Abstract
Cystinosis is a lysosomal storage disorder caused by the accumulation of the amino acid cystine due to genetic defects in the CTNS gene, which encodes cystinosin, the lysosomal cystine transporter. Although many cellular dysfunctions have been described in cystinosis, the mechanisms leading to these defects are not well understood. Here, we show that increased lysosomal overload induced by accumulated cystine leads to cellular abnormalities, including vesicular transport defects and increased endoplasmic reticulum (ER) stress, and that correction of lysosomal transport improves cellular function in cystinosis. We found that Rab27a was expressed in proximal tubular cells (PTCs) and partially colocalized with the lysosomal marker LAMP-1. The expression of Rab27a but not other small GTPases, including Rab3 and Rab7, was downregulated in kidneys from Ctns−/− mice and in human PTCs from cystinotic patients. Using total internal reflection fluorescence microscopy, we found that lysosomal transport is impaired in Ctns−/− cells. Ctns−/− cells showed significant ER expansion and a marked increase in the unfolded protein response-induced chaperones Grp78 and Grp94. Upregulation of the Rab27a-dependent vesicular trafficking mechanisms rescued the defective lysosomal transport phenotype and reduced ER stress in cystinotic cells. Importantly, reconstitution of lysosomal transport mediated by Rab27a led to decreased lysosomal overload, manifested as reduced cystine cellular content. Our data suggest that upregulation of the Rab27a-dependent lysosomal trafficking and secretory pathways contributes to the correction of some of the cellular defects induced by lysosomal overload in cystinosis, including ER stress.
INTRODUCTION
Cystinosis is a lysosomal storage disorder (LSD) resulting from genetic defects in the CTNS gene, which encodes cystinosin, the lysosomal cystine transporter (1–4). As a consequence of this defect, the amino acid cystine is anomalously accumulated in lysosomes. Increased levels of intralysosomal cystine lead to cell malfunction, which is specially manifested in the kidney and eye but also affects other organs (5). Cystinosis affects 1 in 100,000 newborns. The treatment of choice for cystinosis is cysteamine, which reduces the intralysosomal level of cystine. The efficiency of cysteamine in retarding the rate of glomerular deterioration and improvement of linear growth in children with cystinosis demonstrates the effectiveness of cystine-depleting therapies (6–8). However, cysteamine treatment has some disadvantages, including severe side effects and the variable response to treatment, which reflects genetic heterogeneity (5, 8) and patient compliance. It is therefore accepted that new or complementary molecular therapies will help with cystinosis management before and after kidney transplantation.
Transport of lipids and proteins is a highly regulated process, which is required to maintain the integrity of various intracellular organelles and the plasma membrane in eukaryotic cells. Molecular trafficking is mediated by intracellular vesicular transport, a process essential for nearly all aspects of cellular physiology, including lysosomal function regulation. Importantly, defects in vesicular trafficking mechanisms lead to disease in humans (9–11). One of the hypotheses proposed to explain the cellular defects observed in LSDs is that vesicular transport is somehow affected by lysosomal overload (12). However, little is known about the mechanism of lysosomal transport in cystinosis, and the potential beneficial effects of the correction of a putatively defective lysosomal trafficking phenotype in cystinosis have not been explored.
Besides their role as the final degradation compartment of the endocytic pathway, conventional lysosomes are able to engage in the secretory pathway through regulated mechanisms in many cells and tissues (13). These are important mechanisms that help maintain normal cellular homeostasis by, for example, regulating plasma membrane repair and plasma membrane resealing after wounding (14), mediating receptor upregulation at the plasma membrane (15), and regulating secretion of lysosomal phospholipases to mediate intracellular phospholipid remodeling (16). In addition, lysosomal exocytosis is considered a dominant mechanism for cellular elimination of degradation products and is used by eukaryotic cells to avoid accumulation of intracellular debris (13). These processes are of fundamental importance for normal cellular physiology, and defects in lysosomal transport in LSDs could cause significant cellular dysfunction, in addition to the abnormalities induced by the inability of lysosomes to degrade intracellular metabolites.
The efficiency and specificity of vesicular transport processes, including lysosomal transport and regulated secretion, rely on Rab proteins, which are Ras-like small GTPases, and on their specific effectors, a group of diverse molecules that bind to specific Rab proteins and act as membrane organizers (17). Different Rab proteins are distributed in specific cell compartments and, together with their effectors, synchronize and control multiple steps of vesicular transport, including vesicle motility, tethering, and docking to specific compartments of the cell (17, 18). Rab27a and its specific effectors are master regulators of vesicular transport and exocytosis (19, 20). They are expressed in multiple cells and tissues with secretory functions, including the kidney (23–26) and eye (21, 22). Rab27b, which has 72% homology with Rab27a at the amino acid level, is also expressed in the kidney's transitional epithelium and in corneal epithelial cells (23). These secretory factors are also expressed in many other organs that are affected by late cystinosis complications, including in the thyroid glands, pancreas, stomach, large and small intestines, trachea, lung, liver, and heart (23) and in skeletal muscle (27). Although Rab27a was originally associated with the secretory pathway of secretory lysosomes in hematopoietic cells (28, 29), recent studies have highlighted a role for Rab27a in conventional lysosomal trafficking and exocytosis (30).
Several lines of evidence suggest that defects in vesicular transport mechanisms lead to endoplasmic reticulum (ER) stress (31), probably induced by accumulation of misfolded proteins in the ER caused by inefficient protein trafficking. Cells respond to this stress through a transcriptional induction mechanism, the unfolded protein response (UPR), which is initially activated to relieve ER stress. However, if the stress is prolonged or the adaptive response fails, the response switches from prosurvival to prodeath signaling (32). Support for a close regulation between vesicular transport and ER stress was initially revealed by studies showing that accumulation of synucleins inhibits ER-Golgi apparatus transport and induces ER stress, while the correction of intracellular vesicular transport defects was effective in restoring normal cellular function in cells undergoing ER stress (31). Importantly, ER stress has been directly involved in the development of cellular abnormalities associated with LSDs (33, 34), but direct evidence of cross talk between vesicular trafficking mechanisms and ER stress has not been demonstrated in LSDs.
In this work, we investigate the mechanisms of vesicular transport and ER stress in cystinosis and analyze the hypotheses that upregulation of the Rab27a-dependent secretory pathway decreases cystine accumulation in the lysosomes and improves cell function. Our studies characterize defects in Rab27a-dependent mechanisms in cystinosis and utilize the restoration of the lysosomal transport system by upregulation of Rab27a-dependent pathways to improve lysosomal trafficking, upregulate the secretory pathway, and decrease ER stress in cystinotic cells.
MATERIALS AND METHODS
Mice, cells, and cell culture.
C57BL/6 Ctns−/− mice were described before (35). Neonatal skin fibroblasts from Ctns−/− and wild-type mice were prepared by standard procedures (36). Neonatal murine fibroblasts and 293T cells (ATCC) were maintained in Dulbecco modified Eagle medium containing 10% fetal calf serum. Human renal proximal tubular cells (hPTCs) were previously described (37) and were kindly provided by Corinne Antignac. All studies were conducted according to the National Institutes of Health and institutional guidelines and with approval of the animal review board at The Scripps Research Institute.
Constructs, transfections, and transductions.
The wild-type human Rab27a expression vector was obtained from the Missouri S&T cDNA Resource Center (http://www.cdna.org). The constitutively active form of Rab7 (enhanced green fluorescent protein [EGFP]-expressing Rab7Q67L) was obtained from Addgene. The constitutively active mutant Rab27aQ78L was generated by directed mutagenesis of Rab27a on the expression vector pcDNA3.1+ (Life Technologies) using the primer 5′-TGGGACACAGCAGGGCTGGAGAGGTTTCGTAGCT-3′ and a QuikChange multisite-directed mutagenesis kit (Stratagene) as described previously (38). The cDNA corresponding to Rab27aQ78L was subcloned into the lentiviral expression vector pLV-CMV by restriction enzyme digestion (BamHI/XhoI) and ligation by standard procedures. The pLV-GFP expression vector was used as a control. 293T cells were transfected with the pLV constructs along with packaging plasmids pVSVG (Clontech) and pLV-CMV-delta 8.2 (39) using the Lipofectamine LTX reagent (Life Technologies). Virus-containing supernatants were collected at 48 and 72 h posttransfection, filtered, and then either used directly or concentrated by centrifugation at 20,000 rpm for 2 h. Wild-type and Ctns−/− murine fibroblasts were transduced with lentiviruses in the presence of 5 μg/ml Polybrene. After 24 h, the virus-containing medium was replaced with normal growth medium. The transduction efficiency obtained using this procedure was >90% (see Fig. S1 in the supplemental material).
Immunofluorescence and confocal microscopy analysis.
