Abstract
Transcription factor Nrf2 (NF-E2-related factor 2) regulates a broad cytoprotective response to environmental stresses. Keap1 (Kelch-like ECH-associated protein 1) is an adaptor protein for cullin3-based ubiquitin E3 ligase and negatively regulates Nrf2. Whereas the Keap1-Nrf2 system plays important roles in oxidative stress response and metabolism, the roles Nrf2 plays in the prevention of diabetes mellitus remain elusive. Here we show that genetic activation of Nrf2 signaling by Keap1 gene hypomorphic knockdown (Keap1flox/−) markedly suppresses the onset of diabetes. When Keap1flox/− mice were crossed with diabetic db/db mice, blood glucose levels became lower through improvement of both insulin secretion and insulin resistance. Keap1flox/− also prevented high-calorie-diet-induced diabetes. Oral administration of the Nrf2 inducer CDDO-Im {oleanolic acid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl] imidazole} also attenuated diabetes in db/db mice. Nrf2 induction altered antioxidant-, energy consumption-, and gluconeogenesis-related gene expression in metabolic tissues. Thus, the Keap1-Nrf2 system is a critical target for preventing the onset of diabetes mellitus.
INTRODUCTION
Nrf2 (NF-E2-related factor 2) is a basic region-leucine zipper-type transcription factor belonging to the cap ‘n’ collar (CNC) family (1, 2) that tightly interacts with Keap1 (Kelch-like ECH-associated protein 1) (3). Keap1 is an adaptor protein for cullin3-based ubiquitin E3 ligase. Under unstressed conditions, Keap1 inhibits Nrf2 signaling by facilitating ubiquitination and degradation through the ubiquitin-proteasome pathway (4–6). When Keap1 is exposed to electrophiles, reactive oxygen species (ROS), reactive nitrosative species (RNS), or heavy metals, cysteine residues in Keap1 are modified, leading to conformational changes in the complex that, in turn, impede proteasome-mediated degradation of Nrf2. Accordingly, Nrf2 is stabilized, allowing for accumulation in the nucleus and coordinate induction of multiple cellular defense enzymes (7–10). This stress response gene regulation system is called the Keap1-Nrf2 system and acts as a key regulator for protective responses by our body against ROS, RNS, and electrophiles (10, 11).
In addition to cytoprotection, involvement of Nrf2 in diabetes mellitus and obesity has been suggested. For instance, streptozotocin-induced diabetes in Nrf2-null mice exhibits increases in oxidative and nitrosative stress levels (12) as well as elevated blood glucose levels via enhanced expression of hepatic gluconeogenesis-related genes (13). Administration of an Nrf2 inducer, CDDO-Im {oleanolic acid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl] imidazole}, strongly suppressed high-fat-diet-induced obesity: this protection is abrogated by disruption of the Nrf2 gene (14, 15). In addition, Nrf2 is involved in glucose regulation in cancer cells (16). However, direct demonstration of the involvement of the Keap1-Nrf2 system in the onset of diabetes has not been examined. Therefore, we conceived that a genetic model of Nrf2 induction would clarify the importance of the Keap1-Nrf2 system in protection against diabetes mellitus.
We have demonstrated previously that Keap1 negatively regulates Nrf2 activity: Nrf2 is activated by genetic disruption of the Keap1 gene (17, 18). While Keap1-null mice show lethality at 3 weeks after birth, an amenable model for analyzing the effect of enhanced Nrf2 signaling arises in mice harboring the hypomorphic allele of Keap1 (Keap1flox) that allows for graded expression of Keap1 (17). The insertion of two loxP sequences into the Keap1 locus for conditional disruption of the Keap1 gene resulted in reduced expression of Keap1 mRNA. Keap1 gene knockdown (Keap1 KD) by the Keap1flox/− strategy displays constitutive stabilization of Nrf2 and induction of its downstream targets in various tissues. Here we show that genetic activation of Nrf2 signaling by Keap1 KD strongly suppressed the onset of diabetes mellitus. In contrast, genetic ablation of Nrf2 aggravated diabetes mellitus in a murine pancreatic β-cell stress model. Repeated administration of the potent Nrf2 inducer CDDO-Im exerted antidiabetic effects in db/db mice. These genetic and pharmacological results provide direct evidence that the Keap1-Nrf2 system critically contributes to the prevention of onset of diabetes mellitus.
MATERIALS AND METHODS
Mice.
Both db/db::Keap1flox/+ and db/db::Keap1flox/− groups were obtained from ICR background db/+::Keap1flox/flox and db/+::Keap1+/− mating pairs (17, 18). Both control Nrf2+/+ and Nrf2−/− mice were obtained from ICR background Nrf2 heterozygous mating pairs (8). MuCreA mice (19) were kindly supplied by the RIKEN BioResource Center (Tsukuba, Japan). Alb-Cre mice were supplied by Jackson Laboratory (Bar Harbor, ME). For CDDO-Im administration studies, Nrf2−/− mice were backcrossed to C57BL/KsJ db/db mice for more than 8 generations. The db allele was genotyped by the endpoint analysis using TaqMan genotyping master mix (Applied Biosystems) and ABI custom single nucleotide polymorphism (SNP) (forward primer, 5′- CCAACTTCCCAACAGTCCATACAAT -3; reverse primer, 5′- CTCATCAAATGTTATTTCTTAGTCATTCAAACCA -3; probe for wild allele, 5′-VIC- TGATGGAGGGAAACAA -3; probe for db mutant allele, 5′-6-carboxyfluorescein [FAM]- TTGATGGAGGTAAACAA -3′) using a model 7300 real-time PCR system (95°C for 10 min for 1 cycle and 92°C for 15 s and 60°C for 90 s for 50 cycles) (Applied Biosystems).
