Abstract
In contrast to prokaryotes, the precise mechanism of incorporation of ribosomal proteins into ribosomes in eukaryotes is not well understood. For the majority of eukaryotic ribosomal proteins, residues critical for rRNA binding, a key step in the hierarchical assembly of ribosomes, have not been well defined. In this study, we used the mammalian ribosomal protein L13a as a model to investigate the mechanism(s) underlying eukaryotic ribosomal protein incorporation into ribosomes. This work identified the arginine residue at position 68 of L13a as being essential for L13a binding to rRNA and incorporation into ribosomes. We also demonstrated that incorporation of L13a takes place during maturation of the 90S preribosome in the nucleolus, but that translocation of L13a into the nucleolus is not sufficient for its incorporation into ribosomes. Incorporation of L13a into the 90S preribosome was required for rRNA methylation within the 90S complex. However, mutations abolishing ribosomal incorporation of L13a did not affect its ability to be phosphorylated or its extraribosomal function in GAIT element-mediated translational silencing. These results provide new insights into the mechanism of ribosomal incorporation of L13a and will be useful in guiding future studies aimed at fully deciphering mammalian ribosome biogenesis.
INTRODUCTION
Ribosome biogenesis is an essential and highly complex process in all living organisms. Ribosomes are composed of numerous precisely assembled proteins and rRNA molecules; thus, they present a paradigm for RNA-protein recognition/binding and ribonucleoprotein (RNP) folding. In prokaryotes, the highly ordered process of ribosome assembly is relatively well defined and has been shown to involve orchestrated changes in rRNA conformation and protein binding steps (1). In contrast, very little is known about the mechanism of eukaryotic ribosome assembly. Despite the general similarity of eukaryotic and prokaryotic ribosomes, they differ substantially in their size, structure, and subunit compositions (including the numbers and sequences of proteins and rRNAs). Another key difference is that the process of maturation and assembly of eukaryotic ribosomes is a highly compartmentalized process which starts in the nucleolus and finishes in the cytoplasm (2). Factors that are not ribosome components but are involved in the ribosome maturation and assembly process also may differ between prokaryotes and eukaryotes. Production of eukaryotic ribosomes (including export from the nucleolus into the cytoplasm) requires highly synchronized synthesis of four rRNAs, about 80 ribosomal proteins, 150 proteins that serve as assembly factors, and 70 small nucleolar RNAs (snoRNAs) that direct modifications to rRNAs (3, 4). Advances in affinity purification methods using epitope-tagged proteins and mass spectrometry have allowed molecular characterization of several distinct RNP complexes corresponding to nucleolar preribosomes at different stages of assembly (5–7). However, this information was primarily derived from studies in yeast, and it is not yet clear whether it can be directly extrapolated to higher eukaryotes. It should also be noted that ribosomal proteins have not been clearly assigned to specific preribosomal complexes isolated from yeast cells by affinity purification, largely due to studies being confounded by nonspecific protein binding, including binding of ribosomal proteins to complexes not related to ribosome biogenesis (6, 8, 9). Moreover, at present, for the majority of eukaryotic ribosomal proteins, residues critical for rRNA binding have not been well defined.
Mammalian ribosomal protein L13a is a member of the highly conserved L13 family of proteins (10). L13a is known to be a component of the 60S ribosomal subunit and to be present in mature 80S ribosomes; however, its function within the ribosome remains unknown. Nevertheless we have demonstrated that L13a has an important extraribosomal function: translational silencing of a cohort of inflammatory proteins in gamma interferon (IFN-γ)-activated human monocytic U937 cells (11, 12). We demonstrated that this extraribosomal function requires phosphorylation-mediated release of L13a from the 60S ribosomal subunit. The free phosphorylated L13a then binds to gamma interferon-activated inhibitor of translation (GAIT) elements in the 3′ untranslated regions (UTRs) of target mRNAs and blocks their translation by inhibiting recruitment of the 43S preinitiation complex to the eukaryotic translation initiation factor 4F complex (13). We also showed that RNA interference (RNAi)-mediated depletion of L13a does not affect rRNA processing and assembly of mature 80S ribosomes and polyribosomes (14) or their primary function, protein synthesis. These studies did, however, identify a critical role for L13a in rRNA methylation, being required for translation of several cellular internal ribosome entry site (IRES)-containing mRNAs (14, 15). Therefore, while the ribosomal protein L13a appears to be dispensable for the canonical function of ribosomes in protein synthesis, it is important for promoting IRES-dependent translation of particular sets of mRNAs via an effect on rRNA methylation and for mediating translational suppression of GAIT element-containing mRNAs following IFN-γ-induced signaling.
In this study, we sought to gain additional insights into the multifaceted activities of L13a by exploring the mechanism underlying its incorporation into the preribosome during ribosome biogenesis, including definition of which L13a residues mediate its association with rRNA. Using a combination of in silico and ex vivo approaches, we demonstrated that ribosomal incorporation of L13a takes place during the synthesis of 90S preribosomes in the nucleolus and that rRNA methylation in 90S preribosomes is L13a dependent. However, translocation of L13a into the nucleolus is not, in itself, sufficient for its incorporation into ribosomes. In addition, we identified the Arg residue at position 68 of L13a as being essential for L13a association with rRNA and incorporation into ribosomes, while a number of other predicted rRNA binding residues were not required. Three other residues, Arg59, Lys60, and Arg61, were found to be important for nuclear import of the L13a protein. Finally, we show that mutations that abolish ribosomal incorporation of L13a do not affect its ability to be phosphorylated or its extraribosomal activity (GAIT element-mediated translational silencing). To our knowledge, this is the first report providing insights into the mechanism of ribosomal incorporation of a mammalian ribosomal protein and addressing how incorporation into the preribosome or ribosome affects the protein's activities. The findings reported here will be useful in guiding future studies ultimately aimed at a complete description of mammalian ribosome biogenesis.
MATERIALS AND METHODS
Comparative modeling and L13A structural analysis.
Comparative modeling of the human L13a structure was performed using the 3D-PSSM algorithm, version 2.6.0 (and its successor, Phyre2), developed by Kelley and others (16, 17), as well as Swiss-Pdb Viewer V4.0.2, using one- and three-dimensional sequence profiles coupled with secondary structure and solvation potential information. PSSM E value (the score or expectation value of the match; designated percent certainty) was used to compare different model structures, and the structures with the lowest E value (highest percent certainty) were chosen for further analysis. Originally, the L13a model was built using Escherichia coli L13 protein as a template (data not shown). However, the L13 structure was further substantially refined upon availability of the crystal structure of the yeast ribosome and the structure of the yeast L16a protein (structural homolog of human L13a) (10). Structures were visualized using PyMOL v0.98 (DeLano Scientific, San Carlos, CA).
Assay for L13a ribosomal incorporation.
The human L13a cDNA and its mutant variants were cloned into the pFLAG-CMV5a vector (Sigma-Aldrich) as EcoRI-BamHI fragments. HEK 293T cells were transfected with these plasmids, and polyribosomes were isolated from the transfected cells by following our previously published procedure (14). Briefly, 16 h posttransfection, cells were treated with 100 μg/ml cycloheximide for 15 min at 37°C, harvested, and lysed in radioimmunoprecipitation assay (RIPA) lysis buffer containing 150 mM NaCl, 1% NP-40, 0.1% SDS, 50 mM Tris, pH 8.0, 1 mM dithiothreitol (DTT), 50 U of recombinant RNasin (Promega, Madison, WI), and protease and phosphatase inhibitor (Sigma-Aldrich, St. Louis, MO). Fifteen optical density units of cytoplasmic lysate was layered over a 10 to 50% linear sucrose gradient in polyribosome buffer (10 mM HEPES, pH 7.5, 100 mM KCl, 2.5 mM MgCl2, 0.1% Igepal-CA630 [Sigma-Aldrich, St. Louis, MO], 1 mM DTT, 50 U of recombinant RNasin) and centrifuged at 17,000 rpm in a Beckman SW32.1 Ti rotor for 18 h at 4°C. Gradients were fractionated, and 12 fractions were collected by the upward displacement method. Light RNP fractions, 40S, 60S, and 80S, and heavy polyribosome fractions were collected by monitoring through the continuous UV absorption profile at A254. Total protein from each fraction was precipitated using trichloroacetic acid (TCA). The TCA precipitates were subjected to SDS-PAGE followed by immunoblot analysis with anti-Flag antibody (Sigma-Aldrich, St. Louis, MO). Cosedimentation of recombinant L13a (detected by immunoblotting) with ribosomal fractions was used as an indication of ribosomal incorporation of the protein.
Association of L13a with 28S rRNA in vivo.
HEK 293T cells were transfected with plasmids expressing Flag-tagged WT and mutant L13a proteins. Lysates from the transfected cells were used for immunoprecipitation with anti-Flag antibody-coated agarose beads. RNA was extracted from the collected immunoprecipitates with TRIzol and reverse transcribed using random hexamers and Superscript according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). First-strand cDNA was used for reverse transcription-PCR (RT-PCR) with primers specific for human 28S rRNA (NCBI entry NR_003287.2), 5′-GAAGTTTCCCTCAGGATAGCT-3′ (forward) and 5′-GCAGGTGAGTTGTTACACACT-3′ (reverse), producing a PCR product of 355 bp. For quantitative RT-PCR (qRT-PCR) detection of 28S rRNA, first-strand cDNA was amplified using SYBR green PCR master mix (Applied Biosystems, Foster City, CA) and primers 5′-GTTCACCCACTAATAGGGAACGTGA-3′ (forward; position 12403) and 5′-GATTCTGACTTAGAGGCGTTCAGT-3′ (reverse; position 12614), which were based on the human 28S rRNA sequence (GenBank accession number U13369) (18). Data are reported as the ratio of CT (threshold cycle) values obtained with RNA immunoprecipitated using anti-Flag antibody-coupled beads versus noncoupled blank beads.