Murine fibroblasts were seeded at 70% confluence in an eight-well chambered cover glass (pretreated with poly-l-lysine at 0.01% in phosphate-buffered saline [PBS]), fixed with 3.7% paraformaldehyde, permeabilized with 0.01% saponin, and blocked with a solution of 1% bovine serum albumin (BSA) in PBS. Kidneys from wild-type or Ctns−/− mice were fixed in 5% formaldehyde, equilibrated in 20% sucrose overnight, and frozen in Tissue-Tek optimal-cutting-temperature buffer at −80°C (Sakura Finetek). Twelve- to 20-μm-thick sections were permeabilized with 0.01% saponin and blocked with a solution of 1% BSA in PBS. Samples were labeled with the indicated primary antibodies overnight at 4°C and then the appropriate combination of Alexa Fluor (488 and/or 594)-conjugated donkey anti-rabbit, anti-mouse, and/or anti-goat secondary antibodies (Molecular Probes). Nuclei were stained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI). Cells were stored in Fluoromount-G medium (Southern Biotechnology, AL) and analyzed using a Zeiss LSM 710 laser scanning confocal microscope attached to a Zeiss Observer Z1 microscope using 100× or 63× oil Plan Apo (numerical aperture, 1.4) infinity-corrected optics. Images were analyzed using ImageJ software and processed with Adobe Photoshop CS4 software.
Transmission electron microscopy.
Murine fibroblasts were processed for transmission electron microscopy using a modification of the protocol described before (40). Briefly, the samples were fixed in 2.5% glutaraldehyde in 0.1 M Na cacodylate buffer (pH 7.3), washed with buffer, and fixed in 1% osmium tetroxide in 0.1 M Na cacodylate buffer. They were subsequently treated with 0.5% tannic acid, followed by 1% sodium sulfate. The cells were treated with propylene oxide and embedded in Epon-Araldite. Thin sections (70 nm) of the samples were cut on a Reichert Ultracut E ultramicrotome (Leica, Deerfield, IL) using a diamond knife (Diatome; Electron Microscopy Sciences, Hatfield, PA), mounted on Parlodion-coated copper slot grids, and stained in uranyl acetate and lead citrate. Sections were examined on a Philips CM100 transmission electron microscope (FEI, Hillsboro, OR). Images were documented, and measurements were taken using a Megaview III charge-coupled-device camera (Olympus Soft Imaging Solutions, Lakewood, CO). Subsequent processing was performed using Adobe Photoshop CS software. Three-dimensional quantitative analysis of organelle volume density was performed by stereology (41), on the basis of the Delesse principle, which states that the planimetric fraction of a section occupied by sections of a given component corresponds to the fraction of the tissue volume occupied by this component (41). Briefly, a grid probe consisting of squares 0.5 by 0.5 μm was generated over the electron microscopic images using Photoshop software, where line intersections were considered sample points. A systematic lattice of points in the test system instead of random lattices was used to minimize error (41). The number of sample points that coincided with each organelle type was manually counted.
Quantitative analysis of lysosomal transport.
For live-cell total internal reflection fluorescence microscopy (TIRFM) imaging, murine fibroblasts were seeded in eight-well plates with a bottom cover glass (1.5 borosilicate cover glass; Lab-Tek; Nunc) in phenol red-free RPMI medium. Fibroblasts labeled using LysoTracker were transferred to a prewarmed microscope stage. TIRFM experiments were performed using a 100× TIRF objective (numerical aperture, 1.45; Nikon) on a Nikon TE2000U microscope custom modified with a TIRF illumination module. Laser illumination was adjusted to impinge on the coverslip at an angle of incidence that was slightly less than the critical angle required for complete internal reflection (42). This technique generates a highly refracted beam that penetrates the sample slightly deeper than complete TIRFM, thus allowing complete visualization of lysosomes but still maintaining the high signal-to-noise ratio of traditional TIRFM (42). Images were acquired on a 14-bit, cooled charge-coupled-device camera (Hamamatsu) controlled through NIS-Elements software (Nikon, Inc.). The images were recorded at 0.5-s intervals using exposure times of 200 to 600 ms, depending on the intensity of the signal. Images were analyzed using ImageJ (version 1.43) and Imaris (version 7.0) software (Bitplane Scientific Software). All data analysis was performed by tracking granule movement through all frames of the movies. All vesicles that appeared in the TIRFM zone for at least three consecutive frames during the length of the study were included in the analysis.
Gel electrophoresis and Western blotting.
Proteins were separated by gel electrophoresis using NuPAGE gels and MOPS (morpholinepropanesulfonic acid) buffer (Life Technologies). Proteins were transferred onto nitrocellulose membranes, and the membranes were blocked with PBS containing 5% (wt/vol) blotting-grade nonfat dry milk blocker (Bio-Rad). The proteins were detected by probing the membranes with the indicated primary antibodies at appropriate dilutions. The detection system used horseradish peroxidase-conjugated secondary antibodies (Bio-Rad) and a SuperSignal West Pico chemiluminescence substrate system (Thermo). Transferred proteins were visualized using Hyperfilm (Amersham Bioscience).
Antibodies.
The polyclonal antibody against Rab27a was described previously (38). We also used monoclonal anti-Rab27a antibody from Transductions Laboratories. The anti-LAMP-1 and anti-Rab3a antibodies were from Santa Cruz Biotechnology; the monoclonal antibody against KDEL (clone 10C3) was from ENZO Life Sciences. The anti-synaptotagmin 7 (anti-Syt7) was previously described (43). For immunofluorescence, we used anti-Syt7 from Synaptic Systems. Anticalnexin SPA-860 was a gift from L. Wiseman (The Scripps Research Institute). Anti-Rab7 was from Cell Signaling.
Cystine content measurement.
The cystine content in murine fibroblasts was analyzed as previously described (44). Briefly, fibroblasts were resuspended in 750 μl N-ethylmaleimide (Fluka Biochemika) at 650 μg/ml. The samples were sonicated for 9 s, and the proteins were precipitated with 15% 5-sulfosalicylic acid dihydrate (Fluka Biochemika), resuspended in 0.1 N NaOH, and measured using a Bio-Rad protein assay kit. The cystine-containing supernatants were analyzed by mass spectrometry at the Biochemical Genetics Laboratory, University of California, San Diego.
Statistical analysis.
Data are presented as means, and error bars correspond to standard errors of the means (SEMs), unless otherwise indicated. Statistical significance was determined using the unpaired Student's t test or the Mann-Whitney test using GraphPad InStat (version 3) software, and graphs were made using GraphPad Prism (version 4) software. Differences with P values of <0.05 were considered statistically significant.
RESULTS
Cystinotic cells have decreased Rab27a expression.
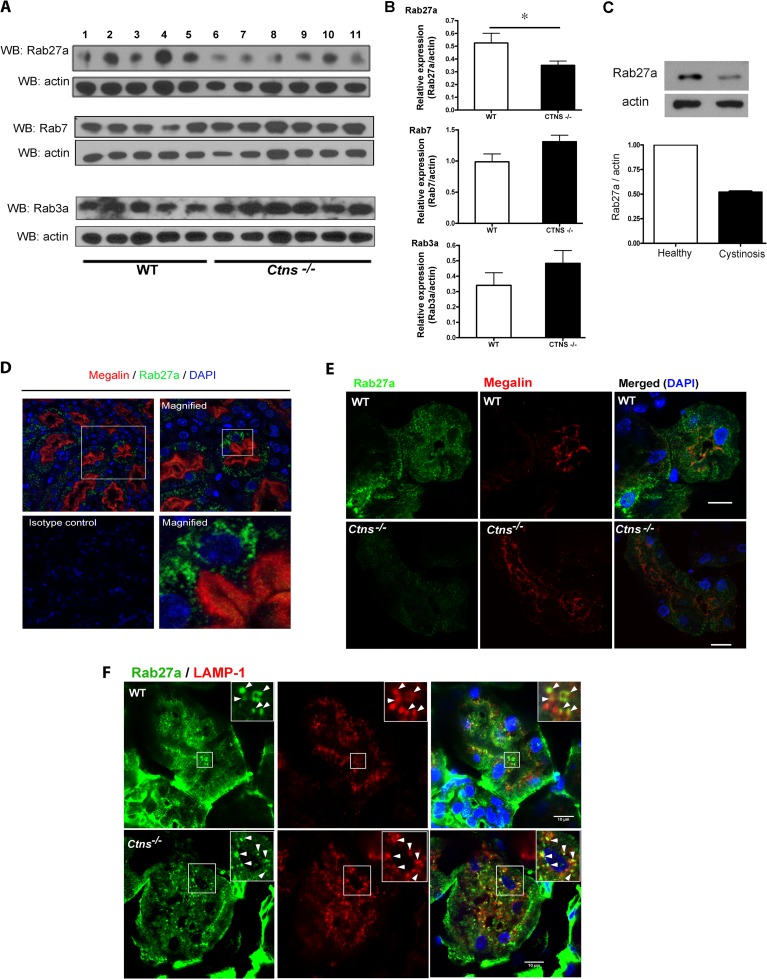
One of the hypotheses proposed to explain the cellular defects observed in lysosomal storage disorders is that vesicular trafficking is affected by lysosomal overload (12). Here, to investigate whether cystinosis is underlined by abnormalities in vesicular trafficking regulators, we analyzed the expression of Rab GTPases that are involved in the endocytic or exocytic pathway of lysosomes. Given the pathophysiological importance of kidneys in cystinosis, we first evaluated Rab GTPase expression using lysates from kidneys of wild-type or Ctns−/− mice. Western blot analysis demonstrated a significant decrease in the level of expression of Rab27a but not other Rab GTPase regulators of the exocytic (Rab3a) or endocytic (Rab7) pathway (Fig. 1A and B). Because defects in renal proximal tubular cells (PTCs) are central to the pathophysiology of late cystinosis, we next determined the expression of Rab27a in PTCs from cystinotic patients, which also showed a significant reduction in Rab27a expression compared to PTCs from healthy controls (Fig. 1C). To analyze the distribution of Rab27a in kidneys in further detail, we performed immunofluorescence analysis of endogenous Rab27a in murine kidneys and identified Rab27a expression in polarized, megalin-positive PTCs (Fig. 1D). Rab27a was distributed at both basal and apical punctate structures resembling vesicular organelles (Fig. 1D). Comparative immunofluorescence analyses of kidneys from wild-type and Ctns−/− mice showed that endogenous Rab27a expression is downregulated in Ctns−/− PTCs (Fig. 1E). A more detailed analysis detected Rab27a colocalized with the lysosomal marker LAMP-1 in kidney PTCs from wild-type and Ctns−/− mice (Fig. 1F).