HCD.
Mice were fed a high-calorie diet (HCD) as demonstrated in Fig. S4A in the supplemental material. Briefly, the standard-diet (SD) group was fed standard chow (medium fat [MF], 3.59 kcal/g; Oriental Yeast) for 8 weeks. The HCD group was fed a high-fat diet (HFD) (5.06 kcal/g, containing 62.2% calories from fat [HFD-60]; Oriental Yeast) alone for 3 weeks from the start of this study to evaluate insulin regulation by HFD; they were then started on oral administration of 20% sucrose in drinking water concomitantly with HFD-60 at 3 weeks from the start of this study to evaluate those with maximum metabolic stress induced by HFD and high carbohydrate.
CDDO-Im administration.
As demonstrated in Fig. S11A, S13A, and S14A in the supplemental material, male db/db mice of the C57BL/KsJ background were orally administered CDDO-Im (10 or 30 μmol/kg of body weight [BW]; obtained from Mochida Pharmaceuticals Co., Ltd.) in 10% dimethyl sulfoxide (DMSO), 10% Cremophor-EL, and phosphate-buffered saline (PBS) as a vehicle or vehicle alone. The animal studies were conducted in accordance with the guiding principles for care and use of research animals promulgated by Tohoku University.
Administration of cAMP analogue.
Mice were fasted for 1 h before the study. Dibutyryl cyclic AMP (dbcAMP; Daiichi-Sankyo; 100 mg/kg of BW) or PBS (as a vehicle control) was intraperitoneally administered twice in 50-mg/kg fractions of dbcAMP with a 2-h interval. Four hours after the first administration of dbcAMP, gluconeogenesis-related gene expression levels in the liver were determined.
Measurement of blood glucose and plasma insulin.
The blood glucose concentrations were determined with the OneTouch Ultra blood glucose analyzer (LifeScan). Plasma insulin and glucagon concentrations were measured using a mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Morinaga Institute) and glucagon enzyme immunoassay (EIA) kit (Yanaihara Institute) according to the manufacturer's instructions. HOMA-IR (homeostasis model assessment as an index of insulin resistance) was calculated as fasting plasma insulin concentration (ng/ml) times fasting blood glucose (mg/dl) divided by 405.
Glucose, insulin, and pyruvate tolerance tests.
For the glucose tolerance test (GTT), mice were fasted for 14 h before the study. Glucose was orally or intraperitoneally administered (2 g/kg of BW). For the insulin tolerance test (ITT), mice were fasted for 0.5, 6, or 14 h before the study. Human regular insulin (Humulin R; Eli Lilly) was intraperitoneally injected (0.75 U/kg of BW). In GTT and ITT, area under the blood concentration-time curve (AUC) was calculated. For the pyruvate tolerance test (PTT), mice were fasted for 16 h before the study. Pyruvate was intraperitoneally administered (2 g/kg of BW).
Preparation of pancreatic islets.
After injection of ice-chilled Hanks' solution containing collagenase type V (Wako Pure Chemical) at a concentration of 2 mg/ml into the common bile duct, pancreata were isolated from the mice as described previously (20).
Measurement of insulin secretion from isolated islets.
Glucose-stimulated insulin secretion (GSIS) was analyzed with the batch of 20 isolated islets. The islets were preincubated for 30 min at 37°C in HEPES-balanced Krebs-Ringer bicarbonate buffer (HKRB) containing 2.8 mmol/liter of glucose and then incubated for another 30 min in the same medium containing various concentrations of glucose as described previously (20). Data were normalized with total protein contents of islets.
RNA preparation and qPCR.
Total RNAs were extracted with Isogen reagent (Nippon Gene). Total RNAs were subjected to a reverse transcription (RT) reaction using PrimeScript RT master mix (TaKaRa Bio). Thereafter, obtained templates were used for quantitative real-time PCR (50°C for 2 min and 95°C for 10 min for 1 cycle; 95°C for 15 s and 60°C for 90 s for 40 cycles) with quantitative PCR (qPCR) master mix (Eurogentec) with a model 7300 real-time PCR system (Applied Biosystems). The qPCR mixture (total, 20 μl) contained qPCR master mix or qPCR SYBR master mix (10 μl), forward and reverse primers (300 nmol/liter each), TaqMan probe (150 nmol/liter), and template cDNA (100 ng of total RNA).
IHC.
Immunohistochemistry (IHC) was performed as previously described (20) with the antibodies raised against insulin (clone K36AC10; Sigma) and glucagon (Cell Signaling Technology). Insulin-positive areas of sections and islet size were determined using ImageJ software (21) by measurement of a 3,3′-diaminobenzidine-stained area of IHC for insulin. The insulin-positive area was normalized with total pancreas area in pancreatic sections.
Protein preparation.
Skeletal muscle (SkM) and liver were homogenized with 1 ml of cell lysis buffer (20 mmol/liter of Tris-HCl [pH 7.5], 5 mmol/liter of EDTA, 10 mmol/liter of Na4P2O7, 1% NP-40, protease inhibitor cocktail [complete; Roche], and phosphatase inhibitor cocktail [PhosSTOP; Roche]) in a Precellys 24 homogenizer (30 s 3 times for skeletal muscle and 15 s once for liver; Bertin Technologies); the lysates of skeletal muscle were incubated for 2 h on ice and sonicated for 45 s. The cell debris was removed by centrifugation at 17,400 × g for 5 min.