Immunofluorescence studies.
HEK 293T cells were transfected with plasmids expressing L13a-hemagglutinin (HA) epitope-tagged protein. At 24 h posttransfection, cells were fixed with methanol, permeabilized with Triton X-100, and incubated with a mouse monoclonal anti-HA antibody (Molecular Probes, Eugene, OR) and a rabbit polyclonal anti-nucleolin antibody (Abcam, Cambridge, MA). Following primary antibody incubation, cells were washed and incubated with goat anti-mouse antibody conjugated with Alexa Fluor 488 (green) or goat anti-rabbit antibody conjugated with Alexa Fluor 594 (red). Cells were costained with 4′,6-diamidino-2-phenylindole (DAPI) (blue) to visualize nuclei. Stained cells were imaged using a Nikon TE-2000-E inverted fluorescence microscope. z-stacks were obtained, and a deconvolution algorithm was used to process the image stacks.
Analysis of L13a association with 90S preribosomes.
90S preribosomes were isolated by following our previously published procedure (14) from HEK 293T cells transfected with plasmids expressing Flag-tagged L13a protein or mutant variants of L13a. Sixteen hours posttransfection, cells were lysed in hypotonic buffer (10 mM HEPES, pH 7.5, 10 mM KCl, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 0.1% Igepal-CA630). Nuclei were collected by centrifugation at 1,400 × g for 5 min and lysed in nuclear lysis buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 0.5% Igepal-CA630, and 1 mM PMSF). The nuclear extracts were resolved by centrifugation through a 10 to 40% sucrose gradient. Fractions corresponding to pre-40S, pre-60S, and 90S preribosomes were collected by monitoring through the continuous UV absorption profile at A254. Total protein from each fraction was TCA precipitated, separated by SDS-PAGE, and immunoblotted with anti-Flag antibody. The authenticity of 90S preribosomes was confirmed by RT-PCR amplification of RNA isolated from the fraction using primers specific for the 5′ external transcribed spacer (ETS) and 18S sequences present in the unprocessed 47S rRNA, the precursor RNA that is a component of the 90S preribosome. ETS primer sequences were 5′-GCGCACGTCCCGTGCTC-3′ (forward) and 5′-GAGGGGGAAGCGGAGGAGG-3′ (reverse). 18S primers were 5′-GCGCTGACCCCCTTCGC-3′ (forward) and 5′-CTCCCCGGGTCGGGAGTG-3′ (reverse).
Analysis of de novo methylation of rRNA.
De novo methylation of rRNA in 90S preribosomes was evaluated by preincubating the cells in methionine-free Dulbecco's modified Eagle medium (DMEM) for 30 min, followed by pulse-labeling with 100 μCi of [3H]methyl methionine for 1 h. Following the pulse, nuclear extracts were prepared and 106 cpm was used. Fractions corresponding to the preribosome fractions were resolved by sucrose gradient centrifugation as described above. Radioactivity in each fraction was determined by scintillation counting.
RESULTS
Comparative modeling and L13A structural analysis.
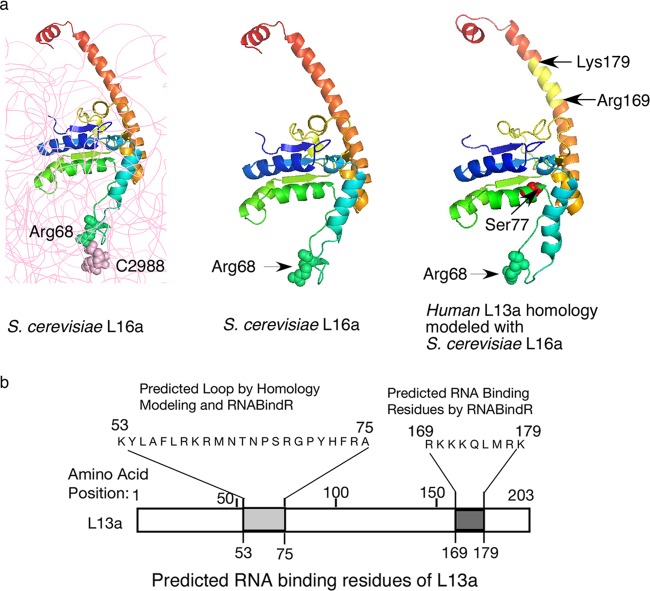
A recent breakthrough in solving the structure of the yeast ribosome at 3.0-Å resolution (19) allowed us to perform comparative modeling and predict the structure of the human L13a protein (using yeast L16a, the structural homologue of L13a, as a template) with very high (∼100%) certainty (Fig. 1a). In this model, Arg68 of L13a was predicted to be located at the tip of the protruding loop region. In yeast L16a, Arg68 interacts with the cytosine at position 2988 (C2988) of the 25S rRNA (Fig. 1a, left and middle) and probably serves to anchor the protein to the rRNA. Interestingly, a Web-based server, RNABindR (http://einstein.cs.iastate.edu/RNABindR/) (20), which employs a distance cutoff to predict amino acids as probable candidates to contact RNA using solved ribonucleoprotein (RNP) structures available in the Protein Data Bank (PDB), also identified Arg68 of L13a as a potential RNA binding candidate within a region comprised of residues 53 to 75. The RNABindR algorithm also predicted a second motif within the region spanning Arg169 to Gln173 as a potential RNA binding site. Binding of a ribosomal protein to rRNA is a possible mechanism for incorporation of the protein into ribosomes during their biogenesis. Therefore, we designed experiments to test the importance of positively charged residues (likely to mediate interaction with negatively charged RNA) within the two potential rRNA binding regions (Lys53 to Ala75 and Arg169 to Gln173) for incorporation of L13a into ribosomes (Fig. 1b).
Fig 1.
L13a protein structure and the putative rRNA binding residues. (a, left and middle) The structure of yeast S. cerevisiae L16a (L13a homologue) depicted as ribbon diagrams based on the crystal structure (Protein Data Bank codes 3U5E and 3O58). In the left panel, rRNA is shown in gray; L16a Arg68 forms van der Waals interactions with 25S rRNA C2988. In the right panel, the predicted structural model of human L13a, generated by using the S. cerevisiae L16a structure as a template for comparative modeling, is shown. van der Waals radii of Arg68 and C2988 are shown. The IFN-γ-mediated phosphorylation site at Ser77 and the RNA binding residues (Arg169 to Lys179) predicted by RNABindR are shown. (b) Potential L13a RNA binding regions predicted by homology modeling using the S. cerevisiae L16a structure and/or the Web-based RNABindR server (http://einstein.cs.iastate.edu/RNABindR/).
Identification of L13a residues required for ribosome incorporation.
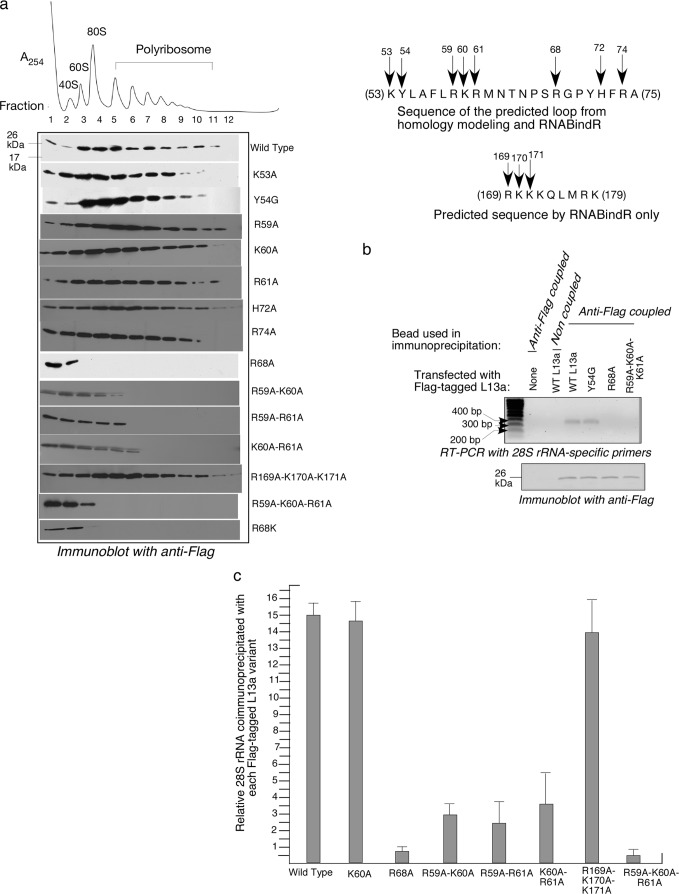
The importance of different L13a residues for ribosomal incorporation of the protein was tested by determining whether L13a variants with particular mutations cosedimented with ribosome subunits and/or ribosomes separated by density gradient centrifugation. To accomplish this, we expressed C-terminally Flag-tagged human L13a protein variants in human 293T cells and assessed (by Western blotting with anti-Flag antibodies) distribution of the expressed protein in ribosomal and polyribosomal fractions resolved by sucrose density gradient sedimentation (Fig. 2a). Flag-tagged wild-type L13a was readily detected in fractions corresponding to 60S ribosomal subunits, 80S ribosomes, and polyribosomes, thus confirming the suitability of this approach (Fig. 2a). L13a proteins containing a number of different single point mutations, namely, K53A, Y54G, R59A, K60A, H72A, R61A, and R74A, were also readily incorporated into ribosomes. In contrast, mutation of Arg68 to alanine completely abolished incorporation of the protein into 60S ribosomal subunits, 80S ribosomes, and polyribosomes (Fig. 2a). These data indicate that Arg68 is essential for ribosomal incorporation of L13a and strongly suggest that it mediates binding of L13a to rRNA. L13a double mutants R59A-K60A, R59A-R61A, and K60A-R61A revealed only mild defects in ribosomal incorporation, and the triple mutant R169A-K170A-K171A showed no incorporation defects (Fig. 2a), suggesting that these residues and regions are not actively involved in rRNA binding (Fig. 2a). However, another triple mutant, R59A-K60A-R61A, showed an incorporation defect similar to that of R68A (Fig. 2a). This finding suggests that the L13a region, comprised of residues 59 to 61, plays an accessory role in anchoring L13a to rRNA or in transport of L13a into the nucleus for ribosome assembly.