Fig 1.
Rab27a is expressed in renal proximal tubular cells and is downregulated in cystinosis. (A) Expression of endogenous Rab GTPases in kidney lysates from wild-type or Ctns−/− mice. Western blot (WB) analysis was performed using 10 μg of protein lysates. Each lane corresponds to kidney lysates from individual wild-type (WT; lanes 1 to 5) or cystinotic (Ctns−/−; lanes 6 to 11) mice. (B) Quantitative densitometry analysis of the immunoblots presented in panel A. Relative expression refers to the ratio between specific Rabs and the actin signal in the same lane. Results are means ± SEMs (error bars). *, P < 0.05 (Mann-Whitney test). (C) Western blot analysis of the expression of Rab27a in human renal proximal tubular cells (PTCs) from a patient with cystinosis and a healthy control. Results are represented as means ± SDs (n = 2). (D) Immunofluorescence analysis showing endogenous Rab27a expression in PTCs from mouse kidneys. (E) Comparative analysis of endogenous Rab27a expression was performed using an affinity-purified anti-Rab27a antibody raised in rabbit. In these experiments, constant laser power and gain were maintained to preserve identical image acquisition conditions for wild-type and Ctns−/− samples. (F) Immunofluorescence analysis of mouse kidney showing colocalization (insets, arrowheads) of Rab27a with the lysosomal marker LAMP-1 in PTCs. Bars = 10 μm.
Cystinotic cells are characterized by impaired lysosomal trafficking.
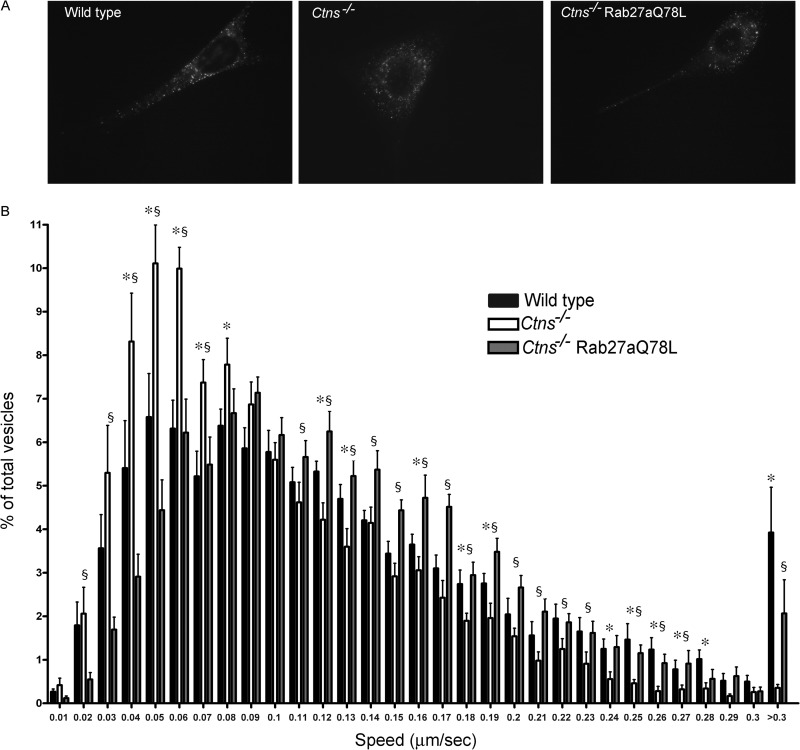
To investigate whether cystinosis is characterized by defective vesicular trafficking, we next analyzed whether Ctns−/− cells have impaired lysosomal transport. To this end, we quantitatively analyzed vesicular dynamics in live wild-type and Ctns−/− murine fibroblasts that were incubated with the acidotropic probe LysoTracker, which labels lysosomes as well as other acidic organelles (45). Labeling of lysosomes in fibroblasts by LysoTracker is further supported by data showing that treatment with lysosomal permeabilization agents dramatically decreases staining (45). Importantly, the lysosomal pH of the cystinotic fibroblast is similar to that of wild-type cells (46). Here, we analyzed vesicular dynamics using TIRFM, a technique that allows highly specific analysis of fluorescent proteins and probes localization by cancelling intracellular background fluorescence from deeper layers in the cytosol (47–49). To facilitate the analysis of organelles that may not necessarily be in areas adjacent to the plasma membrane, we used a modification of this technique named pseudo-TIRFM (oblique illumination) (42). In pseudo-TIRFM, laser illumination is adjusted to impinge on the coverslip at an angle of incidence that is slightly less than the critical angle required for complete internal reflection (42). This technique generates a highly refracted beam that penetrates the sample slightly deeper than complete TIRFM, thus allowing complete visualization of lysosomes but still maintaining the high signal-to-noise ratio of traditional TIRFM (42). Representative images of wild-type and cystinotic cells are shown in Fig. 2A and in Movies S1 to S3 in the supplemental material. Kinetic analyses showed that lysosomes from cystinotic fibroblasts are characterized by impaired dynamics compared to wild-type cells (Fig. 2B). Vesicular dynamics defects are characterized by a significant decrease in the number of acidic organelles moving at high speed and a concomitant increase in the number of these organelles with no or restricted movement in cystinotic cells (Fig. 2B).
Fig 2.
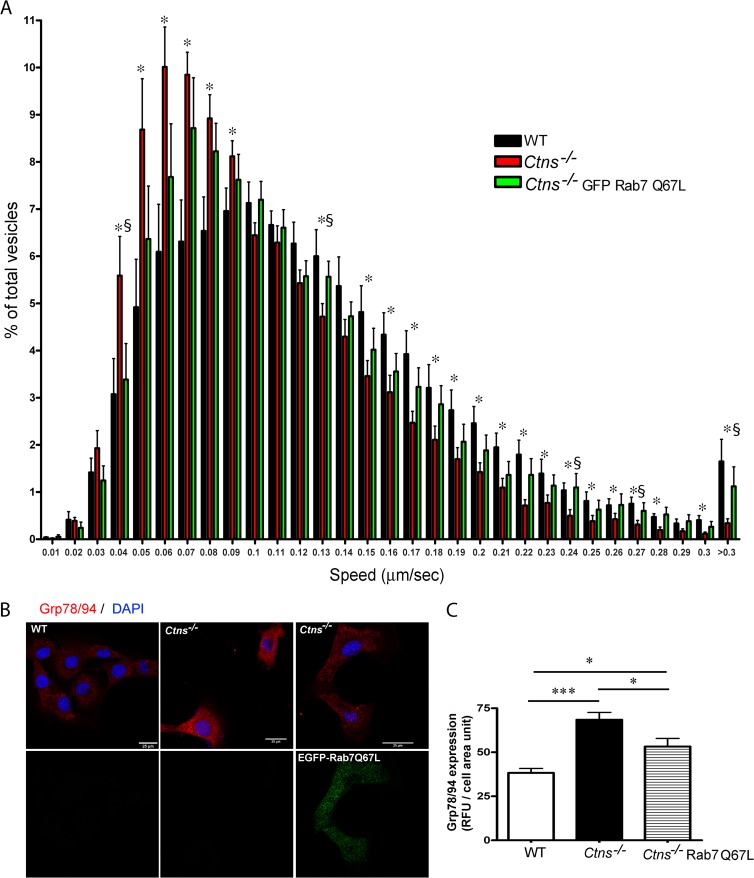
Ctns−/− cells have defective lysosomal trafficking which is rescued by Rab27aQ78L expression. Murine fibroblasts were labeled using LysoTracker, and lysosomal dynamics were analyzed as described in Materials and Methods. (A) Representative images of cells used in vesicular dynamic analyses are shown. The dynamics of the labeled lysosomes were monitored for 2 min and can be seen in Movies S1 to S3 in the supplemental material. (B) The kinetics of lysosomal movement was analyzed using Imaris software. The analysis shown includes the kinetics of 7,304 vesicles from 12 wild-type cells, 8,086 vesicles from 9 Ctns−/− cells, and 11,181 vesicles from 12 Ctns−/− cells expressing Rab27aQ78L. Experiments were repeated three times with similar results. Histograms of lysosomal speeds from wild-type cells, Ctns−/−cells, and Ctns−/− cells expressing constitutively active Rab27a are shown. The speeds of lysosomal movement were binned in 0.01-μm/s increments and plotted as a percentage of the number of total granules for a given cell. Results are represented as means ± SEMs. Ctns−/− cells showed a marked decrease in the number of lysosomes moving at high speed and a concomitant increased number of lysosomes with limited or no motility. This phenotype was rescued by expression of constitutively active Rab27a. *, P < 0.05 for wild-type versus Ctns−/− cells; §, P < 0.05 for Ctns−/− versus Ctns−/− Rab27aQ78L cells. P values were determined by the nonparametric Mann-Whitney test.