Immunoblot analyses.
Immunoblot analyses were conducted as previously described (22–24). Primary antibodies for CPTIB (H-120, 1:1,000; Santa Cruz), CREB (cAMP response element-binding protein) (48H2, 1:1,000; Cell Signaling Technology), PEPCK (ab70358, 1:1,000; Abcam), and antitubulin (1:10,000; Sigma) were used.
Measurement of oxygen consumption.
SkM (gastrocnemius and soleus muscle) and brown adipose tissue (BAT) were removed freshly. Krebs-Ringer bicarbonate buffer was aerated with 5% CO2–95% O2 mixed gas. KRB (1.2 ml) containing 5.6 mmol/liter of glucose in a 24-well SDR sensor OxyDish (PreSens) was preincubated for 10 min at 37°C, and then the SkM and BAT were added to the Krebs-Ringer buffer. Changes of oxygen concentration in buffer were measured for 20 min at 37°C using an SDR2 sensor dish reader (PreSens). Oxygen consumption was determined by oxygen content change in buffer for 20 min normalized with tissue weight.
Plasmids.
The subcloned chimeric construct G6pc-Luc2P, harboring mouse G6pc genomic DNA and luciferase (Luc) cDNA (pGL4.15; Promega), was used for transfection studies. G6pc-Luc2P contains the G6pc 5′ flanking region from −1388 to + 82 relative to the transcription start site upstream of the Luc2P cDNA in pGL4.15. Human CREB cDNA was cloned into the pF9A expression vector (hCREB-A-pF9A). Murine Nrf2 cDNA was cloned into pcDNA3 (Life Technologies) (mNrf2-pcDNA3).
Cell culture.
Murine liver AML12 cells were obtained from the ATCC and grown with a 1:1 mixture of Dulbecco modified Eagle medium (DMEM) and Ham's F-12 medium (Life Technologies), supplemented with 10% fetal bovine serum (FBS), insulin-transferrin-selenium-G supplements (Life Technologies), 100 nmol/liter of dexamethasone (Dex; Wako Pure Chemical), 100 U/ml of penicillin, and 100 μg/ml of streptomycin. For RNA preparation, 5 × 105 AML12 cells were seeded in 6-well plates. Media were changed to a 1:1 mixture of DMEM and Ham's F-12 medium supplemented with 0.5% FBS. The cells were incubated with CDDO-Im for 24 h, Dex or dbcAMP was added, and cells were incubated further for 6 h.
For the transfection experiments, 1 × 105 AML12 cells were seeded in 24-well plates. Media were changed to 0.5 ml of a 1:1 mixture of DMEM and Ham's F-12 medium supplemented with 10% FBS. The cells were then transfected with G6pc-Luc2P, hCREB-A-pF9A, and mNrf2-pcDNA3 using Lipofectamine LTX and Plus reagent (Life Technologies) for 24 h. For stable transfection, 2 × 106 AML12 cells were seeded in a 100-mm dish. These cells were transfected with G6pc-Luc2P and were selected by 600 μg/ml of hygromycin B (Life Technologies).
Open-field test.
Locomotor activity was evaluated by open-field test. Testing was conducted in a 46-cm circular open-field during the light phase. Mouse activity was monitored for 20 min. Two parameters, including moved distance and rated crossing zone, were calculated every 5 min using Viewer-S2 software (Biobserve).
Statistical analyses.
All data are presented as means ± standard errors of means (SEM). Statistical analyses were performed with Student's t test or analysis of variance (ANOVA) followed by Fisher's least-significant-difference post hoc test for multiple comparison. Correlation analysis was done with Pearson's test.
RESULTS
Genetic Nrf2 induction prevents diabetes onset in db/db mice.