Fig 2.
Identification of residues of L13a required for ribosome incorporation. (a) Cosedimentation of L13a variants with ribosomes. HEK 293T cells were transfected with plasmids directing expression of Flag-tagged wild-type L13a or its different mutant variants as indicated to the right of each panel. Ribosomal fractions were resolved by sucrose density gradient centrifugation (10 to 50% sucrose) and analyzed by Western blotting with anti-Flag antibody. The inset on the right shows the sites of the tested L13a mutations. (b) Detection of L13a association with 28S rRNA in vivo by coimmunoprecipitation. The indicated Flag-tagged L13a variants were immunoprecipitated from transfected HEK 293T cells (or nontransfected cells as a control [lane 2]) using anti-Flag-coated agarose beads (or non-antibody-coated blank beads as a control [lane 3]). (Top) RNA present in the immunoprecipitates was extracted and analyzed by RT-PCR with primers specific for human 28S rRNA. (Bottom) Immunoblotting with anti-Flag antibody was used to confirm that equivalent amounts of Flag-tagged protein were present in all immunoprecipitates. (c) Quantitative analysis of L13a association with 28S rRNA. Immunoprecipitates were prepared as described for panel b, using anti-Flag-coupled and noncoupled beads for each L13a variant. Coimmunoprecipitated RNA was isolated and analyzed by qRT-PCR using human 28S rRNA-specific primers. The data are expressed as the ratio of CT values obtained from immunoprecipitates using anti-Flag-coupled beads versus noncoupled beads.
Having found that the residue Arg68 is critical for L13a ribosomal incorporation, we next tested whether this effect is mediated by the positive charge of Arg68 per se or, perhaps, by the charge and structure of the Arg residue in combination. To test this, we generated a mutant L13a variant with Arg68 replaced with another positively charged amino acid, Lys (R68K). When expressed in 293T cells, R68K mutant L13a failed to incorporate into 60S ribosomal subunits, 80S ribosomes, and polyribosomes (Fig. 2a). This demonstrates that the presence of a positively charged amino acid at position 68 of the L13a protein is not sufficient to mediate L13a ribosomal incorporation; rather, other (or additional) aspects of the Arg residue must determine its importance for this process.
As a complementary approach to evaluate the role of different L13a residues in L13a ribosomal incorporation, we directly tested in vivo association of L13a protein variants with 28S rRNA. To this end, we immunoprecipitated L13a complexes from lysates of 293T cells transfected with different Flag-tagged L13a expression constructs using anti-FLAG antibodies and tested them for the presence of 28S rRNA by RT-PCR using 28S rRNA-specific primers. We chose to test three L13a mutants: two incorporation defective (R68A and R59A-K60A-R61A) and one incorporation competent (Y54G). Consistent with the ribosome incorporation results obtained through cosedimentation experiments (Fig. 2a), 28S rRNA was not detected in immunoprecipitates of R68A or R59A-K60A-R61A L13a proteins (Fig. 2b). On the other hand, the immunoprecipitation method did show association of 28S rRNA with both wild-type L13a and the Y54G L13a mutant (shown to be incorporation competent by cosedimentation) (Fig. 2b, upper). Equal efficiency of immunoprecipitation for the different L13a variants was confirmed by immunoblot analysis with anti-Flag antibody (Fig. 2b, lower). Specificity of the coimmunoprecipitation results was confirmed by the absence of amplified 28S rRNA product in immunoprecipitation reactions performed with mock-transfected cells and anti-FLAG-coupled beads or with Flag-tagged wild-type L13a-transfected cells and beads not coupled to an antibody (Fig. 2b, upper, first two lanes).
To quantitatively compare the relative efficiencies of 28S rRNA association of L13a and its different mutants, we performed qRT-PCR analysis of RNA recovered from the anti-Flag immunoprecipitates described above. These results were entirely consistent with the results of cosedimentation experiments (Fig. 2a), confirming that the triple mutant R59A-K60A-R61A and the single mutant R68A have similar severe defects in association with 28S rRNA (Fig. 2c). In addition, the double mutants R59A-K60A, R59A-R61A, and K60A-R61A showed very weak association with rRNA compared to wild-type L13a and the K60A and R169A-K170A-K171A mutants. This defect might explain why the double mutant proteins were not observed in heavier polyribosome fractions (Fig. 2a).
Subcellular localization of L13a during ribosome biogenesis and its relationship to ribosome incorporation.
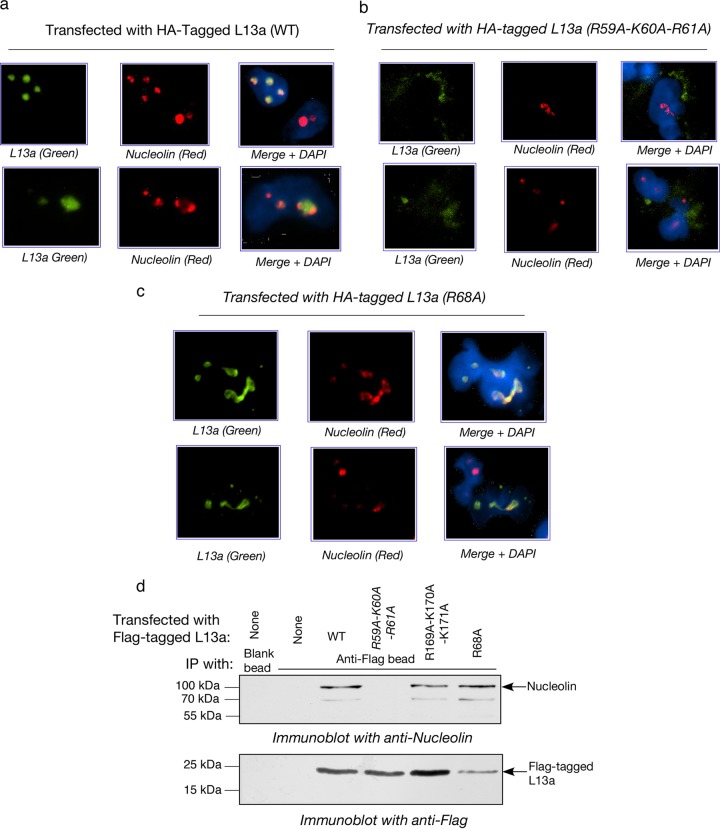
The absence of association of L13a R68A and R59A-K60A-R61A mutants with ribosomes and rRNA described above could be due to (i) inability of the mutant proteins to directly associate with rRNA, and/or (ii) inability of the mutant proteins to reach the nucleolar compartment(s) where most of the ribosome assembly events take place in eukaryotes (21). To distinguish between these possibilities, we used immunofluorescence to monitor the intracellular distribution of L13a and determine whether mutations that affect ribosomal incorporation of the protein also affect its import into the nucleolus. HEK 293T cells were transfected with the HA-tagged wild type or incorporation-defective (R68A and R59A-K60A-R61A) L13a mutants, and 24 h later they were fixed and stained with antibodies detecting the HA tag (Alexa Fluor-488; green) or nucleolin (a marker of the nucleolus; Alexa Fluor 594; red). Cells were counterstained with DAPI to visualize nuclei. In these experiments, both wild-type L13a (Fig. 3a) and R68A mutant L13a (Fig. 3c) translocated into the nucleolus (green and red colors merged), while the R59A-K60A-R61A mutant protein failed to translocate even into the nucleus (Fig. 3b). The R59A-K60A-R61A L13a variant was retained in the cytoplasm and accumulated at the nuclear periphery, suggesting that these mutations destroyed a nuclear localization signal in L13a. Although both wild-type and R68A mutant L13a were found to be present in nucleoli, the mutant protein appeared to alter nucleolar structure. In contrast to cells expressing wild-type L13a, those expressing the R68A variant had more diffuse nucleoli with irregular shapes (Fig. 3, compare a to c). This finding presents the possibility that presence of L13a in the nucleolus without incorporation into preribosomes causes a change in nucleolar structure and/or function.
Fig 3.
Colocalization of L13a and nucleolin in the nucleolus. (a to c) Immunofluorescence-based detection of HA-tagged L13a and nucleolin in HEK 293T cells transfected with HA-tagged wild type (a) or mutant L13a variants (R59A-K60A-R61A in panel b, R68A in panel c). At 24 h posttransfection, cells were fixed and stained with antibodies against the HA tag (green) and nucleolin (red) and with DAPI (blue; to visualize nuclei). (d) In vivo association of L13a and the ribosome incorporation-defective L13a mutant R68A with nucleolin. Immunoprecipitation (IP) analysis was performed as described for Fig. 2b. L13a proteins were immunoprecipitated from HEK 293T cells transfected with the indicated Flag-tagged L13a variants using anti-Flag-coupled beads (or noncoupled beads as a control). Immunoprecipitates were analyzed by immunoblotting with antinucleolin (top panel) and anti-Flag (bottom panel) antibodies.