Upregulation of Rab27a rescues the vesicular transport phenotype in cystinotic cells.
Rab27a is a master regulator of vesicular transport and secretory pathways of secretory lysosomes in hematopoietic cells (19) and has recently been suggested to regulate the exocytic pathway of conventional lysosomes in other cell types (30). Here, we tested the hypothesis that upregulation of Rab27a-mediated mechanisms would restore normal lysosomal transport in cystinotic cells. To this end, we expressed the constitutively active form of the small GTPase Rab27a, Rab27aQ78L, in cystinotic fibroblasts (see Fig. S2 in the supplemental material). This mutant, by lacking GTPase activity, remains in the GTP-bound form, which favors its binding to endogenous Rab27a effectors (38, 50). Lentiviral vectors were used to stably introduce the constitutively active mutant of Rab27a in the cells. Active Rab27a expression in fibroblasts was evident at as early as 2 to 3 days postinfection, and expression was maintained for several passages. Rab27aQ78L-expressing cells were used in lysosomal dynamic assays and analyzed by pseudo-TIRFM. Here, we show, for the first time, that upregulation of the Rab27a-dependent pathway improves the mechanism of lysosomal transport and rescues the defective trafficking phenotype in cystinotic cells (Fig. 2B; see Movies S1 to S3 in the supplemental material). Importantly, a similar rescue of the defective trafficking phenotype was observed in cystinotic cells transfected for the expression of EGFP-Rab27aQ78L (data not shown). Altogether, these data indicated that Rab27a is a regulator of conventional lysosomal transport and suggested that dysfunctional trafficking mechanisms and associated cellular defects in cystinosis may be corrected by upregulation of the Rab27a-dependent lysosomal trafficking pathway.
Cystinotic cells have upregulated ER stress.
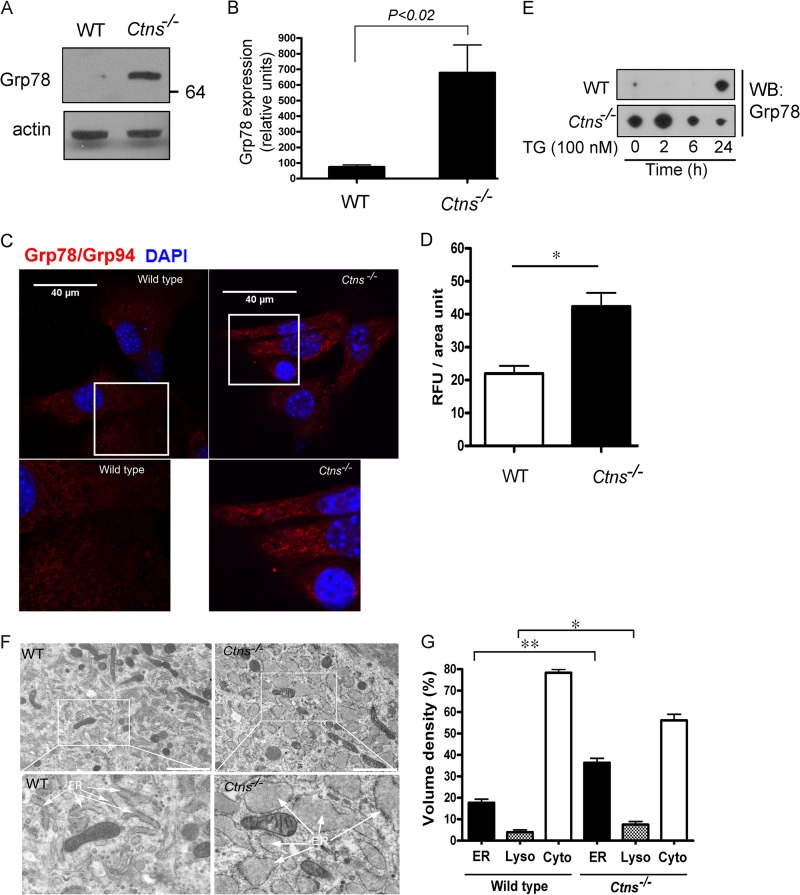
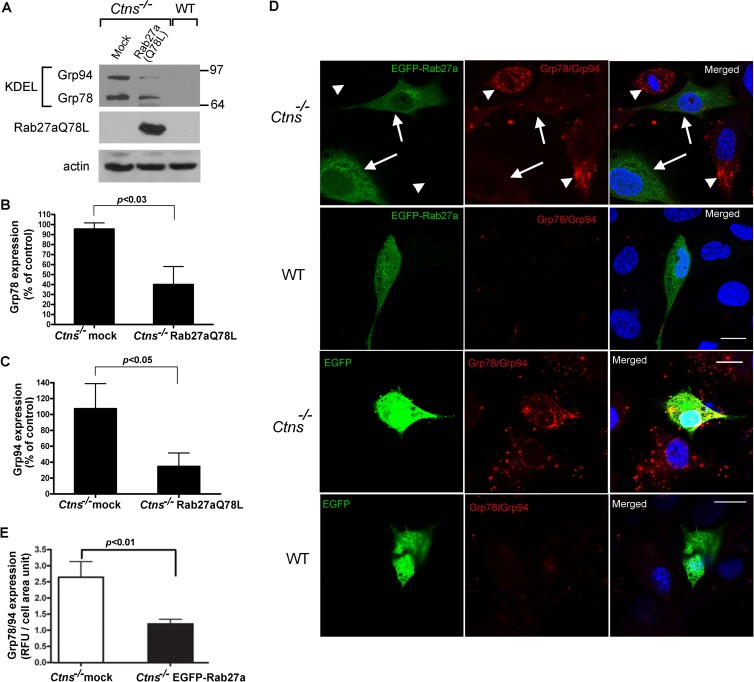
Defects in vesicular transport mechanisms may lead to the development of ER stress (31, 51, 52), and ER stress is upregulated in some lysosomal storage disorders (34). To evaluate whether Ctns−/− cells have an upregulated unfolded protein response, we first analyzed the expression level of the established UPR target genes Grp78 (Bip) and Grp94, which are transcriptionally upregulated and play a central role during the response to conditions of ER stress (53, 54). In Fig. 3, we show that the expression of endogenous UPR-induced chaperones is upregulated in Ctns−/− mouse embryonic fibroblasts. Immunoblotting analysis showed significantly increased expression levels of the KDEL-reactive protein Grp78 in Ctns−/− cells (Fig. 3A and B). These data correlated with those from immunofluorescence analyses showing significantly increased staining for UPR chaperones in cystinotic cells (Fig. 3C and D). In addition, cystinotic cells showed marked Grp78 expression upregulation when treated with the noncompetitive inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) thapsigargin for 2 h, while wild-type cells showed upregulated expression of the chaperone only after 24 h treatment (Fig. 3E).
Fig 3.
Ctns−/− cells are characterized by marked upregulation of the UPR. (A) The level of expression of the established UPR target gene Grp78, which is induced during conditions of ER stress, was analyzed in Ctns−/− and wild-type fibroblasts by Western blotting, using a mouse monoclonal anti-KDEL antibody. The data are representative of at least four independent experiments with similar results. (B) Quantitative analysis was performed using the nonparametric Mann-Whitney test (n = 4). (C) Immunofluorescence analysis of endogenous chaperone Grp78 and Grp94 expression was performed by confocal microscopy using an anti-KDEL antibody. Nuclei were stained with DAPI. (D) Quantitative analysis of fluorescence intensity (in relative fluorescence units [RFU]) for Grp78/Grp94 (KDEL) from 65 wild-type and 65 cystinotic cells from four experiments. Results are represented as means ± SEMs (error bars). *, P < 0.001 (Mann-Whitney test). (E) Marked upregulation of Grp78 expression after treatment with thapsigargin. Wild-type and Ctns−/− were treated with thapsigargin (TG; 100 nM) for the indicated times. Cells were harvested and lysed, and Grp78 expression was evaluated by Western blotting (WB). (F and G) Electron microscopy analysis shows ER expansion in Ctns−/− cells. (F) Primary fibroblasts from Ctns−/− or wild-type mice were analyzed by electron microscopy. The arrows point to the ER. An aberrantly enlarged ER (ER expansion), a characteristic of ER stress, was observed in all Ctns−/− cells analyzed. Bar = 2 μm. (G) Organelle volume density quantitative analysis by stereology. At least 5 to 10 cells were included in the analysis. A total of 2,030 and 890 points were counted for wild-type and Ctns−/− cells, respectively. Lyso, lysosomes; Cyto, cytosol. *, P < 0.05; **, P < 0.001. P values were determined by the Mann-Whitney test.