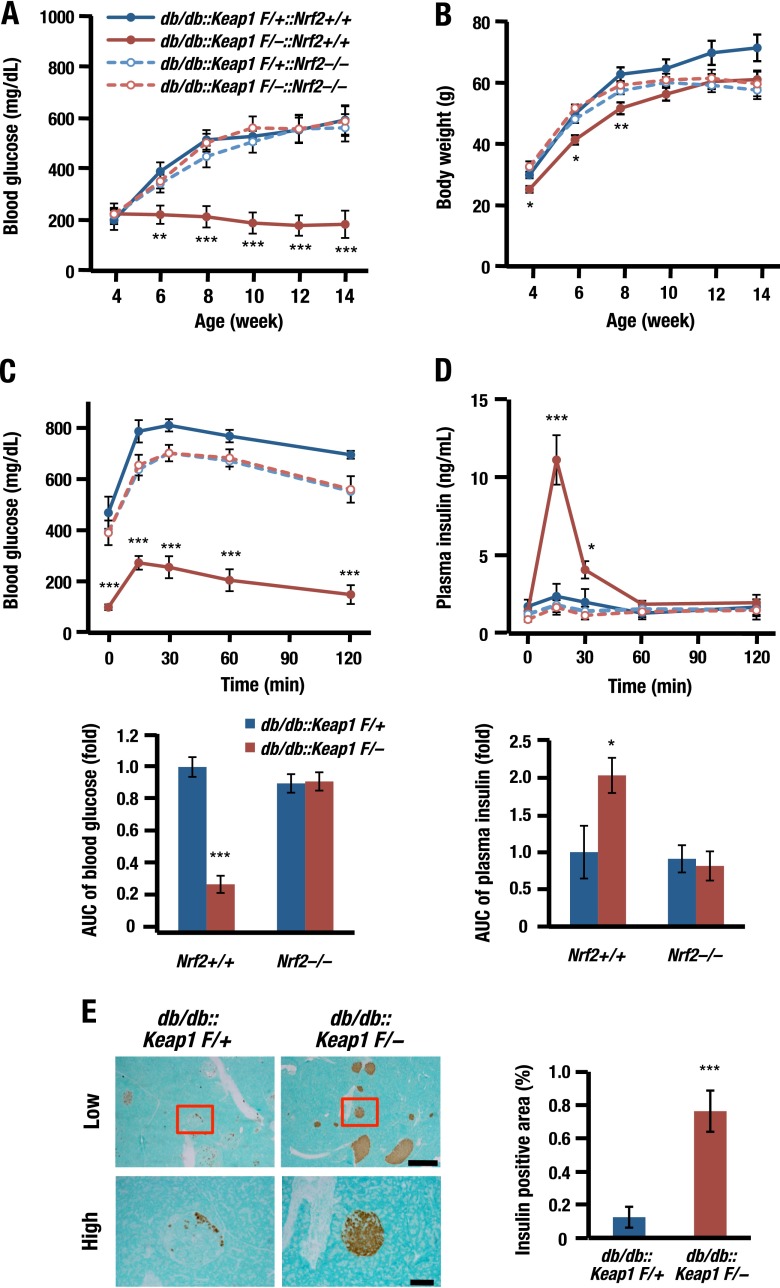
We first evaluated the impact of genetic induction of Nrf2 signaling on diabetes mellitus using Keap1 KD mice crossed with db/db mice (db/db::Keap1flox/−). Whereas db/db::Keap1flox/+ littermates used as controls for this experiment showed severe hyperglycemia, db/db::Keap1flox/− mice did not show such hyperglycemia at any point throughout the experimental period, indicating that Nrf2 strongly affects glucose metabolism (Fig. 1A). The body weights of juvenile db/db::Keap1flox/− mice were marginally lower than those of littermate control mice (Fig. 1B), indicating that the suppression of blood glucose levels in db/db::Keap1flox/− mice was not important for body weight gain.
Fig 1.
Nrf2 prevents diabetes in db/db mice. (A and B) Blood glucose levels (A) and body weights (B) of db/db::Keap1flox/+ (control) or db/db::Keap1flox/− mice crossed with Nrf2+/+ or Nrf2−/− mice (n = 9 to 20) and fed ad libitum. Also shown are blood glucose (C) and plasma insulin (D) levels in the OGTT. Glucose (2 g/kg of BW) was orally administered to 13- to 18-week-old mice after 14 h of fasting (n = 9 to 20). The lower graphs demonstrate AUC. (E) IHC of insulin in pancreatic sections (low, 5× objective; high, 20× objective) of 18-week-old control or db/db::Keap1flox/− mice (left) and the quantified insulin-positive area in these sections (right; n = 9 to 12). Scale bars, 500 μm (low) and 100 μm (high). P < 0.001 (***), P < 0.01 (**), and P < 0.05 (*) versus db/db::Keap1flox/+. Error bars, SEM.
Surprisingly, when we determined glucose tolerance by means of oral GTT (OGTT), blood glucose levels were found to be markedly lower in db/db::Keap1flox/− mice than in control mice, and AUC was sharply decreased (Fig. 1C). An increase in plasma insulin was fully preserved in db/db::Keap1flox/− mice, while in contrast, insulin levels of control mice were diminished (Fig. 1D). Of note, the improvement of both blood glucose and insulin secretion in db/db::Keap1flox/− mice was not observed in those with Nrf2-null background (Fig. 1A to D, dashed lines).
We found remarkable preservation of β-cell mass in pancreata of db/db::Keap1flox/− mice compared to that in control mice as measured by insulin IHC (Fig. 1E). The insulin-positive area was approximately 8-fold higher in db/db::Keap1flox/− mice than in control mice, demonstrating that Nrf2 induction preserves the β-cell mass and function and prevents diabetes onset in db/db mice.
Nrf2 increases insulin sensitivity.
We surmise that the observed antidiabetic phenotype provoked by the genetic activation of Nrf2 signaling could be explained by protection of β-cell function and/or increased insulin sensitivity in the peripheral tissues. To address this point, we examined changes in insulin sensitivity by measuring insulin resistance using HOMA-IR. HOMA-IR was markedly decreased in db/db::Keap1flox/− mice compared with control mice (see Fig. S1A and B in the supplemental material). Blood glucose levels in ITTs of db/db::Keap1flox/− mice were markedly lower than those of control mice both before and after insulin administration at any time point (see Fig. S1B), and those percent changes at 120 min were also lower than those of control mice (see Fig. S1C). These data support the idea that Nrf2 enhances insulin sensitivity in db/db mice. We consider the fact that the insulin sensitivity has not been fully evaluated by the ITT to be due to the great difference of blood glucose levels before insulin administration between these mice. In order to accurately evaluate insulin sensitivity, we changed the mouse model from db/db mice to lean mice.