Nucleolin is a ubiquitous RNA and protein binding protein that is mainly localized to the dense fibrillar region as well as the granular compartment of the nucleolus, where rRNA processing and modifications and ribosome assembly take place (22, 23). Nucleolin has multiple functions within the cell (24) and the nucleus (25), including promotion of rRNA processing (26) and import of nuclear and ribosomal proteins into the nucleolus (27, 28). Given this background and our immunofluorescence data showing colocalization of L13a and nucleolin, we investigated whether the two proteins interact directly. Immunoprecipitation of Flag-tagged L13a proteins expressed in HEK 293T cells using anti-Flag antibody-conjugated beads resulted in coimmunoprecipitation of nucleolin with wild-type L13a, the incorporation-defective R68A L13a mutant, and the incorporation-competent R169A-K170A-K171A triple mutant (Fig. 3d). However, the R59A-K60A-R61A triple mutant, shown to be defective in nuclear import (Fig. 3b), was not found to be associated with nucleolin by immunoprecipitation (Fig. 3d). Together, these experiments show that L13a translocates into the nucleolus during ribosome biogenesis in association with nucleolin, but that is not sufficient for ribosome incorporation. Arg68 appears to be crucial for L13a incorporation into ribosomes but not for L13a-nucleolin association or translocation of L13a into the nucleolus.
Methylation of rRNA within 90S precursor ribosomes during ribosome biogenesis is L13a dependent.
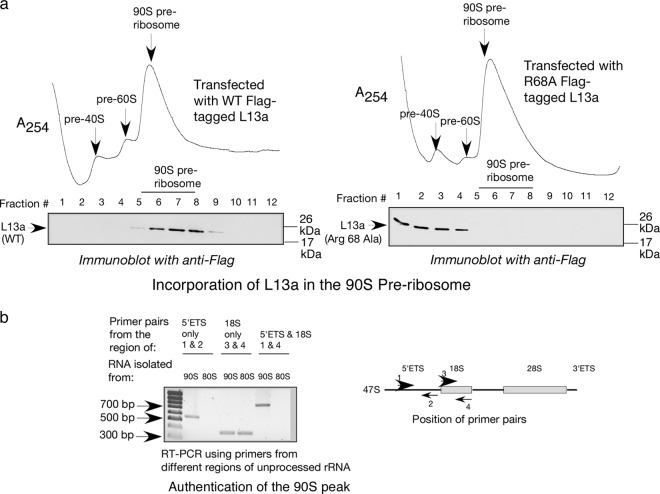
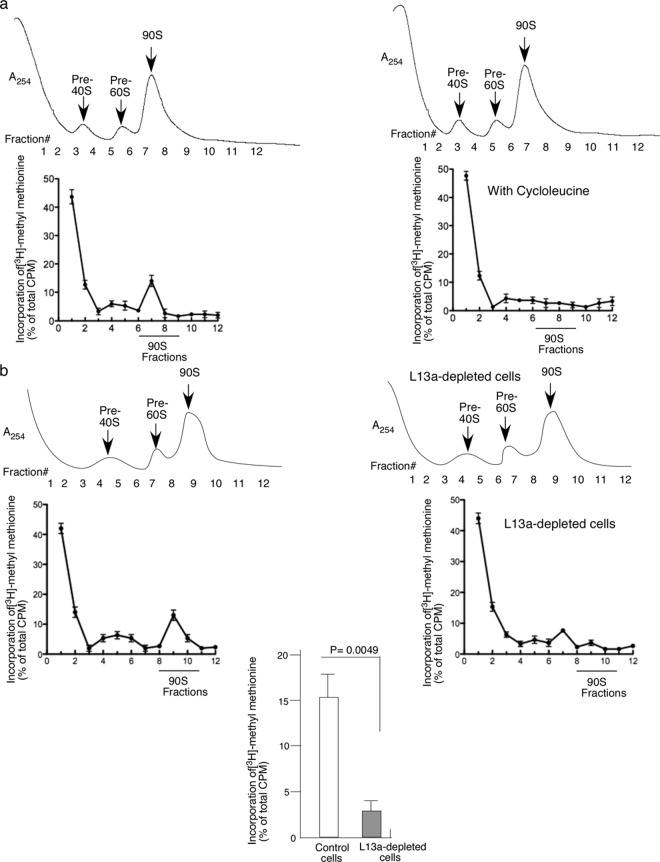
Previous studies, mostly conducted in yeast, identified the 90S preribosome as a major site for rRNA processing (2, 5) and modification (29, 30). Our earlier studies demonstrated that L13a is required for rRNA methylation (14) and identified L13a as a part of the fibrillarin and snoRNA-containing C/D box snoRNP complex (15) that is responsible for rRNA methylation in mammalian cells (31, 32). Therefore, here we tested whether L13-dependent rRNA methylation occurs in 90S preribosomes and whether binding of L13a to rRNA affects rRNA methylation. To accomplish this, we prepared nuclear lysates from HEK 293T cells transfected with expression constructs for Flag-tagged wild-type or R68A mutant L13a proteins and resolved pre-40S, pre-60S, and 90S fractions by sucrose density gradient centrifugation. The presence of L13a protein in fractions was monitored by immunoblot analysis with anti-Flag antibody (Fig. 4). The authenticity of the 90S fractions was confirmed by RT-PCR using primers specific for the 5′ external transcribed spacer (ETS) and 18S sequences present in the unprocessed 47S rRNA that is a component of the 90S preribosome (Fig. 4b). As expected, no product was obtained using the 5′ETS primers from the RNA sample obtained from the 80S fraction due to absence of the 5′ETS (Fig. 4b). Fractions confirmed to contain 90S preribosomes using this RT-PCR assay were found to contain L13a protein when the fractions were from cells expressing wild-type L13a but not when the fractions were from cells expressing the R68A mutant (Fig. 4a). This indicates that wild-type L13a, but not R68A mutant L13a, incorporates into 90S preribosomes (Fig. 4a). Metabolic labeling with [3H]methyl methionine next was used to measure de novo methylation of rRNA in 90S preribosomes and the requirement for L13a in this process. The results presented in Fig. 5a show rRNA methylation occurring in 90S fractions from HEK 293T cells and its sensitivity to cycloleucine, an inhibitor of rRNA methylation. The level of rRNA methylation in different fractions was determined by scintillation counting of 3H following pulse-labeling of cells with [3H]methyl methionine. Data were collected from triplicate samples and are expressed as a percentage of the total cpm. To test whether L13a is required for rRNA methylation in the 90S preribosome, we performed the same analysis on HEK 293T cells depleted of L13a by RNAi (14). The extent of L13a depletion by RNAi (>80%) was checked by immunoblot analysis using anti-L13a antibody (see Fig. S1a in the supplemental material). We found that rRNA methylation in 90S preribosomes was significantly (P = 0.0049 by two-tailed paired Student t test) inhibited upon RNAi-mediated L13a depletion (Fig. 5b, bottom). Together, these results indicate that L13a incorporates into the 90S preribosome during biogenesis and is required for efficient rRNA methylation of the 90S preribosome.
Fig 4.
L13a mutant variant R68A is not incorporated into 90S preribosomes during ribosome biogenesis. (a) Western blotting with anti-Flag antibody of nuclear fractions containing ribosome precursor complexes resolved by sucrose density gradient centrifugation (10 to 40% sucrose). Fractions were obtained from HEK 293T cells expressing Flag-tagged wild-type L13a (left panel) or R68A mutant L13A (right panel). (b) Confirmation of the authenticity of the 90S preribosome fraction. Total RNA was extracted from 90S peak fractions prepared from nuclear lysates and 80S peak fractions prepared from cellular lysates of untransfected HEK 293T cells. RT-PCR analysis was performed using primers specific to the 5′ external transcribed spacer (ETS) and 18S sequence present in the unprocessed 47S rRNA component of 90S preribosomes (primer positions are shown in the right panel). The rRNA in 80S ribosomes is processed such that the 5′ETS sequence is removed while the 18S sequence is preserved. Bands observed upon agarose gel electrophoresis were of the predicted sizes.
Fig 5.
rRNA methylation occurs in 90S preribosomes during ribosome biogenesis and requires the presence of L13a. (a) rRNA methylation occurs in 90S preribosomes. HEK 293T cells were pulse-labeled with [3H]methyl methionine as described in Materials and Methods in the absence (left panel) or presence (right panel) of cycloleucine. Nuclear lysates from these cells were subjected to 10 to 40% sucrose density gradient centrifugation. Radioactivity incorporated into preribosomal fractions resolved by centrifugation was determined by liquid scintillation counting. Data shown are the averages from three experiments. (b) rRNA methylation in 90S preribosomes requires L13a. rRNA methylation was assessed using [3H]methyl methionine pulse-labeling of cells as described for panel a. Cells were unaltered HEK 293T cells (top left panel) or HEK 293T cells depleted of L13a (top right panel) through expression of a short hairpin RNA against L13a as previously described (14). The extent of L13a depletion in these cells (>80%) was confirmed by immunoblot analysis using an anti-L13a antibody (see Fig. S1 in the supplemental material). The reduction in rRNA methylation upon L13a depletion was statistically significant (P = 0.0049 by paired Student t test, two-tailed; lower panel).
Ribosome incorporation-defective L13a variants retain GAIT element-mediated translational silencing activity.