Next, to confirm that cystinotic cells are under marked ER stress, we analyzed their ultrastructure by electron microscopy. We found that cystinotic cells are characterized by a pronounced enlargement of the endoplasmic reticulum (ER expansion [55]) (Fig. 3F), which correlates with chronic rather than acute ER stress (53). In order to determine quantitative differences between wild-type and cystinotic cells, ER expansion was quantified by stereology (56). In Fig. 3G, we show that the volume density of the ER is significantly increased in Ctns−/− fibroblasts. Importantly, our quantitative analyses also showed a moderate but significant increment in lysosomal volume density in cystinotic cells that is in line with the condition of lysosomal overload in cystinosis (Fig. 3G).
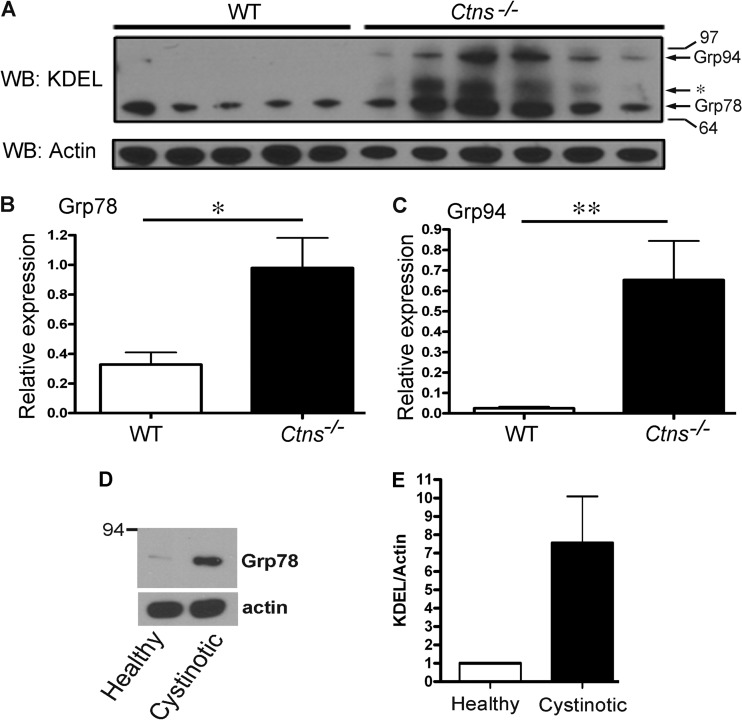
To determine whether the ER stress phenotype is recapitulated in vivo, we next analyzed UPR chaperone expression in kidneys from cystinotic mice. Western blot analysis showed significant upregulation of both Grp78 and Grp94 expression in lysates from kidneys from Ctns−/− mice and low levels of these chaperones in lysates from kidneys from wild-type mice. (Fig. 4A to C). Next, we analyzed the expression of calnexin, a chaperone previously associated with stress-induced apoptosis (57, 58). Immunofluorescence analysis demonstrated significantly higher levels of expression of calnexin in PTCs from Ctns−/− mouse kidneys (see Fig. S3 in the supplemental material). The increment in calnexin expression was less pronounced than that of Grp78/94 chaperones, which correlates with the observation that, in other systems, calnexin is an ire-1/xbp-1-dependent gene, albeit less sensitive than other UPR targets (59). Importantly, we also found significant upregulation of UPR-induced chaperones in human PTCs from cystinotic patients (Fig. 4D and E). Altogether, our data indicate that the ER stress phenotype observed in cystinotic fibroblasts correlates with a similar phenotype in cystinosis target organs and Ctns-deficient human PTCs.
Fig 4.
In vivo upregulation of the UPR in cystinosis. (A) Western blot analyses of the expression of Grp94 and Grp78 in kidney lysates from wild-type and Ctns−/− mice. *, KDEL-responsive unidentified protein. (A) Each lane corresponds to an individual sample from independent mice. (B and C) Chaperone expression (relative to the actin signal in the same lane) was quantified by densitometry. The actin signal in this figure is identical to that presented in Fig. 1A for Rab27a, as it corresponds to the same membrane. Results are represented as means ± SEMs (error bars). *, P < 0.05; **, P < 0.005. P values were determined by the Mann-Whitney test. (D and E) Western blot and quantification analyses of the expression of Grp78 in human proximal tubular cells (PTCs). Results are represented as means ± SDs (n = 2).
Upregulation of lysosomal transport decreases the UPR in cystinosis.
To analyze whether correction of intracellular vesicular transport is an effective mechanism to improve cellular function in Ctns−/− cells, we next tested the hypothesis that upregulation of the Rab27a-dependent trafficking pathway protects cystinotic cells and decreases ER stress. To this end, we analyzed the UPR in wild-type and cystinotic cells expressing constitutively active Rab27a. Using Western blot analysis, we showed that constitutively active Rab27a expression significantly reduces the expression of both the Grp78 and Grp94 UPR chaperones (Fig. 5A to C). Using immunofluorescence analysis and confocal microscopy, we also found that Ctns−/− cells in which Rab27a-dependent lysosomal trafficking was upregulated have a marked reduction in the expression of UPR-induced chaperones (Fig. 5D and E). Altogether, our data suggest that the upregulation of lysosomal trafficking mediated by Rab27a reduces endoplasmic reticulum stress and decreases the need for the UPR in cystinotic cells and support the idea that the correction of vesicular transport mechanisms in LSDs has the potential to improve cellular function.
Fig 5.
Upregulation of the Rab27a-dependent trafficking pathway decreases ER stress in Ctns−/− cells. (A) Western blot analysis of Grp78 and Grp94 expression in Ctns−/− cells expressing the constitutively active form of Rab27a (Rab27aQ78L). (B and C) Quantitative analysis of Grp78 and Grp94 expression from three to four independent experiments (means ± SEMs). Results are expressed as percent relative to the results for mock-infected Ctns−/− control cells. (D) Immunofluorescence analysis of Grp78 and Grp94 expression in Ctns−/− or wild-type cells expressing EGFP-Rab27a. Primary Ctns−/− or wild-type fibroblasts were transfected with the expression vector EGFP-Rab27a or with the EGFP control expression vector. Immunofluorescence analysis of the expression of Grp78/94 was performed as described in Materials and Methods. Nuclei were stained with DAPI. The level of expression of the UPR target genes Grp78 and Grp94, which are upregulated in Ctns−/− cells (arrowheads), was significantly decreased in cells expressing EGFP-Rab27a (arrows). Bars = 40 μm. (E) Fluorescence in the red channel (KDEL) was quantified using ImageJ and expressed as the ratio of the mean fluorescence intensity/cell area. Chaperone expression in EGFP-Rab27a-expressing Ctns−/− cells is compared to the expression in control Ctns−/− cells that do not express EGFP-Rab27a (mock). Eleven to 13 cells of each type were included in the analysis. Results are represented as means ± SEMs.
Lysosomal trafficking mediated by Rab7 contributes to the improvement of the cellular phenotype in cystinosis.
Next, we determined whether Rab7, a Rab known to mediate lysosomal bidirectional microtubule-associated movement through interaction with its effectors, RILP and FYCO1 (60, 61), could contribute to the rescue of the defective phenotype in cystinosis. To this end, we analyzed the effect of constitutively active Rab7 expression on lysosomal kinetics and ER stress in Ctns−/− cells. Here, we showed that the expression of constitutively active Rab7 increases lysosomal trafficking in Ctns−/− cells (Fig. 6A and B; see Movie S4 in the supplemental material). This is in agreement with the findings of a previous study showing the trafficking repair mechanisms induced by Rab7 in a different storage disease (62). Furthermore, active Rab7 expression significantly reduced the UPR (Fig. 6C), albeit to a lesser extent than the reduction observed in cells overexpressing Rab27a. These data support the idea that upregulation of the lysosomal transport system has the potential to reduce cell defects induced by lysosomal overload. The data also suggest that Rab27a, a GTPase with dual roles in trafficking and secretion, may have additional positive effects over Rab7, which regulates lysosomal trafficking but not exocytosis.
Fig 6.
Upregulation of lysosomal trafficking by Rab7 in Ctns−/− cells. Murine fibroblasts were labeled using LysoTracker, and lysosomal dynamics were analyzed by TIRFM, as described in the legend to Fig. 2. (A) The dynamics of the labeled lysosomes were monitored for 2 min. The kinetics of lysosomal movement was analyzed using Imaris software. Experiments were repeated three times. Histograms of lysosomal speeds from wild-type cells, Ctns−/− cells, and Ctns−/− cells expressing constitutively active Rab7 are shown. The speeds of lysosomal movement were binned in 0.01-μm/s increments and plotted as a percentage of the total number of granules for a given cell. Results are represented as means ± SEMs. *, P < 0.05 for Ctns−/− versus wild-type cells; §, P < 0.05 for Ctns−/− Rab7Q67L versus Ctns−/− cells. (B) Immunofluorescence analysis of the expression of Grp78/94 was performed as described in Materials and Methods. Nuclei were stained with DAPI. The level of expression of the UPR target genes Grp78 and Grp94, which are upregulated in Ctns−/− cells (middle), was significantly decreased in cells expressing EGFP-Rab7Q67L (right), although not to the basal levels shown in wild-type cells (left). (C) Quantification of the immunofluorescence shown in panel B. *, P < 0.05; ***, P < 0.001. P values were determined by one-way analysis of variance.