To clarify whether insulin sensitivity was enhanced in lean Keap1flox/− mice, we calculated HOMA-IR of lean Keap1flox/− mice. HOMA-IR was decreased in lean Keap1flox/− mice compared with control littermate lean Keap1flox/+ mice (see Fig. S1D in the supplemental material). Both blood glucose levels and plasma insulin levels of Keap1flox/− mice were also decreased after 10 weeks of age (Fig. 2A and B). Importantly, the blood glucose levels were not decreased in Keap1flox/− mice crossed with Nrf2−/− mice (Fig. 2C).
Fig 2.
Nrf2 enhances insulin sensitivity and prevents obesity. (A and B) Blood glucose (n = 5) (A) and plasma insulin levels (n = 10) (B) of male lean Keap1flox/+ (control) or Keap1flox/− mice fed ad libitum. ***, P < 0.001 versus control. (C) Blood glucose levels of male lean control or Keap1flox/− mice crossed with Nrf2+/+ or Nrf2−/− mice fed ad libitum (10 weeks old; n = 9 to 19). (D) Body weights of male lean control or Keap1flox/− mice crossed with Nrf2+/+ (n = 5) or Nrf2−/− (n = 4 or 5) mice. ***, P < 0.001 versus control. (E) Blood glucose levels of male lean control or Keap1flox/− mice crossed with Nrf2+/+ (n = 10) or Nrf2−/− (n = 9 to 11) mice in the ipGTT. Glucose (2 g/kg of BW) was administered after 14 h of fasting (mice were 8 to 9 weeks old). ***, P < 0.001 versus control. (F) Blood glucose levels of male lean control or Keap1flox/− mice crossed with Nrf2+/+ (n = 9 or 10) or Nrf2−/− (n = 6 or 7) mice in the ITT. Regular insulin (0.75 U/kg of BW) was administered after 0.5 h of fasting (mice were 9 to 10 weeks old). ***, P < 0.001 versus control. (G and H) Blood glucose levels of male lean Keap1flox/flox (control) or tissue-specific Keap1 knockout mice in SkM (G) (Keap1 MuKO, n = 10 to 12) and liver (H) (Keap1 LKO, n = 8) in the ITT. Regular insulin (0.75 U/kg of BW) was administered after 0.5 h of fasting (mice were 8 to 9 weeks old). P < 0.001 (***) and P < 0.05 (*) versus control. (I) Blood glucose levels of male lean control, Keap1 MuKO, and Keap1 LKO mice fed ad libitum. ***, P < 0.001 versus control. (J to L) Body weight changes (J and K) and blood glucose levels (L) of male Keap1flox/+ mice fed the SD (Keap1 F/+ SD), Keap1flox/+ mice fed the HCD (HFD with 20% sucrose in drinking water; Keap1 F/+ HCD), Keap1flox/− mice fed the SD (Keap1 F/− SD), and Keap1flox/− mice fed the HCD (Keap1 F/− HCD) crossed with Nrf2+/+ (J and L) (n = 5) or Nrf2−/− (K) (n = 4 to 6) mice. P < 0.001 (***) and P < 0.01 (**) versus Keap1 F/− SD. Error bars, SEM.
Body weights of Keap1flox/− mice were lower than those of control littermates after 6 weeks of age and did not increase substantially thereafter (Fig. 2D). However, food intake normalized to body weight was higher in Keap1flox/− mice than in control mice (see Fig. S1E in the supplemental material), indicating that the low body weight gain in the Keap1flox/− mice was not caused by a decrease in food intake but rather was reflective of significant metabolic reprogramming. Indeed, in Keap1flox/− mice, blood glucose levels were decreased in the intraperitoneal GTT (ipGTT) (Fig. 2E). Plasma insulin levels were not affected during the first 30 min but decreased 60 min after glucose administration to Keap1flox/− mice (see Fig. S1D). Blood glucose levels of Keap1flox/− mice were also decreased in an ITT (Fig. 2F). The lowering of body weights and glucose levels in the GTT/ITT in Keap1flox/− mice was diminished by the Nrf2 gene knockout (Fig. 2D to F, dashed lines). Thus, the results in lean mice unequivocally demonstrate that the amplification of Nrf2 signaling increases insulin sensitivity, which in combination with the preservation of β-cell function overcomes the db/db phenotype.
Graded expression of Keap1 and insulin sensitivity.
Keap1flox/flox and Keap1flox/− mice show graded expression of Keap1, and Keap1flox/flox mutation elicits lower Keap1 expression than Keap1flox/+ but higher expression than Keap1flox/− (17). A negative correlation between the graded expression of Keap1 and Nrf2 accumulation to the nucleus has also been observed (17). Since these graded Keap1 expression levels faithfully reflect the Nrf2 activity within the cells, we evaluated body weight and insulin sensitivity of Keap1flox/flox mice along with those of Keap1flox/− mice. Whereas Keap1flox/flox mice did not show any substantial alterations of body weight and blood glucose level in the ITT, Keap1flox/− mice showed much lower levels in these two parameters (see Fig. S2A and B in the supplemental material). These results thus indicate that Nrf2 activation in Keap1flox/− mice decreased both body weight and blood glucose level by giving rise to more intensive induction of Nrf2 than that in Keap1flox/flox mice.
SkM contributes the enhancement of insulin sensitivity by Nrf2.