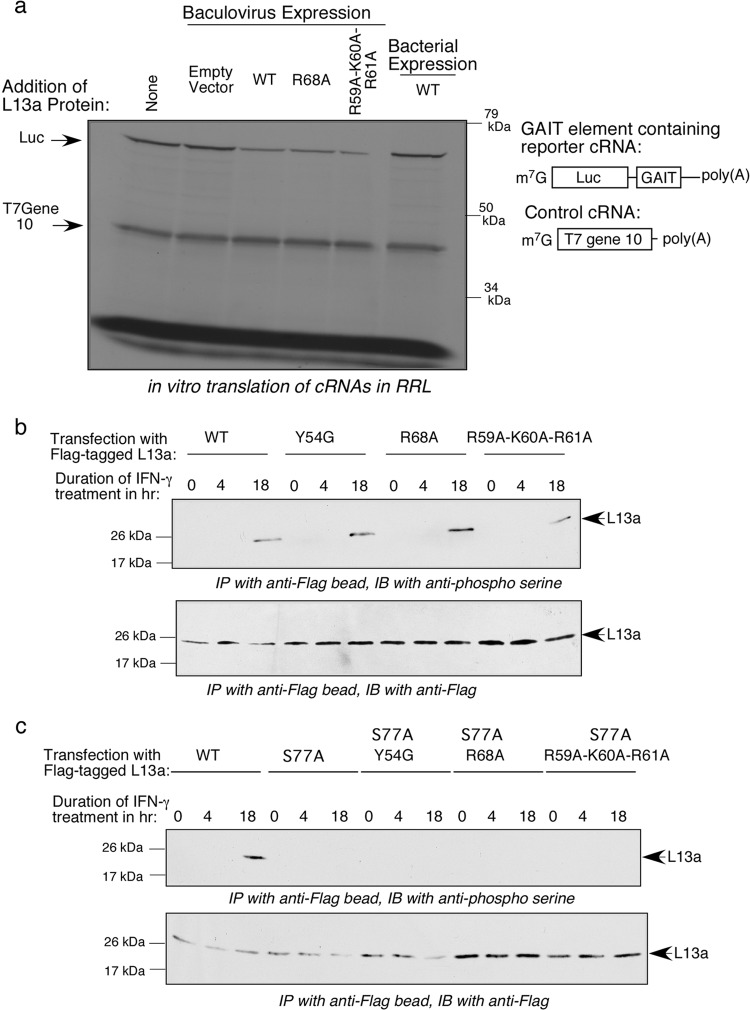
We previously defined an extraribosomal activity of L13a: IFN-γ-induced translational silencing of a cohort of mRNAs encoding inflammatory proteins and containing a GAIT element in their 3′-UTRs (11, 12, 33, 34). This activity requires phosphorylation-mediated release of L13a from the 60S ribosomal subunit, which occurs in response to IFN-γ (13, 35). However, the mechanism of L13a assembly into the GAIT complex remains poorly understood. Thus, we decided to test whether ribosome incorporation-defective L13a mutants retain GAIT element-mediated translational silencing activity. This was done by monitoring the translation of a GAIT element-containing reporter RNA in a rabbit reticulocyte lysate (RRL) in vitro translation system. Translation of T7 gene reporter mRNA 10 without a GAIT element was used as a control for nonspecific inhibition as previously described (13). Both tested incorporation-defective L13a mutants, R68A and R59A-K60A-R61A, showed activity similar to that of wild-type L13a in silencing translation of the GAIT element-containing reporter RNA (Fig. 6a). These data strongly suggest that ribosomal incorporation of L13a is not a prerequisite for its translational silencing activity, and that a residue(s) of L13a required for its ribosome incorporation is not required for its assembly into the GAIT complex.
Fig 6.
Ribosome incorporation-defective L13a variants retain GAIT element-mediated translational silencing activity and are subject to IFN-γ-induced phosphorylation on Ser77. (a) Purified recombinant His-tagged wild-type L13a and its mutant variants, R68A and R59A-K60A-R61A, were produced in a baculovirus expression system and used in in vitro translation reactions with rabbit reticulocyte lysate (RRL). Wild-type L13a protein produced in a bacterial expression system was also tested. Reporter mRNAs containing a GAIT element (Luc) or lacking a GAIT element (T7 gene 10; control) were cotranslated in the RRL translation system for 1 h in the presence or absence of L13a variants. 35S-radiolabeled translation products were resolved on 10% SDS-PAGE gels and visualized by autoradiography. (b) IFN-γ-induced serine phosphorylation of L13a is preserved in mutant variants of L13a. U937 cells were transfected with Flag-tagged L13a protein variants and treated with IFN-γ for 0, 4, and 18 h. Cell lysates were used for immunoprecipitation with anti-Flag antibody. Immunoprecipitates were analyzed by immunoblotting (IB) with antiphosphoserine antibody (top panel) or anti-Flag antibody (bottom panel). (c) IFN-γ-induced Ser phosphorylation of L13a is restricted to Ser77. U937 cells were transfected with Flag-tagged L13a protein variants harboring an additional S77A substitution on the backgrounds used for panel b, namely, the wild type and Y54G, R68A, and R59A-K60A-R61A variants. IFN-induced serine phosphorylation was evaluated as described for panel b.
Ribosomal incorporation of L13a is not a prerequisite for IFN-γ-induced phosphorylation of L13a Ser77.
IFN-γ-induced phosphorylation of L13a by death-associated protein kinase–zipper-interacting protein kinase (DAPK-ZIPK) at Ser77 is known to be critical for its release from the 60S ribosomal subunit (36). However, it is unknown whether the ribosomal location of L13a is a prerequisite for this phosphorylation event. To address this question, we evaluated whether L13a mutants found to be ribosome incorporation defective still can be phosphorylated through this mechanism. HEK 293T cells were transfected with expression constructs for Flag-tagged and incorporation-defective (R68A and R59A-K60A-R61A) or incorporation-competent (wild type and Y54G) L13a proteins and treated with IFN-γ to activate the DAPK-ZIPK phosphorylation cascade. Phosphorylation of L13a proteins was detected by immunoprecipitation with anti-Flag antibodies followed by immunoblotting with a phosphoserine-specific antibody (Fig. 6b, upper). The extent of immunoprecipitation of the Flag-tagged protein was monitored by immunoblotting with anti-Flag antibody (Fig. 6b, lower). Consistent with our previous findings (11), we observed delayed phosphorylation of wild-type L13a occurring after 18 h of IFN-γ treatment. Interestingly, the two incorporation-defective mutants (R68A and R59A-K60A-R61A) and one incorporation-competent mutant (Y54G) all showed levels and time dependence of phosphorylation equivalent to those of the wild-type protein (Fig. 6b). It remained possible, however, that the mutant L13a proteins were phosphorylated on another Ser residue(s) besides Ser77. To exclude this possibility, we performed the same analysis of IFN-γ-induced serine phosphorylation in cells expressing L13a with Ser77 mutated to Ala in addition to the other mutations (Y54G, R68A, and R59A-K60A-R61A) (Fig. 6c). In all cases, the S77A mutation abrogated phosphorylation, strongly suggesting that Ser77 is a major phosphorylation site in all of the L13a mutants, as is the case for the wild-type protein. The finding that IFN-γ-induced phosphorylation of Ser77 occurs equally in both ribosome incorporation-competent and -incompetent variants of L13a indicates that the ribosomal location of L13a is not a prerequisite for this phosphorylation event.
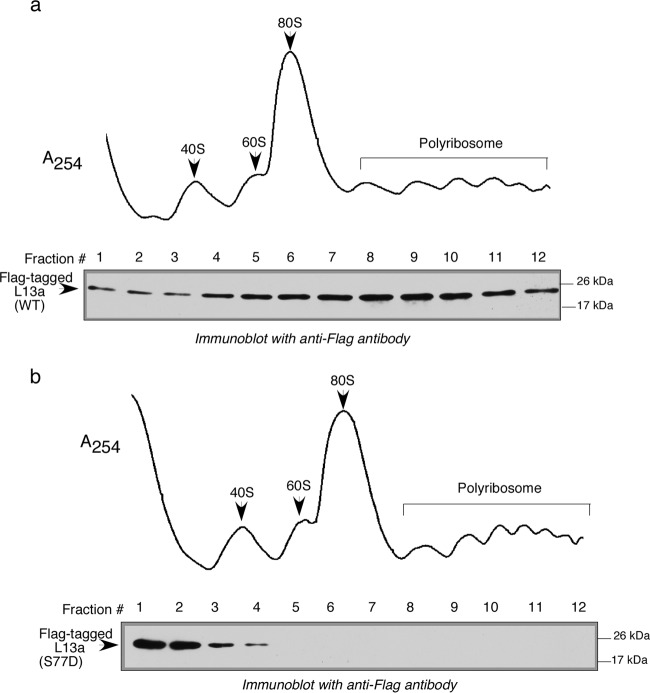
Phosphomimetic mutation of the serine at position 77 of L13a to aspartic acid prevents incorporation of L13a into ribosomes.
