISG15, IFIT1, MX1, OAS1, and USP18 genes, predicts a good prognosis in glioblastoma multiforme (26). The expression of these genes was substantially suppressed, with changes of >2.0-fold in telomere-elongated PC-3 xenografts (see Data set S1 in the supplemental material). Taken together, these observations suggest that tumor malignancy in vivo is reduced by telomere elongation.
In the current study, we demonstrated that telomere elongation in cancer cells resulted in the formation of duct-like structures and induced well-differentiated tumors in vivo. Suppression of N-cadherin expression in telomere-elongated PC-3 xenografts not only mediates prostate cancer metastasis but also acts as a marker of undifferentiated carcinoma (24). Moreover, the innate immune system-related genes are often involved in cell differentiation, since the system is one of the signal transmission processes in multicellular organisms. For example, STAT1 inhibits the differentiation of osteoblasts through an interferon-dependent pathway (27). In addition, the expression of immune cell response-related genes, including the IFITM1, IFITM2, IFITM3, GBP1, OAS1, OAS2, and IFNGR1 genes, is strongly downregulated during differentiation of multipotent human hematopoietic stem cells into erythrocyte precursors (28). In our study, expression of these genes was substantially suppressed in PC-3 xenografts with elongated telomeres, with the exception of the IFNGR1 gene (see Fig. S5B in the supplemental material). Since IFNGR1 expression is similar in cancer and normal tissue samples according to the Oncomine database (see Fig. S5C in the supplemental material), IFNGR1 may not be involved in determining the differentiation state of cancer cells, unlike normal cells. These observations suggest that cellular differentiation might be related to the expressions of N-cadherin and innate immune/cellular defense system genes in several different settings.
Whereas telomere-elongated xenograft tumors showed a more differentiated state, there was no considerable difference in tumor volume between control and telomere-elongated xenograft tumors (see Fig. S1C in the supplemental material). One possibility is that the differential growth of those tumors is seen at much later phases of the xenografts: we collected tumor tissues for approximately 1 month postinoculation in order to prevent necrotic cell death of the tumors, which would disturb precise examination of the tumor histology. Because the transcriptional response of the innate immune-related genes was observed only in vivo, such a response might be enhanced at later periods after the inoculation. Meanwhile, we assume that PC-3 cancer cells themselves induce the expression of innate immune-related genes in vivo in tumors, since we detected very few host-derived macrophages in telomere-elongated PC-3 xenograft tumors as well as in control xenograft tumors by means of immunohistochemistry (our unpublished observations).
The expression of many genes was changed by telomere elongation in this study. Because N-cadherin expression in telomere-elongated PC-3 cells was also reduced in culture dishes before subcutaneous injection, N-cadherin might play a critical role in various effects of telomere elongation in human cancer cells. We also found many changes of gene expression levels by telomere elongation in xenograft tumors. One possibility is that telomere elongation influenced the dynamics of the telomere-specific shelterin proteins and that shelterin altered the transcription profile with genome-wide TTAGGG elements. In practice, it has recently been proposed that shelterin participate in gene regulation networks by binding to genome-wide nontelomeric sequences (29, 30).
Senescent cells preferentially secrete a characteristic set of proteins, which is presumably controlled at the transcriptional level and is known as the senescence-associated secretory phenotype (SASP) (31). Coppé et al. found 39 SASPs of human prostate cells, including PC-3 (31). We present a list of the gene expression profiles of these 39 genes in control and the telomere-elongated tumors in Fig. S7C in the supplemental material. Among those genes, only CXCL11 expression was changed >2.0-fold, but others were not affected by telomere elongation. In fact, we did not detect an enhanced γH2AX signal and substantial differences in γH2AX expression in the PC-3-derived tumors (see Fig. S7B in the supplemental material), indicating that short telomeres in PC-3 cells did not elicit the SASP in vivo. Collectively, these observations exclude the possibility that the morphological changes in PC-3 tumors due to telomere elongation are caused merely by repair of short dysfunctional telomeres.
Among the genes affected by telomere elongation in PC-3 cells, the ISG15 gene is located near the p-arm telomere of chromosome 1 (1p36.33). Intriguingly, the ISG15 gene is the first human gene that has been identified whose expression is inversely correlated with telomere length (32). Because ISG15 is implicated in inflammation (33) and ISG15 expression is strikingly increased in various types of cancer (Fig. 4D), especially in human prostate cancer (34), its upregulation by short telomeres may contribute to the malignant progression of cancer by eliciting a chronic inflammatory environment. However, neither TPE nor telomere dysfunction-induced DNA damage is involved in the transcriptional regulation of ISG15 (32). Additionally, the genetic loci affected by telomere elongation in PC-3 cells were not concentrated near the chromosome ends (see Fig. S7A in the supplemental material). Although there is the possibility that TPE affected the expression of master regulators of innate immune-related genes, TPE does not seem to account for the direct transcriptional repression of these genes. Consistently, a recent report by Arnoult et al. demonstrated that telomere elongation does not decrease the expression levels of genes located near telomeres (35). These observations suggest that telomere length can modulate gene expression in a TPE-independent manner.