In order to determine which organ contributes to the increase of insulin sensitivity brought by Nrf2, we have evaluated insulin sensitivity by exploiting two tissue-specific Keap1 conditional knockout mouse lines. We have utilized the MuCreA Cre-recombinase system for skeletal muscle (SkM) (Keap1flox/flox::MuCreA; Keap1 MuKO) and the Alb-Cre system for the liver (Keap1flox/flox::Alb-Cre; Keap1 LKO) and compared the results of these specific knockouts with those of Keap1flox/flox (control) mice. We found that the Keap1 level was strongly suppressed but the Nqo1 level was induced in SkM of Keap1 MuKO mice (see Fig. S3A in the supplemental material) and in the livers of Keap1 LKO mice (see Fig. S3B).
Body weights of Keap1 MuKO mice were mildly lower than those of littermate control mice, but those of LKO mice were not altered (see Fig. S3C in the supplemental material). Importantly, blood glucose levels in the ITT were strikingly decreased in Keap1 MuKO mice (Fig. 2G; see also Fig. S3D) but not in LKO mice (Fig. 2H; see also Fig. S3D). These results thus demonstrate that the genetic Nrf2 induction provokes the increase of insulin sensitivity in SkM, and this mainly contributes to the lower blood glucose levels in ITTs of lean Keap1flox/− mice.
In contrast, in Keap1 LKO mice, blood glucose levels of mice fed ad libitum were slightly decreased, but they were not decreased in MuKO mice (Fig. 2I). These results suggest that the Nrf2 induction in the liver may contribute to some extent to the decrease of blood glucose level in Keap1-deficient mice.
Nrf2 prevents HCD-induced diabetes and obesity.
We next examined how Nrf2 counteracts HCD-mediated diabetes and obesity. In this analysis, both Keap1flox/− and control Keap1flox/+ littermates received an SD (3.59 kcal/g) or an HCD (an HFD supplemented with 20% sucrose in the drinking water) from 12 weeks of age for 8 weeks (see Fig. S4A in the supplemental material). Of note, as shown in Fig. 2J, while the body weights of Keap1flox/+ mice fed the HCD increased significantly compared with those of mice fed the SD, Keap1flox/− mice fed the HCD showed a modest loss of body weight that was similar to that in mice fed the SD. Furthermore, the prevention of body weight gain of Keap1flox/− mice was canceled by Nrf2 gene knockout in both the SD and HCD groups (Fig. 2K). Whereas blood glucose levels of Keap1flox/+ HCD-fed mice were significantly elevated after 6 weeks, the blood glucose levels did not increase in Keap1flox/− mice fed the HCD (Fig. 2L). These results thus unequivocally demonstrate that Nrf2 suppressed blood glucose levels and body weight gain even with an increase of food intake, demonstrating that Nrf2 functions to strongly oppose the onset of HCD-induced diabetes and obesity.
Consistent with this notion, epididymal fat (EF) pad volume was markedly decreased in Keap1flox/− SD-fed mice, and HCD feeding partially canceled this fat loss (see Fig. S4B in the supplemental material). The EF loss in Keap1flox/− mice was abrogated by the Nrf2 gene knockout (see Fig. S4B). Plasma insulin levels of Keap1flox/+ mice fed the HCD were much higher than in Keap1flox/+ mice fed the SD after 3 weeks, but those of both Keap1flox/− HCD-fed and Keap1flox/− SD-fed mice were very low and did not increase during the experimental period (see Fig. S4C).
Nrf2 stimulates energy consumption.
We explored mechanisms underlying the increase in insulin sensitivity and the loss of body weight in the Keap1flox/− mice. To confirm constitutive Nrf2 signaling in insulin-sensitive tissues of the Keap1flox/− mice, we measured expression levels of a prototypic Nrf2 target gene, Nqo1. Expression of Nqo1 mRNA was significantly upregulated in the liver, SkM, EF, and brown adipose tissue (BAT) of Keap1flox/− mice (see Fig. S5A in the supplemental material). Since it has been reported that Nrf2 is abundantly (2) or poorly (25) expressed in SkM, we also evaluated Nrf2 expression levels in SkM in our study. Nrf2 mRNA was abundantly expressed in SkM of 8-week-old male ICR mice but less abundantly in C57BL/6J mice (see Fig. S5B), suggesting that these strain differences may explain the discrepancy of the previous reports. Based on this observation, we examined expression of energy consumption-related genes in liver, SkM, and fat tissues of ICR strain Keap1flox/− mice by quantitative PCR analysis. The expression level of Cpt1b (carnitine palmitoyltransferase 1B gene) was upregulated in SkM, while Glut1 was increased in BAT (Fig. 3A).
Fig 3.
Nrf2 increases energy consumption-related genes in SkM. (A) Energy consumption-related gene expression in liver, SkM, white adipose tissue (WAT), and BAT of male 8-week-old lean Keap1flox/+ (control) or Keap1flox/− mice. Data are normalized with Hprt (n = 5 or 6). **, P < 0.01 versus Keap1flox/+. (B and C) Plasma NEFA (B) and TAG (C) levels in male lean 20-week-old control or Keap1flox/− mice crossed with Nrf2+/+ or Nrf2−/− mice (n = 5 or 6). ***, P < 0.001; *, P < 0.05. (D) Cpt1b expression in SkM of 20-week-old male control or Keap1flox/− mice fed the HCD for 8 weeks crossed with Nrf2+/+ or Nrf2−/− (n = 5 or 6) mice. Data are normalized with Hprt. ***, P < 0.001; *, P < 0.05. (E) Immunoblot analysis for CPTIB in SkM of 20-week-old male control or Keap1flox/− mice fed the HCD and quantified protein expression level normalized with α-tubulin (n = 4). **, P < 0.01. (F) Oxygen consumption levels in SkM of male (n = 5) or female (n = 6) 8-week-old control or Keap1flox/− mice fed the HCD for 2 weeks. **, P < 0.01 versus control. Error bars, SEM.