As mentioned above, IFN-induced phosphorylation of L13a at Ser77 causes release of the phosphorylated protein from the 60S ribosomal subunit (36). It has also been shown that phosphorylation of L13a at Ser77 severely compromises subsequent association of the phosphoprotein with the mature ribosome (36). However, whether phosphorylation of Ser77 blocks incorporation of L13a into the ribosome during biogenesis was not previously studied. To directly address this question, we tested the effect of phosphomimetic mutation of Ser77 to Asp (S77D) on incorporation of L13a into ribosomes in vivo. Flag-tagged wild-type L13a and S77D mutant L13a proteins were expressed in HEK 293T cells, and immunoblotting with anti-Flag antibody was used to assess the presence of the L13a proteins in different ribosomal fractions, which was resolved by density gradient centrifugation. As shown in Fig. 7, mutation of L13a Ser77 to Asp clearly abrogates ribosomal incorporation of the protein. This result suggests that phosphorylation of Ser77 occurring in vivo prevents incorporation of L13a into the ribosome during ribosomal biogenesis. Another possibility that cannot be excluded at this stage is that Ser77 itself is required for association of L13a with rRNA (and, thus, its ribosomal incorporation as well); therefore, it would not tolerate any charge alteration either by phosphorylation or by phosphomimetic mutation.
Fig 7.
Phosphomimetic alteration of Ser at position 77 of L13a to Asp abrogates ribosome incorporation of L13a. HEK 293T cells were transfected with Flag-tagged wild-type L13a (a) and/or its mutant variant S77D (b). Total protein from different ribosomal fractions, resolved by sucrose density gradient centrifugation (10 to 50% sucrose), was analyzed by Western blotting with anti-Flag antibody.
DISCUSSION
Assembly of prokaryotic ribosomes is a complex process in which ribosomal proteins bind to rRNA in a highly organized, hierarchical manner. The assembly process is facilitated by a number of cofactors that catalyze rRNA processing, aid in rRNP remodeling, and may serve as sensors at checkpoints during the ribosome assembly (1). Existing studies of eukaryotes, primarily performed in yeast, have revealed an even more complex mechanism of ribosome assembly requiring numerous additional cofactors, such as ATPases (37), endo- and exoribonucleases (23), RNA helicases (38, 39), accessory proteins (7, 40, 41), and snoRNPs (42, 43). The greater number of ribosomal proteins and involvement of both cytoplasmic and nuclear/nucleolar compartments also contribute to the greater complexity of eukaryotic ribosome assembly. This process appears to be highly organized, proceeding in a stepwise manner in various nuclear/cellular compartments (3, 4). For both prokaryotes and eukaryotes, the exact details of the mechanism(s) by which rRNA and ribosomal proteins organize themselves into functional three-dimensional ribosomal structures remain unknown.
Ribosome assembly in eukaryotes involves pre-rRNA processing, modification(s), and folding steps accompanied by binding of ribosomal proteins; thus, it proceeds through distinct intermediates (preribosomal particles). Tandem affinity purification approaches have been used to isolate and characterize these intermediates (44–46). The biogenesis of both the 40S and 60S eukaryotic subunits involves a 90S preribosome containing a common precursor rRNA (35S pre-rRNA), accessory proteins, and (mostly) small ribosomal subunit proteins (7, 47). Subsequently, the 35S pre-rRNA within the 90S preribosome is cleaved into the 20S pre-rRNA and 27S pre-rRNA that serve as cores for assembly of pre-40S and pre-60S ribosomal subunits, respectively. Successive processing and maturation steps taking place in the nucleolus, nucleus, and cytoplasm create mature 40S and 60S subunits.
Identification of residues responsible for ribosomal protein attachment to rRNA would be helpful in deciphering the details of how proteins are incorporated into ribosomes during their biogenesis. However, for most eukaryotic ribosomal proteins, these critical residues have not been mapped. In this study, we used the mammalian ribosomal protein L13a as a model to study incorporation of ribosomal proteins. As detailed below, we succeeded in identifying a specific residue (Arg68) required for ribosomal incorporation of L13a and used mutation of this residue to determine the relationship between L13a ribosomal incorporation and other aspects of ribosome biogenesis (e.g., nucleolar import) and L13a function (e.g., rRNA methylation and GAIT element-mediated translational silencing). Although the result from the homology modeling (Fig. 1a) is consistent with the notion that Arg68 directly interacts with rRNA, at this stage we could not completely rule out the possibility that it also is required for an early step of the multistep process of ribosome biogenesis. Our results provide a number of new insights into the mechanism of eukaryotic ribosome biogenesis and will serve as a foundation for future investigations aimed at, for example, defining the hierarchical relationship between other ribosomal proteins and accessory proteins in the process.
A key finding of this work was identification of a residue of L13a (Arg68) that is essential for L13a incorporation into ribosomes and association with 28S rRNA (Fig. 2). The concurrence of results obtained by (i) immunoblot analysis of L13a presence in ribosomal fractions resolved by density gradient centrifugation and (ii) RT-PCR-based detection of 28S rRNA in L13a immunoprecipitates strengthens our conclusion that L13a Arg68 is a critical mediator of this step of ribosome assembly. The predicted location of this residue at the tip of the protruding loop region (Fig. 1) and its extreme conservation among all eukaryotic L13a family proteins (data not shown) suggest that it serves to attach L13a to rRNA in all eukaryotic organisms. This activity is not, however, simply due to the presence of a positive charge at position 68 of the L13a amino acid sequence, since replacement of Arg68 with Lys abrogated ribosomal incorporation (Fig. 2a). We also found that Arg59, Lys60, and Arg61 may form part of the L13a nuclear localization signal, since their mutation impeded L13a nuclear import (Fig. 3b). A number of other L13a residues predicted to bind rRNA were determined to play no significant role in L13a ribosomal incorporation.
Our data support a model in which L13a attachment to rRNA takes place in the nucleolus (Fig. 3a) in the context of the 90S preribosome (Fig. 5). However, nucleolar import of L13a is not, in itself, sufficient to drive its ribosomal incorporation, as indicated by the normal nuclear translocation of the incorporation-defective R68A L13a mutant (Fig. 2 and 3c). Interestingly, previous studies suggested that proteins of the large ribosomal subunit predominantly become associated with the pre-60S particle after it has been cleaved and separated from the 90S preribosome (7, 47). However, our studies clearly indicate that this is not the case for L13a (Fig. 5).
While we have established that L13a associates with 90S preribosomes, it remains unclear whether it can also become incorporated into mature 60S subunits. This point is of interest based on previous studies showing that phosphorylation of L13a following IFN-γ treatment causes release of the phosphorylated L13a protein from the 60S ribosomal subunit into the cytoplasm (11). It was not determined whether unphosphorylated L13a protein can incorporate or reassociate with mature 60S ribosomal subunits devoid of L13a. Here, we present preliminary evidence indicating that unphosphorylated L13a does not associate with mature 60S subunits (see Fig. S1 in the supplemental material). This tentative conclusion is based on our finding that bacterially expressed purified recombinant His-tagged L13a failed to associate with purified 60S subunits from L13a-depleted cells in an in vitro binding assay, while the same His-tagged L13a protein was incorporated into ribosomes in vivo when expressed in HEK 293T cells. Additional experiments are needed to confirm that the negative in vitro results are biologically relevant.
It should be noted that while L13a is a verified component of the 60S ribosomal subunit, its function within the ribosome remains unknown. In contrast to many other ribosomal proteins of both the small and large ribosomal subunits (48–54), L13a does not affect ribosome biogenesis and maturation or global protein synthesis (14). We have, however, previously demonstrated that L13a is required for rRNA methylation, one of the rRNA modifications that takes place during ribosome biogenesis (15). Here, we confirm that L13a is required for rRNA methylation and demonstrate that this occurs within the 90S preribosome (Fig. 6). The importance of L13a for rRNA methylation in the 90S preribosome is consistent with our previous finding that L13a is a component of the fibrillarin-containing C/D box snoRNP complex responsible for methyltransferase activity (15). Another demonstrated function of L13a occurs outside the ribosome. We (11, 13) and others (36) have shown that the phosphorylation-dependent release of L13a from 60S subunits in response to extracellular stimuli, such as IFN-γ treatment, leads to incorporation of L13a into the so-called GAIT complex (11, 13, 55) and translational silencing of a cohort of mRNAs harboring GAIT elements in their 3′-UTRs (12). Here, we show that the mechanisms of incorporation of L13a protein into the ribosome and into the GAIT complex are distinct. Our data clearly indicate that L13a mutants that are defective in ribosome incorporation (R68A) or both nuclear import and ribosome incorporation (R59A-K60A-R61A) retain the ability to participate in GAIT-mediated translational silencing (Fig. 6a). We further show that mutations that impede L13a ribosomal incorporation do not affect IFN-γ-induced phosphorylation of L13a on Ser77, the event that leads to L13a release from 60S ribosomes in the process of GAIT element-mediated translational silencing (Fig. 6b). This suggests that phosphorylation of L13a does not require the protein to be ribosome bound.
Phosphorylation of ribosomal proteins has been implicated in several important cellular functions, such as regulation of cell size (56), glucose homeostasis (57), poly(U) translation in prokaryotes (58), and diurnal protein synthesis in plants (59). Therefore, we are also interested in understanding the mechanism(s) underlying the release of L13a from the ribosome upon phosphorylation. L13a Ser77 is located at the edge of the loop region containing Arg68 (Fig. 1a, right). Phosphorylation of Ser77 adds a double negative charge, which can be predicted to create repulsive electrostatic forces capable of overcoming van der Waals and electrostatic interactions of Arg68 with rRNA. However, as mentioned above, the positive charge of Arg68 does not fully account for its role in rRNA binding, as replacement of Arg68 with Lys prevented L13a ribosomal incorporation (Fig. 2a). In addition, phosphomimetic mutation of Ser77 to Asp abrogated ribosomal incorporation of L13a (Fig. 7). We cannot, however, exclude the possibility that phosphorylation of Ser77 or mutation of Ser77 into Asp alters the conformation of the loop region harboring Arg68, causing the release of the protein from the ribosome. Future studies aimed at monitoring conformational changes within the L13a protein upon phosphorylation should resolve this issue.