In the current study, we demonstrated that forced elongation of telomeres in cancer cells promotes their differentiation and may reduce tumor malignancy in vivo. Considering that the degree of tumor malignancy is strongly associated with the level of cellular differentiation in clinical diagnosis, our results suggest that telomere elongation in cancer cells palliates tumor behavior. In other words, cancer cells may maintain short telomeres to maintain their undifferentiated state through enhanced expression of N-cadherin and innate immune/cellular defense system-related genes. In conclusion, the present observations suggest that shortened telomeres in cancer cells play a functional role in the maintenance of malignancy, implicating them as targets for telomere-directed cancer therapeutics.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kimie Nomura and Hironori Murayama (JFCR Cancer Institute) for technical assistance regarding the cytochemistry, immunohistochemistry, and preparation of tumor sections. We thank Takashi Tsuruo, Mitsuaki Yoshida, and Haruo Sugano for critical comments and discussions.
This work was supported by a Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) Fellowship (no. 20-11574) and a Grant-in-Aid for Scientific Research (B), JSPS (no. 22300341).
K.H. and H.S. designed the research program; K.H., T.M., S.S., and Y.M. performed the research; K.H., T.M., Y.I., and H.S. analyzed the data; and K.H. and H.S. wrote the manuscript.
Footnotes
Published ahead of print 28 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00136-13.
REFERENCES
- 1.Hanahan D, Weinberg RA. 2000. The hallmarks of cancer. Cell 100:57–70 [DOI] [PubMed] [Google Scholar]
- 2.Niida H, Matsumoto T, Satoh H, Shiwa M, Tokutake Y, Furuichi Y, Shinkai Y. 1998. Severe growth defect in mouse cells lacking the telomerase RNA component. Nat. Genet. 19:203–206 [DOI] [PubMed] [Google Scholar]
- 3.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015 [DOI] [PubMed] [Google Scholar]
- 4.Maser RS, DePinho RA. 2002. Connecting chromosomes, crisis, and cancer. Science 297:565–569 [DOI] [PubMed] [Google Scholar]
- 5.Meeker AK, Hicks JL, Platz EA, March GE, Bennett CJ, Delannoy MJ, De Marzo AM. 2002. Telomere shortening is an early somatic DNA alteration in human prostate tumourigenesis. Cancer Res. 62:6405–6409 [PubMed] [Google Scholar]
- 6.Sommerfeld HJ, Meeker AK, Piatyszek MA, Bova GS, Shay JW, Coffey DS. 1996. Telomerase activity: a prevalent marker of malignant human prostate tissue. Cancer Res. 56:218–222 [PubMed] [Google Scholar]
- 7.de Lange T. 2005. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19:2100–2110 [DOI] [PubMed] [Google Scholar]
- 8.Marcand S, Gilson E, Shore D. 1997. A protein-counting mechanism for telomere length regulation in yeast. Science 275:986–990 [DOI] [PubMed] [Google Scholar]
- 9.Greenberg RA, Chin L, Femino A, Lee KH, Gottlieb GJ, Singer RH, Greider CW, DePinho RA. 1999. Short dysfunctional telomeres impair tumourigenesis in the INK4a(delta2/3) cancer-prone mouse. Cell 97:515–525 [DOI] [PubMed] [Google Scholar]
- 10.González-Suárez E, Samper E, Flores JM, Blasco MA. 2000. Telomerase-deficient mice with short telomeres are resistant to skin tumourigenesis. Nat. Genet. 26:114–117 [DOI] [PubMed] [Google Scholar]
- 11.Feldser DM, Greider CW. 2007. Short telomeres limit tumour progression in vivo by inducing senescence. Cancer Cell 11:461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taboski MA, Sealey DC, Dorrens J, Tayade C, Betts DH, Harrington L. 2012. Long telomeres bypass the requirement for telomere maintenance in human tumourigenesis. Cell Rep. 1:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. 1999. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97:527–538 [DOI] [PubMed] [Google Scholar]
- 14.Baur JA, Zou Y, Shay JW, Wright WE. 2001. Telomere position effect in human cells. Science 292:2075–2077 [DOI] [PubMed] [Google Scholar]