Calcium-induced exocytosis of readily releasable lysosomes is not affected in cystinotic cells.
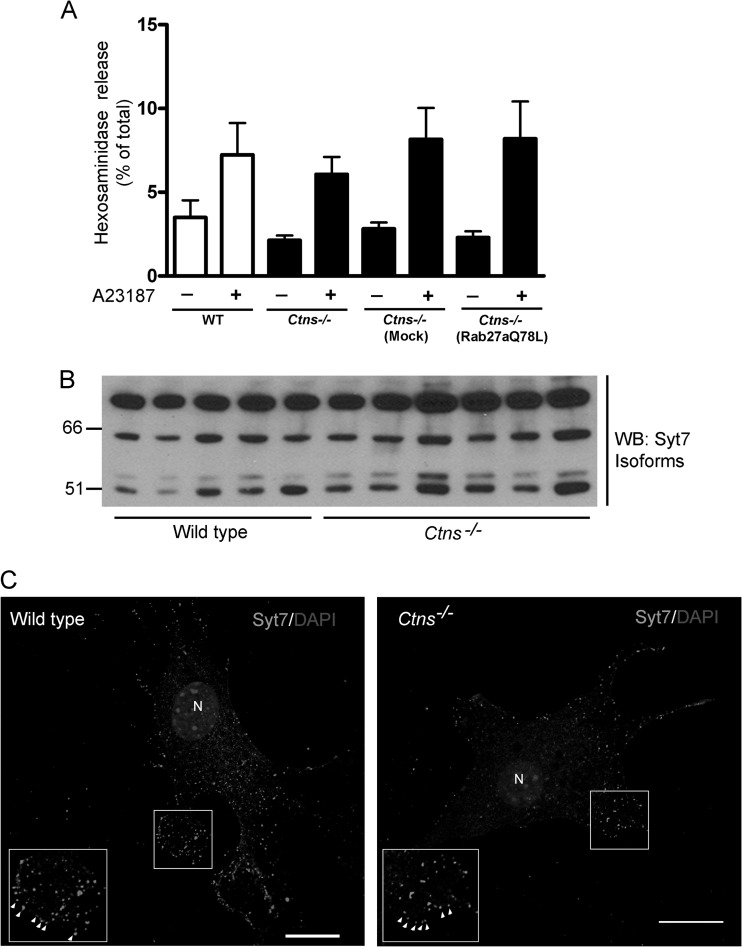
Exocytosis has been proposed to decrease lysosomal overload in lysosomal storage disorders (63). To determine whether the lysosomal exocytic pathway is impaired in cystinotic cells, we first evaluated calcium-induced exocytosis of readily releasable lysosomes in Ctns−/− fibroblasts. Here, we showed that exocytosis of these lysosomal pools is not impaired in cystinotic fibroblasts stimulated with calcium ionophores (Fig. 7A), which induce exocytosis of plasma membrane proximal lysosomes (64). Moreover, although Rab27a was proposed to regulate both trafficking and exocytosis of secretory organelles (65), including lysosomes (30), cells overexpressing constitutively active Rab27a showed no significant upregulation of readily releasable lysosome exocytosis in response to calcium ionophores (Fig. 7A). In addition, the expression of synaptotagmin 7 (Syt7), an exocytosis regulatory protein of the readily releasable lysosomal pool (66), was not downregulated in cystinosis. Thus, all Syt7 isoforms (43, 67) were expressed in cystinotic kidney lysates at levels similar to or higher than those observed in wild-type cells (Fig. 7B). Next, we showed that the subcellular localization of Syt7 in cystinotic fibroblasts has a distribution pattern similar to that observed in wild-type cells (Fig. 7C). Thus, Syt7 was observed in punctate internally distributed structures as well as in close proximity to or at the plasma membrane, most likely representing readily releasable lysosomes (Fig. 7C). Altogether, our data rule out putative defects in Syt7 expression or localization, exclude exocytosis of membrane proximal lysosomes as an impaired mechanism in cystinosis, and suggest that Rab27a does not play a major role in calcium-induced secretion of readily releasable proximal lysosomes in cystinotic cells.
Fig 7.
The Syt7-dependent lysosomal exocytic pathway is not impaired in cystinotic cells. (A) Lysosomal exocytosis was evaluated in fibroblasts stimulated with the ionophore A23187 in the presence of 1 mM Ca2+. Hexosaminidase in the supernatants and cell lysates was analyzed as described in Materials and Methods. Results are represented as mean ± SEMs (n = 3). (B) Analysis of the expression of Syt7 in kidneys from wild-type and Ctns−/− mice. Each lane corresponds to an individual sample from independent mice. Similar to previous studies (43), several isoforms of Syt7 were identified. None of these isoforms were downregulated in Ctns−/− cells. (C) The subcellular localization of Syt7 was further analyzed by immunofluorescence. Endogenous Syt7 was detected in punctate structures in proximity to the plasma membrane of fibroblasts (insets, arrowheads), as well as internally, in both wild-type and cystinotic cells. N, nucleus. Bars = 20 μm.
Expression of constitutively active Rab27a decreases cystine content in Ctns−/− cells.
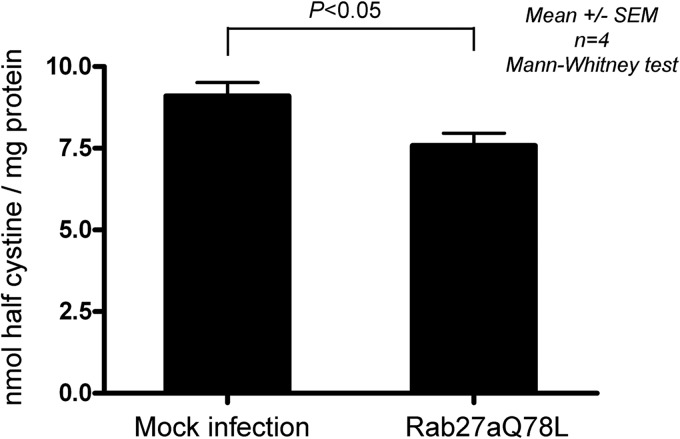
Next, we hypothesized that upregulation of vesicular transport mechanisms mediated by Rab27a has the potential to contribute to a net decrease in lysosomal overload by engaging lysosomes in a slow rather than a rapid secretory pathway. In particular, we evaluated whether the expression of the active form of Rab27a has the ability to reduce the amount of cystine accumulated in the lysosomes of otherwise unstimulated cystinotic cells. To this end, we determined the cystine content in cystinotic cells that expressed the mutant Rab27aQ78L by mass spectrometry. Our results showed for the first time that upregulation of the Rab27a-dependent mechanisms induces a significant reduction in the intracellular level of the metabolite accumulated in lysosomes in cystinotic cells (Fig. 8), thus decreasing lysosomal overload. Since exocytosis of the membrane proximal readily releasable pool of lysosomes is not involved in this process (Fig. 7A), our data suggest that Rab27a favors the mobilization and secretion of lysosomes from a deeper intracellular pool.
Fig 8.
Reduced cystine content in Ctns−/− cells expressing constitutively active Rab27a. Ctns−/− cells expressing constitutively active Rab27a (Rab27aQ78L) or mock infected were analyzed for lysosomal cystine content by mass spectrometry, as described in Materials and Methods. Results are represented as means ± SEMs (n = 4). The P value was determined by the Mann-Whitney test.
DISCUSSION
In this work, we make two independent but individually important observations. First, we show that cystinotic cells are characterized by impaired lysosomal transport and increased ER stress. Second, we demonstrate that reconstitution of lysosomal trafficking by expression of the constitutively active form of the small GTPase Rab27a increases the ability of these cells to eliminate accumulated metabolites and improves cellular function, manifested as a net decrease in the endoplasmic reticulum stress-associated response. Our data suggest that impaired vesicular transport induced by lysosomal overload contributes to the cellular defects observed in cystinosis. Our findings highlight the importance of the correction of vesicular trafficking for the normalization of cellular functions in LSDs and suggest that at least some of the cellular defects observed in lysosomal storage disorders can potentially be repaired by reconstitution of the normal intracellular lysosomal transport system.
In this work, we show that upregulation of Rab27a corrects lysosomal trafficking, decreases ER stress, and reduces lysosomal overload in the lysosomal storage disorder cystinosis. In this context, the observation that Rab27a expression is significantly decreased in kidneys from Ctns−/− mice and in cystinotic human PTCs is highly significant. The defect is specific for this GTPase, as the expression of other Rab GTPases involved in either exocytic or endocytic pathways was not affected in cystinotic cells. Despite its relatively low expression, endogenous Rab27a was not mislocalized in cystinotic cells. These data suggest that reduced expression of Rab27a but not dysfunction of the GTPase due to mislocalization or deficient posttranslational modification is affected in kidney cystinotic cells in vivo. Overexpression of constitutively active Rab7 also increased lysosomal transport and decreased the UPR. However, the effects of Rab7 on vesicular trafficking and ER stress, although significant, were less marked than those elicited by Rab27a expression. This is most likely explained by the dual role of Rab27a in trafficking and exocytosis.