To further address Nrf2 regulation of the expression of energy consumption-related genes in SkM, we also examined plasma levels of nonesterified free fatty acid (NEFA) and triacylglycerol (TAG). Both plasma NEFA and TAG levels were lower in the Keap1flox/− mice than in the Keap1flox/+ littermates. This lowering effect was nicely canceled by simultaneous Nrf2 knockout (Fig. 3B and C), indicating an accelerated utilization of NEFA and TAG in the SkM of Keap1flox/− mice. We also examined mRNA levels of the other energy consumption-related genes in the SkM of Keap1flox/− HCD mice and found that Cpt1b, PGC1α, and Ucp3 mRNAs were all strongly induced, but these inductions were diminished by the concomitant knockout of Nrf2 (Fig. 3D; see also Fig. S5C in the supplemental material). The CPT1B protein level was also upregulated in SkM of Keap1flox/− mice fed the HCD (Fig. 3E). These results thus demonstrate that Nrf2 stimulates the expression of energy consumption-related genes.
We next evaluated oxygen consumption levels in SkM and BAT of Keap1flox/− mice fed the HCD. Oxygen consumption levels in SkM of both male and female Keap1flox/− mice were higher than those of Keap1flox/+ mice, and this difference was most noticeable in SkM of female Keap1flox/− mice (Fig. 3F). In addition to SkM, oxygen consumption was increased in BAT of Keap1flox/− mice (see Fig. S5D in the supplemental material). Consistent with the results in Fig. 2, body weights of Keap1flox/− mice fed the HCD for 2 weeks were lower than in Keap1flox/+ mice treated similarly (see Fig. S5E). Tissue weights of SkM (gastrocnemius and soleus muscles) normalized with body weight were slightly increased in Keap1flox/− mice, but there were not statistically significance differences between these groups (see Fig. S5F). In contrast, the tissue weight of BAT of Keap1flox/− mice was similar to that in Keap1flox/+ mice (see Fig. S5G).
To confirm the contribution of energy consumption to suppression of obesity, we also evaluated locomotor activity of lean Keap1flox/− mice in an open-field test (26, 27). Since open-field activity was influenced by exploration immediately after starting (26), we recorded open-field locomotor activity in every 5 min for 20 min. Two parameters, moved distance and rated crossing zone number, were rapidly reduced during the 5- to 10-min period compared with those in the 0- to 5-min period in both Keap1flox/+ and Keap1flox/− mice (see Fig. S6A and B in the supplemental material). These data suggest that exploration behavior was strong in the first 5-min period. We thus evaluated spontaneous locomotor activity by parameters of open-field test in the 15- to 20-min period. Both parameters of Keap1flox/− mice were similar to those of Keap1flox/+ littermates (see Fig. S6A and B), indicating that Nrf2 did not affect the spontaneous locomotor activity. These results support our contention that upregulation of the energy consumption-related genes in SkM and BAT of Keap1flox/− mice leads to an increase in oxygen consumption without changing locomotor activity and eventually to the protection from HCD-mediated obesity.
Nrf2 induction in SkM suppresses HFD-induced obesity.
In the previous section, we showed that energy expenditure-related genes were increased in Keap1flox/− mice. Therefore, in order to closely clarify relationships of glucose metabolism and Nrf2 induction in SkM, we next conducted analyses of HFD-induced obesity of Keap1 MuKO mice. Keap1flox/flox and Keap1 MuKO mice were fed the HFD for 8 weeks (see Fig. S7A in the supplemental material). While body weights of SD-fed Keap1 MuKO mice were lower than those of littermate control Keap1flox/flox mice at 8 weeks of age (see Fig. S3C in the supplemental material), calculated body weight change after 10 weeks of age was not changed significantly in with the SD (Fig. 4A). In contrast and importantly, HFD-induced body weight gain was clearly ameliorated in Keap1 MuKO mice compared with littermate control HFD-fed mice (Fig. 4A).
Fig 4.
Nrf2 induction in SkM prevents HFD-induced obesity. (A) Body weight changes of male Keap1flox/flox (control) or Keap1 MuKO mice fed the SD (n = 4) or the HFD (n = 11). (B) Blood glucose levels of male control or Keap1 MuKO mice 8 weeks after being fed the SD (n = 4) or HFD (n = 11) ad libitum. (C) Plasma insulin levels of male control or Keap1 MuKO mice 8 weeks after being fed the HFD (n = 5 each) ad libitum. (D and E) Locomotor activity in the open-field study of male control or Keap1 MuKO mice 8 weeks after being fed the HFD (n = 5). Moved distance and rated crossing zone number were quantified every 5 min (0 to 5, 5 to 10, 10 to 15, and 15 to 20 min) or through the whole testing period (0 to 20 min). Data are represented moved distance (D) and rated crossing zone number (E) per minute. (F and G) Correlation between locomotor activity and body weight. Locomotor activities are represented as moved distance (F) and rated crossing zone number (G) in the 15- to 20-min period of the open-field test. P < 0.001 (***) and P < 0.05 (*) versus control. Error bars, SEM.