- 15.Seimiya H, Muramatsu Y, Ohishi T, Tsuruo T. 2005. Tankyrase 1 as a target for telomere-directed molecular cancer therapeutics. Cancer Cell 7:25–37 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Kagawa H, Yamanashi Y, Sata T, Kawaguchi Y. 2003. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo construction of an excisable bac. J. Virol. 77:1382–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meeker AK, Gage WR, Hicks JL, Simon I, Coffman JR, Platz EA, March GE, De Marzo AM. 2002. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am. J. Pathol. 160:1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cookson JC, Dai F, Smith V, Heald RA, Laughton CA, Stevens MF, Burger AM. 2005. Pharmacodynamics of the G-quadruplex-stabilizing in human tumour cells correlates with telomere length and can be enhanced, or antagonized, with cytotoxic agents. Mol. Pharmacol. 68:1551–1558 [DOI] [PubMed] [Google Scholar]
- 19.Nugent CI, Lundblad V. 1998. The telomerase reverse transcriptase: components and regulation. Genes Dev. 12:1073–1085 [DOI] [PubMed] [Google Scholar]
- 20.Choi J, Southworth LK, Sarin KY, Venteicher AS, Ma W, Chang W, Cheung P, Jun S, Artandi MK, Shah N, Kim SK, Artandi SE. 2008. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 4:e10. 10.1371/journal.pgen.0040010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maida Y, Yasukawa M, Furuuchi M, Lassmann T, Possemato R, Okamoto N, Kasim V, Hayashizaki Y, Hahn WC, Masutomi K. 2009. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature 461:230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim M, Xu L, Blackburn EH. 2003. Catalytically active human telomerase mutants with allele-specific biological properties. Exp. Cell Res. 288:277–287 [DOI] [PubMed] [Google Scholar]
- 23.Nagy A. 2000. Cre recombinase: the universal reagent for genome tailoring. Genesis 26:99–109 [PubMed] [Google Scholar]
- 24.Derycke LD, Bracke ME. 2004. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int. J. Dev. Biol. 48:463–476 [DOI] [PubMed] [Google Scholar]
- 25.Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, Shimomura T, Fazli L, Wada R, Huang J, Vessella RL, An J, Horvath S, Gleave M, Rettig MB, Wainberg ZA, Reiter RE. 2010. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat. Med. 16:1414–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duarte CW, Willey CD, Zhi D, Cui X, Harris JJ, Vaughan LK, Mehta T, McCubrey RO, Khodarev NN, Weichselbaum RR, Gillespie GY. 2012. Expression signature of IFN/STAT1 signaling genes predicts poor survival outcome in glioblastoma multiforme in a subtype-specific manner. PLoS One 7:e29653. 10.1371/journal.pone.0029653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Koga T, Isobe M, Kern BE, Yokochi T, Chin YE, Karsenty G, Taniguchi T, Takayanagi H. Stat1 functions as a cytoplasmic attenuator of Runx2 in the transcriptional program of osteoblast differentiation. Gene Dev. 3:1979–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui K, Zang C, Roh TY, Schones DE, Childs RW, Peng W, Zhao K. 2009. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell 4:80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez P, Thanasoula M, Carlos AR, Go‘mez-Lo'pez G, Tejera AM, Schoeftner S, Dominguez O, Pisano DG, Tarsounas M, Blasco MA. 2010. Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nat. Cell Biol. 12:768–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simonet T, Zaragosi LE, Philippe C, Lebrigand K, Schouteden C, Augereau A, Bauwens S, Ye J, Santagostino M, Giulotto E, Magdinier F, Horard B, Barbry P, Waldmann R, Gilson E. 2011. The human TTAGGG repeat factors 1 and 2 bind to a subset of interstitial telomeric sequences and satellite repeats. Cell Res. 21:1028–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumour suppressor. PLoS Biol. 6:2853–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lou Z, Wei J, Riethman H, Baur JA, Voglauer R, Shay JW, Wright WE. 2009. Telomere length regulates ISG15 expression in human cells. Aging 1:608–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen JB, Hassel BA. 2006. The interferon regulated ubiquitin-like protein, ISG15, in tumourigenesis: friend or foe? Cytokine Growth Factor Rev. 17:411–421 [DOI] [PubMed] [Google Scholar]
- 34.Satake H, Tamura K, Furihata M, Anchi T, Sakoda H, Kawada C, Iiyama T, Ashida S, Shuin T. 2010. The ubiquitin-like molecule interferon-stimulated gene 15 is overexpressed in human prostate cancer. Onc. Rep. 23:11–16 [PubMed] [Google Scholar]
- 35.Arnoult N, Van Beneden A, Decottignies A. 2012. Telomere length regulates TERRA levels through increased trimethylation of telomeric H3K9 and HP1α. Nat. Struct. Mol. Biol. 19:948–956 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.