A recent report suggested that Rab27a regulates exocytosis of conventional lysosomes in otherwise considered nonsecretory epidermoid carcinoma cells (30). Lysosomal exocytosis involves many steps, including calcium-independent trafficking of lysosomes to the docking site at the plasma membrane and the subsequent fusion of vesicular membranes with the plasma membrane in a calcium-dependent manner. In this work, we found that although Rab27a decreases lysosomal overload, Rab27a expression does not affect the rapid exocytosis of membrane proximal, calcium-regulated secretion of readily releasable lysosomes, suggesting that Rab27a engages a deeper lysosomal pool in an alternative secretory pathway in cells with lysosomal overload. Our observations that endogenous Rab27a colocalizes with LAMP-1-positive organelles in PTCs and that overexpression of constitutively active Rab27a increases lysosomal trafficking and decreases the cystine content in cystinotic cells, together with the results obtained using calcium ionophores, suggest that Rab27a regulates calcium-independent trafficking and secretory events rather than the calcium-dependent final step of membrane proximal lysosome-regulated secretion.
Here, we show that Rab27a expression is downregulated in the cystinotic kidney. In melanocytes, Rab27a expression is regulated at the transcriptional level by the transcription factor microphthalmia-associated transcription factor (MITF), and downregulation of MITF decreases melanosome transport due to decreased Rab27a expression (68). In addition, TFEB, which shares a strong homology with MITF (68) and is expressed in renal cells (69), is a master regulator of lysosomal biogenesis and lysosomal degradative function (70). Upregulation of TFEB increases lysosomal exocytosis and induces cellular clearance in other LSDs (63). Interestingly, three proximal conserved E boxes described in the promoter regions of the mouse and human rab27a genes are important for MITF binding in melanocytes and were proposed to be possible TFEB-mediated regulatory elements in other cells (68). On the basis of these data, it is possible that downregulation of Rab27a expression in cystinosis is caused by TFEB dysfunction, although further studies are necessary to confirm this hypothesis.
The mechanism for the development of ER stress in LSDs is not well understood. It is possible that the development of ER stress is consequential to the impaired vesicular trafficking in cystinotic cells. This is supported by the observation that the unfolded protein response decreases by upregulation of the lysosomal transport and the secretory pathways. Because cystinotic cells are constantly exposed to lysosomal overload and impaired lysosomal transport, Ctns−/− cells are likely under chronic rather than acute ER stress. This is consistent with our result showing maintained upregulation of Grp78 and Grp94 chaperones, together with the observed morphological abnormalities of the ER, a characteristic that is absent in cells that have adapted to stress (53). The observation that upregulation of the lysosomal trafficking and exocytic pathway reduces the UPR indicates that the beneficial changes in Ctns−/− cells induced by trafficking upregulation reduce the necessity for a compensatory response to stress, likely by increasing the excretion of unfolded proteins through the secretory pathway.
In addition, a net decrease in the amount of metabolites accumulated in lysosomes by increased exocytosis would be beneficial for cell function and may directly contribute to the downregulation of ER stress. This is in agreement with the findings of a previous study showing that lysosomal exocytosis may directly modulate cellular clearance in LSDs (63). Importantly, in our assays, cystine content is significantly but moderately reduced by constitutively active Rab27a expression. Although it may be possible to further reduce the cystine content from cystinotic cells by upregulating additional secretory proteins, including Rab27a effectors or enhancers of the fusion process, it is also possible that enhanced lysosomal trafficking is sufficient to reduce cellular stress. In support of this, upregulation of Rab7, which regulates lysosomal trafficking but not lysosomal exocytosis, decreases ER stress. Finally, upregulation of lysosomal transport has been associated with the improvement of many cellular functions, including phospholipid remodeling (16), receptor recycling (15), and plasma membrane repair (14), which may also contribute, directly or indirectly, to reduce cellular stress in cystinosis.
Altogether, our data suggest that improvement of lysosomal trafficking is beneficial for the correction of the cellular defects that characterize cells affected by lysosomal overload. These results have direct implications for cystinosis and for other lysosomal storage disorders. We suggest that the proteins that regulate the lysosomal transport system are potential targets of novel therapeutic approaches for the treatment of cystinosis and other LSDs.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a fellowship from the Cystinosis Research Foundation (CRF) to G. Napolitano and by a research grant from CRF to S. D. Catz. This work was also supported by U.S. Public Health Service grants HL088256 and GM105894 to S. D. Catz and by NIH/NCRR grant number UL1 RR025774. J. Monfregola is a postdoctoral fellow of the American Heart Association.
We thank Malcolm Wood for help with electron microscopy. We are thankful to Corinne Antignac for providing human PTCs.
Footnotes
Published ahead of print 28 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00417-13.
REFERENCES
- 1.Cherqui S, Kalatzis V, Trugnan G, Antignac C. 2001. The targeting of cystinosin to the lysosomal membrane requires a tyrosine-based signal and a novel sorting motif. J. Biol. Chem. 276:13314–13321 [DOI] [PubMed] [Google Scholar]
- 2.Kalatzis V, Cherqui S, Antignac C, Gasnier B. 2001. Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter. EMBO J. 20:5940–5949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taranta A, Wilmer MJ, van den Heuvel LP, Bencivenga P, Bellomo F, Levtchenko EN, Emma F. 2010. Analysis of CTNS gene transcripts in nephropathic cystinosis. Pediatr. Nephrol. 25:1263–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, van't Hoff W, Antignac C. 1998. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat. Genet. 18:319–324 [DOI] [PubMed] [Google Scholar]
- 5.Gahl WA, Thoene JG, Schneider JA. 2002. Cystinosis. N. Engl. J. Med. 347:111–121 [DOI] [PubMed] [Google Scholar]
- 6.da Silva VA, Zurbrugg RP, Lavanchy P, Blumberg A, Suter H, Wyss SR, Luthy CM, Oetliker OH. 1985. Long-term treatment of infantile nephropathic cystinosis with cysteamine. N. Engl. J. Med. 313:1460–1463 [DOI] [PubMed] [Google Scholar]
- 7.Gahl WA, Reed GF, Thoene JG, Schulman JD, Rizzo WB, Jonas AJ, Denman DW, Schlesselman JJ, Corden BJ, Schneider JA. 1987. Cysteamine therapy for children with nephropathic cystinosis. N. Engl. J. Med. 316:971–977 [DOI] [PubMed] [Google Scholar]
- 8.Nesterova G, Gahl W. 2008. Nephropathic cystinosis: late complications of a multisystemic disease. Pediatr. Nephrol. 23:863–878 [DOI] [PubMed] [Google Scholar]
- 9.Klein C, Philippe N, Le Deist F, Fraitag S, Prost C, Durandy A, Fischer A, Griscelli C. 1994. Partial albinism with immunodeficiency (Griscelli syndrome). J. Pediatr. 125:886–895 [DOI] [PubMed] [Google Scholar]
- 10.Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint BG. 2000. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 25:173–176 [DOI] [PubMed] [Google Scholar]
- 11.Seabra MC, Mules EH, Hume AN. 2002. Rab GTPases, intracellular traffic and disease. Trends Mol. Med. 8:23–30 [DOI] [PubMed] [Google Scholar]
- 12.Futerman AH, van Meer G. 2004. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 5:554–565 [DOI] [PubMed] [Google Scholar]
- 13.Andrews NW. 2000. Regulated secretion of conventional lysosomes. Trends Cell Biol. 10:316–321 [DOI] [PubMed] [Google Scholar]
- 14.Reddy A, Caler EV, Andrews NW. 2001. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell 106:157–169 [DOI] [PubMed] [Google Scholar]
- 15.Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado RD. 2007. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 120:3838–3849 [DOI] [PubMed] [Google Scholar]
- 16.Abe A, Kelly R, Kollmeyer J, Hiraoka M, Lu Y, Shayman JA. 2008. The secretion and uptake of lysosomal phospholipase A2 by alveolar macrophages. J. Immunol. 181:7873–7881 [DOI] [PubMed] [Google Scholar]
- 17.Zerial M, McBride H. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107–117 [DOI] [PubMed] [Google Scholar]
- 18.Hammer JA, III, Wu XS. 2002. Rabs grab motors: defining the connections between Rab GTPases and motor proteins. Curr. Opin. Cell Biol. 14:69–75 [DOI] [PubMed] [Google Scholar]
- 19.Bossi G, Griffiths GM. 2005. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin. Immunol. 17:87–94 [DOI] [PubMed] [Google Scholar]
- 20.Brzezinska AA, Johnson JL, Munafo DB, Crozat K, Beutler B, Kiosses WB, Ellis BA, Catz SD. 2008. The Rab27a effectors JFC1/Slp1 and Munc13-4 regulate exocytosis of neutrophil granules. Traffic 9:2151–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Futter CE, Ramalho JS, Jaissle GB, Seeliger MW, Seabra MC. 2004. The role of Rab27a in the regulation of melanosome distribution within retinal pigment epithelial cells. Mol. Biol. Cell 15:2264–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbs D, Azarian SM, Lillo C, Kitamoto J, Klomp AE, Steel KP, Libby RT, Williams DS. 2004. Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes. J. Cell Sci. 117:6473–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomi H, Mori K, Itohara S, Izumi T. 2007. Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane. Mol. Biol. Cell 18:4377–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch H, Hofmann K, Brose N. 2000. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem. J. 349:247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAdara-Berkowitz JK, Catz SD, Johnson JL, Ruedi JM, Thon V, Babior BM. 2001. JFC1, a novel tandem C2 domain-containing protein associated with the leukocyte NADPH oxidase. J. Biol. Chem. 276:18855–18862 [DOI] [PubMed] [Google Scholar]