Interestingly, recent studies point to the possibility of defects in ribosome biogenesis and ribosomal protein incorporation having physiological effects that are detrimental to human health. For example, mutations and haploinsufficiencies of a number of ribosomal proteins lead to defects in the development of the erythroid lineage from the bone marrow, causing Diamond-Blackfan anemia (DBA) (60). Mutations causing haploinsufficiency of S19 (61), S24 (62), S17 (63), L35a (64), L5, and L11 (65) have been identified in DBA patients and shown to inhibit synthesis of mature ribosomes (60). A different study showed that mutation causing loss of function of L38 led to a defect in tissue patterning during vertebrate development (66). These findings support the possibility that L13a mutations abrogating L13a ribosomal incorporation occur and have a physiological impact.
Our previous studies showed that depletion of L13a by RNAi does not cause any significant defects in global protein synthesis; however, it was shown to affect the expression of a cohort of cellular IRES-containing mRNAs (13, 14). These data indicate that L13a has an important function in the cell in addition to its extraribosomal activity. Complete L13a knockout as well as L13a incorporation-defective knock-in mouse models may provide further insights into the role played by L13a protein in animal cellular physiology and development.
Supplementary Material
ACKNOWLEDGMENTS
These studies were supported by PHS grant NIH HL79164 to B.M., AHA Predoctoral Fellowship grants 0815407D and 11PRE7660008 to P.D. and D.P., respectively, NIH predoctoral training fellowship T32HL076125 to the Center for Lung Biology, University of South Alabama, for J.A., NIH AI 059267 to S.B., HFSP RGP0024 to A.A.K., and the Center for Gene Regulation in Health and Disease, sponsored by a Third Frontier grant from the State of Ohio.
We are grateful to Patricia Stanhope Baker for help with manuscript editing and proofreading.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 20 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00250-13.
REFERENCES
- 1.Shajani Z, Sykes MT, Williamson JR. 2011. Assembly of bacterial ribosomes. Annu. Rev. Biochem. 80:501–526 [DOI] [PubMed] [Google Scholar]
- 2.Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI. 2007. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 8:574–585 [DOI] [PubMed] [Google Scholar]
- 3.Henras AK, Soudet J, Gerus M, Lebaron S, Caizergues-Ferrer M, Mougin A, Henry Y. 2008. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell. Mol. Life Sci. 65:2334–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fromont-Racine M, Senger B, Saveanu C, Fasiolo F. 2003. Ribosome assembly in eukaryotes. Gene 313:17–42 [DOI] [PubMed] [Google Scholar]
- 5.Tschochner H, Hurt E. 2003. Pre-ribosomes on the road from the nucleolus to the cytoplasm. Trends Cell Biol. 13:255–263 [DOI] [PubMed] [Google Scholar]
- 6.Milkereit P, Kuhn H, Gas N, Tschochner H. 2003. The pre-ribosomal network. Nucleic Acids Res. 31:799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saveanu C, Namane A, Gleizes PE, Lebreton A, Rousselle JC, Noaillac-Depeyre J, Gas N, Jacquier A, Fromont-Racine M. 2003. Sequential protein association with nascent 60S ribosomal particles. Mol. Cell. Biol. 23:4449–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavin AC, Bosche M, Krause R, Grandi P, Marzioch M, Bauer A, Schultz J, Rick JM, Michon AM, Cruciat CM, Remor M, Hofert C, Schelder M, Brajenovic M, Ruffner H, Merino A, Klein K, Hudak M, Dickson D, Rudi T, Gnau V, Bauch A, Bastuck S, Huhse B, Leutwein C, Heurtier MA, Copley RR, Edelmann A, Querfurth E, Rybin V, Drewes G, Raida M, Bouwmeester T, Bork P, Seraphin B, Kuster B, Neubauer G, Superti-Furga G. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141–147 [DOI] [PubMed] [Google Scholar]
- 9.Ho Y, Gruhler A, Heilbut A, Bader GD, Moore L, Adams SL, Millar A, Taylor P, Bennett K, Boutilier K, Yang L, Wolting C, Donaldson I, Schandorff S, Shewnarane J, Vo M, Taggart J, Goudreault M, Muskat B, Alfarano C, Dewar D, Lin Z, Michalickova K, Willems AR, Sassi H, Nielsen PA, Rasmussen KJ, Andersen JR, Johansen LE, Hansen LH, Jespersen H, Podtelejnikov A, Nielsen E, Crawford J, Poulsen V, Sorensen BD, Matthiesen J, Hendrickson RC, Gleeson F, Pawson T, Moran MF, Durocher D, Mann M, Hogue CW, Figeys D, Tyers M. 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415:180–183 [DOI] [PubMed] [Google Scholar]
- 10.Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M. 2011. The structure of the eukaryotic ribosome at 3.0 A resolution. Science 334:1524–1529 [DOI] [PubMed] [Google Scholar]
- 11.Mazumder B, Sampath P, Seshadri V, Maitra RK, DiCorleto PE, Fox PL. 2003. Regulated release of L13a from the 60S ribosomal subunit as a mechanism of transcript-specific translational control. Cell 115:187–198 [DOI] [PubMed] [Google Scholar]
- 12.Vyas K, Chaudhuri S, Leaman DW, Komar AA, Musiyenko A, Barik S, Mazumder B. 2009. Genome-wide polysome profiling reveals an inflammation-responsive posttranscriptional operon in gamma interferon-activated monocytes. Mol. Cell. Biol. 29:458–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapasi P, Chaudhuri S, Vyas K, Baus D, Komar AA, Fox PL, Merrick WC, Mazumder B. 2007. L13a blocks 48S assembly: role of a general initiation factor in mRNA-specific translational control. Mol. Cell 25:113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhuri S, Vyas K, Kapasi P, Komar AA, Dinman JD, Barik S, Mazumder B. 2007. Human ribosomal protein L13a is dispensable for canonical ribosome function but indispensable for efficient rRNA methylation. RNA 13:2224–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu A, Das P, Chaudhuri S, Bevilacqua E, Andrews J, Barik S, Hatzoglou M, Komar AA, Mazumder B. 2011. Requirement of rRNA methylation for 80S ribosome assembly on a cohort of cellular internal ribosome entry sites. Mol. Cell. Biol. 31:4482–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley LA, Maccallum R, Sternberg MJE. 1999. Recognition of remote protein homologies using three-dimensional information to generate a position specific scoring matrix in the program 3D-PSSM, p 218–225 In Pevzner ISP, Waterman M. (ed), RECOMB 99, the Third Annual Conference on Computational Molecular Biology. The Association for Computing Machinery, New York, NY [Google Scholar]
- 17.Kelley LA, Sternberg MJ. 2009. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4:363–371 [DOI] [PubMed] [Google Scholar]
- 18.Munaut C, Noel A, Hougrand O, Foidart JM, Boniver J, Deprez M. 2003. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int. J. Cancer 106:848–855 [DOI] [PubMed] [Google Scholar]
- 19.Ben-Shem A, Jenner L, Yusupova G, Yusupov M. 2010. Crystal structure of the eukaryotic ribosome. Science 330:1203–1209 [DOI] [PubMed] [Google Scholar]
- 20.Walia RR, Caragea C, Lewis BA, Towfic FG, Terribilini M, El-Manzalawy Y, Dobbs D, Honavar V. 2012. Protein-RNA interface residue prediction using machine learning: an assessment of the state of the art. BMC Bioinformatics 13:89. 10.1186/1471-2105-13-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarzacher HG, Mosgoeller W. 2000. Ribosome biogenesis in man: current views on nucleolar structures and function. Cytogenet. Cell Genet. 91:243–252 [DOI] [PubMed] [Google Scholar]
- 22.Leary DJ, Huang S. 2001. Regulation of ribosome biogenesis within the nucleolus. FEBS Lett. 509:145–150 [DOI] [PubMed] [Google Scholar]
- 23.Chen D, Huang S. 2001. Nucleolar components involved in ribosome biogenesis cycle between the nucleolus and nucleoplasm in interphase cells. J. Cell Biol. 153:169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava M, Pollard HB. 1999. Molecular dissection of nucleolin's role in growth and cell proliferation: new insights. FASEB J. 13:1911–1922 [PubMed] [Google Scholar]
- 25.Mongelard F, Bouvet P. 2007. Nucleolin: a multiFACeTed protein. Trends Cell Biol. 17:80–86 [DOI] [PubMed] [Google Scholar]
- 26.Ginisty H, Amalric F, Bouvet P. 1998. Nucleolin functions in the first step of ribosomal RNA processing. EMBO J. 17:1476–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouvet P, Diaz JJ, Kindbeiter K, Madjar JJ, Amalric F. 1998. Nucleolin interacts with several ribosomal proteins through its RGG domain. J. Biol. Chem. 273:19025–19029 [DOI] [PubMed] [Google Scholar]
- 28.Ginisty H, Sicard H, Roger B, Bouvet P. 1999. Structure and functions of nucleolin. J. Cell Sci. 112(Part 6):761–772 [DOI] [PubMed] [Google Scholar]
- 29.Cavaille J, Nicoloso M, Bachellerie JP. 1996. Targeted ribose methylation of RNA in vivo directed by tailored antisense RNA guides. Nature 383:732–735 [DOI] [PubMed] [Google Scholar]
- 30.Kiss-Laszlo Z, Henry Y, Bachellerie JP, Caizergues-Ferrer M, Kiss T. 1996. Site-specific ribose methylation of preribosomal RNA: a novel function for small nucleolar RNAs. Cell 85:1077–1088 [DOI] [PubMed] [Google Scholar]