- 26.Wilson SM, Yip R, Swing DA, O'Sullivan TN, Zhang Y, Novak EK, Swank RT, Russell LB, Copeland NG, Jenkins NA. 2000. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc. Natl. Acad. Sci. U. S. A. 97:7933–7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kesari A, Fukuda M, Knoblach S, Bashir R, Nader GA, Rao D, Nagaraju K, Hoffman EP. 2008. Dysferlin deficiency shows compensatory induction of Rab27A/Slp2a that may contribute to inflammatory onset. Am. J. Pathol. 173:1476–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson JL, Hong H, Monfregola J, Kiosses WB, Catz SD. 2011. MUNC13-4 restricts motility of RAB27A-expressing vesicles to facilitate lipopolysaccharide-induced priming of exocytosis in neutrophils. J. Biol. Chem. 286:5647–5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. 2001. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 152:825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laulagnier K, Schieber NL, Maritzen T, Haucke V, Parton RG, Gruenberg J. 2011. Role of AP1 and Gadkin in the traffic of secretory endo-lysosomes. Mol. Biol. Cell 22:2068–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. 2006. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 313:324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szegezdi E, Logue SE, Gorman AM, Samali A. 2006. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7:880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vitner EB, Platt FM, Futerman AH. 2010. Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem. 285:20423–20427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei H, Kim SJ, Zhang Z, Tsai PC, Wisniewski KE, Mukherjee AB. 2008. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum. Mol. Genet. 17:469–477 [DOI] [PubMed] [Google Scholar]
- 35.Nevo N, Chol M, Bailleux A, Kalatzis V, Morisset L, Devuyst O, Gubler MC, Antignac C. 2010. Renal phenotype of the cystinosis mouse model is dependent upon genetic background. Nephrol. Dial. Transplant. 25:1059–1066 [DOI] [PubMed] [Google Scholar]
- 36.Min LJ, Cui TX, Yahata Y, Yamasaki K, Shiuchi T, Liu HW, Chen R, Li JM, Okumura M, Jinno T, Wu L, Iwai M, Nahmias C, Hashimoto K, Horiuchi M. 2004. Regulation of collagen synthesis in mouse skin fibroblasts by distinct angiotensin II receptor subtypes. Endocrinology 145:253–260 [DOI] [PubMed] [Google Scholar]
- 37.Racusen LC, Wilson PD, Hartz PA, Fivush BA, Burrow CR. 1995. Renal proximal tubular epithelium from patients with nephropathic cystinosis: immortalized cell lines as in vitro model systems. Kidney Int. 48:536–543 [DOI] [PubMed] [Google Scholar]
- 38.Johnson JL, Ellis BA, Noack D, Seabra MC, Catz SD. 2005. The Rab27a binding protein JFC1 regulates androgen-dependent secretion of prostate specific antigen and prostate specific acid phosphatase. Biochem. J. 391:699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuzawa A, Tseng PH, Vallabhapurapu S, Luo JL, Zhang W, Wang H, Vignali DA, Gallagher E, Karin M. 2008. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science 321:663–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson JL, Brzezinska AA, Tolmachova T, Munafo DB, Ellis BA, Seabra MC, Hong H, Catz SD. 2010. Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms. Traffic 11:533–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weibel ER, Kistler GS, Scherle WF. 1966. Practical stereological methods for morphometric cytology. J. Cell Biol. 30:23–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mudrakola HV, Zhang K, Cui B. 2009. Optically resolving individual microtubules in live axons. Structure 17:1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugita S, Han W, Butz S, Liu X, Fernandez-Chacon R, Lao Y, Sudhof TC. 2001. Synaptotagmin VII as a plasma membrane Ca(2+) sensor in exocytosis. Neuron 30:459–473 [DOI] [PubMed] [Google Scholar]
- 44.Syres K, Harrison F, Tadlock M, Jester JV, Simpson J, Roy S, Salomon DR, Cherqui S. 2009. Successful treatment of the murine model of cystinosis using bone marrow cell transplantation. Blood 114:2542–2552 [DOI] [PubMed] [Google Scholar]
- 45.Kilpatrick BS, Eden ER, Schapira AH, Futter CE, Patel S. 2013. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J. Cell Sci. 126:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oude Elferink RP, Harms E, Strijland A, Tager JM. 1983. The intralysosomal pH in cultured human skin fibroblasts in relation to cystine accumulation in patients with cystinosis. Biochem. Biophys. Res. Commun. 116:154–161 [DOI] [PubMed] [Google Scholar]
- 47.Axelrod D. 2003. Total internal reflection fluorescence microscopy in cell biology. Methods Enzymol. 361:1–33 [DOI] [PubMed] [Google Scholar]
- 48.Kuhn JR, Pollard TD. 2005. Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophys. J. 88:1387–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steyer JA, Almers W. 2001. A real-time view of life within 100 nm of the plasma membrane. Nat. Rev. Mol. Cell Biol. 2:268–275 [DOI] [PubMed] [Google Scholar]
- 50.Strom M, Hume AN, Tarafder AK, Barkagianni E, Seabra MC. 2002. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 277:25423–25430 [DOI] [PubMed] [Google Scholar]
- 51.Aridor M, Hannan LA. 2000. Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic 1:836–851 [DOI] [PubMed] [Google Scholar]
- 52.Preston AM, Gurisik E, Bartley C, Laybutt DR, Biden TJ. 2009. Reduced endoplasmic reticulum (ER)-to-Golgi protein trafficking contributes to ER stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia 52:2369–2373 [DOI] [PubMed] [Google Scholar]
- 53.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. 2006. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 4:e374. 10.1371/journal.pbio.0040374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen J, Chen X, Hendershot L, Prywes R. 2002. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3:99–111 [DOI] [PubMed] [Google Scholar]
- 55.Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ, Brewer JW. 2009. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J. Cell Sci. 122:1626–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Briarty LG. 1975. Stereology: methods for quantitative light and electron microscopy. Sci. Prog. 62:1–32 [PubMed] [Google Scholar]
- 57.Delom F, Fessart D, Chevet E. 2007. Regulation of calnexin sub-cellular localization modulates endoplasmic reticulum stress-induced apoptosis in MCF-7 cells. Apoptosis 12:293–305 [DOI] [PubMed] [Google Scholar]
- 58.Guerin R, Arseneault G, Dumont S, Rokeach LA. 2008. Calnexin is involved in apoptosis induced by endoplasmic reticulum stress in the fission yeast. Mol. Biol. Cell 19:4404–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen X, Ellis RE, Sakaki K, Kaufman RJ. 2005. Genetic interactions due to constitutive and inducible gene regulation mediated by the unfolded protein response in C. elegans. PLoS Genet. 1:e37. 10.1371/journal.pgen.0010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cantalupo G, Alifano P, Roberti V, Bruni CB, Bucci C. 2001. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20:683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A, Bjorkoy G, Johansen T. 2010. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J. Cell Biol. 188:253–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choudhury A, Dominguez M, Puri V, Sharma DK, Narita K, Wheatley CL, Marks DL, Pagano RE. 2002. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 109:1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, Palmieri M, Polishchuk R, Puertollano R, Ballabio A. 2011. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 21:421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaiswal JK, Andrews NW, Simon SM. 2002. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J. Cell Biol. 159:625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tolmachova T, Anders R, Stinchcombe J, Bossi G, Griffiths GM, Huxley C, Seabra MC. 2004. A general role for Rab27a in secretory cells. Mol. Biol. Cell 15:332–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. 2000. Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J. Cell Biol. 148:1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukuda M, Ogata Y, Saegusa C, Kanno E, Mikoshiba K. 2002. Alternative splicing isoforms of synaptotagmin VII in the mouse, rat and human. Biochem. J. 365:173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chiaverini C, Beuret L, Flori E, Busca R, Abbe P, Bille K, Bahadoran P, Ortonne JP, Bertolotto C, Ballotti R. 2008. Microphthalmia-associated transcription factor regulates RAB27A gene expression and controls melanosome transport. J. Biol. Chem. 283:12635–12642 [DOI] [PubMed] [Google Scholar]
- 69.Medendorp K, van Groningen JJ, Schepens M, Vreede L, Thijssen J, Schoenmakers EF, van den Hurk WH, Geurts van Kessel A, Kuiper RP. 2007. Molecular mechanisms underlying the MiT translocation subgroup of renal cell carcinomas. Cytogenet. Genome Res. 118:157–165 [DOI] [PubMed] [Google Scholar]
- 70.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. 2009. A gene network regulating lysosomal biogenesis and function. Science 325:473–477 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.