- 31.Omer AD, Ziesche S, Ebhardt H, Dennis PP. 2002. In vitro reconstitution and activity of a C/D box methylation guide ribonucleoprotein complex. Proc. Natl. Acad. Sci. U. S. A. 99:5289–5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin J, Lai S, Jia R, Xu A, Zhang L, Lu J, Ye K. 2011. Structural basis for site-specific ribose methylation by box C/D RNA protein complexes. Nature 469:559–563 [DOI] [PubMed] [Google Scholar]
- 33.Mazumder B, Fox PL. 1999. Delayed translational silencing of ceruloplasmin transcript in gamma interferon-activated U937 monocytic cells: role of the 3′ untranslated region. Mol. Cell. Biol. 19:6898–6905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazumder B, Li X, Barik S. 2010. Translation control: a multifaceted regulator of inflammatory response. J. Immunol. 184:3311–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazumder B, Seshadri V, Imataka H, Sonenberg N, Fox PL. 2001. Translational silencing of ceruloplasmin requires the essential elements of mRNA circularization: poly(A) tail, poly(A) binding protein, and eukaryotic translation initiation factor 4G. Mol. Cell. Biol. 21:6440–6449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukhopadhyay R, Ray PS, Arif A, Brady AK, Kinter M, Fox PL. 2008. DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol. Cell 32:371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bassler J, Kallas M, Pertschy B, Ulbrich C, Thoms M, Hurt E. 2010. The AAA-ATPase Rea1 drives removal of biogenesis factors during multiple stages of 60S ribosome assembly. Mol. Cell 38:712–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Day CL, Chavanikamannil F, Abelson J. 1996. 18S rRNA processing requires the RNA helicase-like protein Rrp3. Nucleic Acids Res. 24:3201–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charollais J, Dreyfus M, Iost I. 2004. CsdA, a cold-shock RNA helicase from Escherichia coli, is involved in the biogenesis of 50S ribosomal subunit. Nucleic Acids Res. 32:2751–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo KY, Li Z, Bussiere C, Bresson S, Marcotte EM, Johnson AW. 2010. Defining the pathway of cytoplasmic maturation of the 60S ribosomal subunit. Mol. Cell 39:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kruger T, Zentgraf H, Scheer U. 2007. Intranucleolar sites of ribosome biogenesis defined by the localization of early binding ribosomal proteins. J. Cell Biol. 177:573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni J, Tien AL, Fournier MJ. 1997. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell 89:565–573 [DOI] [PubMed] [Google Scholar]
- 43.Dragon F, Gallagher JE, Compagnone-Post PA, Mitchell BM, Porwancher KA, Wehner KA, Wormsley S, Settlage RE, Shabanowitz J, Osheim Y, Beyer AL, Hunt DF, Baserga SJ. 2002. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature 417:967–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harnpicharnchai P, Jakovljevic J, Horsey E, Miles T, Roman J, Rout M, Meagher D, Imai B, Guo Y, Brame CJ, Shabanowitz J, Hunt DF, Woolford JL., Jr 2001. Composition and functional characterization of yeast 66S ribosome assembly intermediates. Mol. Cell 8:505–515 [DOI] [PubMed] [Google Scholar]
- 45.Saveanu C, Bienvenu D, Namane A, Gleizes PE, Gas N, Jacquier A, Fromont-Racine M. 2001. Nog2p, a putative GTPase associated with pre-60S subunits and required for late 60S maturation steps. EMBO J. 20:6475–6484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fatica A, Cronshaw AD, Dlakic M, Tollervey D. 2002. Ssf1p prevents premature processing of an early pre-60S ribosomal particle. Mol. Cell 9:341–351 [DOI] [PubMed] [Google Scholar]
- 47.Grandi P, Rybin V, Bassler J, Petfalski E, Strauss D, Marzioch M, Schafer T, Kuster B, Tschochner H, Tollervey D, Gavin AC, Hurt E. 2002. 90S pre-ribosomes include the 35S pre-rRNA, the U3 snoRNP, and 40S subunit processing factors but predominantly lack 60S synthesis factors. Mol. Cell 10:105–115 [DOI] [PubMed] [Google Scholar]
- 48.Robledo S, Idol RA, Crimmins DL, Ladenson JH, Mason PJ, Bessler M. 2008. The role of human ribosomal proteins in the maturation of rRNA and ribosome production. RNA 14:1918–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jakovljevic J, de Mayolo PA, Miles TD, Nguyen TM, Leger-Silvestre I, Gas N, Woolford JL., Jr 2004. The carboxy-terminal extension of yeast ribosomal protein S14 is necessary for maturation of 43S preribosomes. Mol. Cell 14:331–342 [DOI] [PubMed] [Google Scholar]
- 50.Hedges J, West M, Johnson AW. 2005. Release of the export adapter, Nmd3p, from the 60S ribosomal subunit requires Rpl10p and the cytoplasmic GTPase Lsg1p. EMBO J. 24:567–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dresios J, Chan YL, Wool IG. 2002. The role of the zinc finger motif and of the residues at the amino terminus in the function of yeast ribosomal protein YL37a. J. Mol. Biol. 316:475–488 [DOI] [PubMed] [Google Scholar]
- 52.Dresios J, Derkatch IL, Liebman SW, Synetos D. 2000. Yeast ribosomal protein L24 affects the kinetics of protein synthesis and ribosomal protein L39 improves translational accuracy, while mutants lacking both remain viable. Biochemistry 39:7236–7244 [DOI] [PubMed] [Google Scholar]
- 53.Meskauskas A, Dinman JD. 2001. Ribosomal protein L5 helps anchor peptidyl-tRNA to the P-site in Saccharomyces cerevisiae. RNA 7:1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meskauskas A, Dinman JD. 2007. Ribosomal protein L3: gatekeeper to the A site. Mol. Cell 25:877–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sampath P, Mazumder B, Seshadri V, Gerber CA, Chavatte L, Kinter M, Ting SM, Dignam JD, Kim S, Driscoll DM, Fox PL. 2004. Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell 119:195–208 [DOI] [PubMed] [Google Scholar]
- 56.Ruvinsky I, Meyuhas O. 2006. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem. Sci. 31:342–348 [DOI] [PubMed] [Google Scholar]
- 57.Ruvinsky I, Katz M, Dreazen A, Gielchinsky Y, Saada A, Freedman N, Mishani E, Zimmerman G, Kasir J, Meyuhas O. 2009. Mice deficient in ribosomal protein S6 phosphorylation suffer from muscle weakness that reflects a growth defect and energy deficit. PLoS One 4:e5618. 10.1371/journal.pone.0005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mikulik K, Bobek J, Zikova A, Smetakova M, Bezouskova S. 2011. Phosphorylation of ribosomal proteins influences subunit association and translation of poly (U) in Streptomyces coelicolor. Mol. Biosyst. 7:817–823 [DOI] [PubMed] [Google Scholar]
- 59.Turkina MV, Klang Arstrand H, Vener AV. 2011. Differential phosphorylation of ribosomal proteins in Arabidopsis thaliana plants during day and night. PLoS One 6:e29307. 10.1371/journal.pone.0029307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Narla A, Ebert BL. 2010. Ribosomopathies: human disorders of ribosome dysfunction. Blood 115:3196–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Da Costa L, Tchernia G, Gascard P, Lo A, Meerpohl J, Niemeyer C, Chasis JA, Fixler J, Mohandas N. 2003. Nucleolar localization of RPS19 protein in normal cells and mislocalization due to mutations in the nucleolar localization signals in 2 Diamond-Blackfan anemia patients: potential insights into pathophysiology. Blood 101:5039–5045 [DOI] [PubMed] [Google Scholar]
- 62.Gazda HT, Grabowska A, Merida-Long LB, Latawiec E, Schneider HE, Lipton JM, Vlachos A, Atsidaftos E, Ball SE, Orfali KA, Niewiadomska E, Da Costa L, Tchernia G, Niemeyer C, Meerpohl JJ, Stahl J, Schratt G, Glader B, Backer K, Wong C, Nathan DG, Beggs AH, Sieff CA. 2006. Ribosomal protein S24 gene is mutated in Diamond-Blackfan anemia. Am. J. Hum. Genet. 79:1110–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cmejla R, Cmejlova J, Handrkova H, Petrak J, Pospisilova D. 2007. Ribosomal protein S17 gene (RPS17) is mutated in Diamond-Blackfan anemia. Hum. Mutat. 28:1178–1182 [DOI] [PubMed] [Google Scholar]
- 64.Farrar JE, Nater M, Caywood E, McDevitt MA, Kowalski J, Takemoto CM, Talbot CC, Jr, Meltzer P, Esposito D, Beggs AH, Schneider HE, Grabowska A, Ball SE, Niewiadomska E, Sieff CA, Vlachos A, Atsidaftos E, Ellis SR, Lipton JM, Gazda HT, Arceci RJ. 2008. Abnormalities of the large ribosomal subunit protein, Rpl35a, in Diamond-Blackfan anemia. Blood 112:1582–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gazda HT, Sheen MR, Vlachos A, Choesmel V, O'Donohue MF, Schneider H, Darras N, Hasman C, Sieff CA, Newburger PE, Ball SE, Niewiadomska E, Matysiak M, Zaucha JM, Glader B, Niemeyer C, Meerpohl JJ, Atsidaftos E, Lipton JM, Gleizes PE, Beggs AH. 2008. Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan anemia patients. Am. J. Hum. Genet. 83:769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kondrashov N, Pusic A, Stumpf CR, Shimizu K, Hsieh AC, Xue S, Ishijima J, Shiroishi T, Barna M. 2011. Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell 145:383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.