Blood glucose levels of Keap1 MuKO mice fed the SD ad libitum were at levels almost similar to those of littermate controls at 18 weeks of age (Fig. 4B), and this result is consistent with that of 8-week-old mice (Fig. 2I). In contrast, blood glucose levels of Keap1 MuKO mice fed the HFD ad libitum were significantly lower than those of control mice (Fig. 4B). Plasma insulin levels of HFD-fed Keap1 MuKO mice were also lower than those of HFD-fed littermate control mice (Fig. 4C). These data indicate that induction of Nrf2 in SkM markedly ameliorates HFD-induced impairment of glucose metabolism and protects mice from obesity.
To delineate mechanisms of how Nrf2 induction in SkM protects mice from HFD-induced obesity, we evaluated food intake and locomotor activity of Keap1 MuKO mice. We found that food intakes of Keap1 MuKO mice were at levels comparable with those of control mice (see Fig. S7B in the supplemental material). The locomotor activity was determined by means of open-field test. We found that two parameters of the locomotor activity, moved distance and rated zone crossing number, were not significantly changed in the 0- to 5-min period for HFD-fed Keap1 MuKO mice and control mice, indicating that Nrf2 induction in SkM does not affect the exploration behavior of HFD-fed mice. While these parameters were markedly diminished in HFD-fed control mice in the three succeeding 5-min periods after the first 5-min period, the impairment of locomotor activity was nicely protected in the Keap1 MuKO mice (Fig. 4D and E). Particularly, both parameters of Keap1 MuKO mice were significantly higher than those of control mice in the 15- to 20-min period.
To clarify effects of the improved locomotor activity on the antiobesity phenotype, we analyzed correlations between the locomotor activity and body weight; we found that both the moved distance (r = −0.695; P = 0.026 [Fig. 4F]) and the rated zone crossing number (r = −0.763; P = 0.01 [Fig. 4G]) are negatively correlated with body weight. These data thus support our contention that upregulation of Nrf2 activity in SkM preserves HFD-induced impairment of locomotor activity and attenuates progression of diet-induced obesity in the mice.
Nrf2 suppresses gluconeogenesis.
Since we have observed that the liver also contributes to the decrease of blood glucose levels (Fig. 2I), we next focused on glucose metabolism in the db/db mouse liver. We evaluated glucose metabolism-related genes in db/db::Keap1flox/− mouse livers and found that expression levels of gluconeogenesis-related G6pc, Fbp1, PGC1α, and Nr4a2 mRNAs were all downregulated in db/db::Keap1flox/− mouse livers (Fig. 5A). Of note, the expression of the PGC1α gene was strongly enfeebled by the absence of Nrf2. These results thus demonstrate that gluconeogenesis-related genes are under negative regulation by Nrf2.
Fig 5.
Nrf2 induction decreases gluconeogenesis-related genes in liver. (A) Expression levels of gluconeogenesis-related genes in livers of 14- to 18-week-old db/db::Keap1flox/+ (control) or db/db::Keap1flox/− mice crossed with Nrf2+/+ or Nrf2−/− mice (n = 13 to 18). Data are normalized with Hprt. **, P < 0.01; *, P < 0.05. (B) Blood glucose levels in the PTT. Pyruvate (1.5 g/kg of BW) was intraperitoneally administered after 24 h of fasting to 8- to 10-week-old control or db/db::Keap1flox/− mice (n = 12 to 21). ***, P < 0.001 versus control. (C) Effect of CDDO-Im on G6pc mRNA expression in AML12 cells. After a 24-h incubation with 10 nmol/liter of CDDO-Im, cells were stimulated with 1 μmol/liter of Dex or 1 mmol/liter of dbcAMP for 6 h. Data are represented as G6pc expression levels and normalized with β-actin (n = 4). ***, P < 0.001. (D) Effect of CDDO-Im on G6pc regulatory activities in AML12 cells stably transfected with G6pc-Luc2P. After a 24-h incubation with 10 nmol/liter of CDDO-Im, cells were stimulated with 1 mmol/liter of dbcAMP for 9 h. Data are represented as luciferase activities after normalization to the total cellular protein amounts (n = 5). **, P < 0.01. (E) Role of Nrf2 in G6pc regulatory activities in AML12 cells transiently transfected with G6pc-Luc2P. Data are represented as firefly luciferase activities after normalization to Renilla luciferase activities (n = 6). P < 0.001 (***) and P < 0.01 (**) versus CREB. (F) Immunoblot analysis for CREB in liver and the quantified protein expression levels normalized with α-tubulin of 18-week-old control or db/db::Keap1flox/− mice (n = 5). ***, P < 0.001. (G) Plasma glucagon concentrations of male 18-week-old Keap1flox/− or Keap1flox/+ lean or db/db mice fed ad libitum (n = 3). **, P < 0.01 versus lean mice. Error bars, SEM.
We next a conducted pyruvate tolerance test (PTT) to clarify the contribution of the downregulation of gluconeogenesis-related genes to the prevention of diabetes. The blood glucose levels of Keap1flox/− mice after pyruvate loading were markedly lower than those of Keap1flox/+ mice at any time point (Fig. 5B). We calculated amounts of changes in blood glucose of Keap1flox/− mice, and they were significantly lower than those in Keap1flox/+ mice after pyruvate administration (see Fig. S8A in the supplemental material). Simultaneous Nrf2 gene knockout completely canceled the decrease of blood glucose levels in the PTTs of Keap1flox/− mice.
As various hormones intensely regulate gluconeogenesis genes, we determined the roles Nrf2 plays in the suppression of gluconeogenesis genes in hepatic cell cultures. Since AML12 cells were derived from ICR background mice and we have mainly analyzed gluconeogenesis using mice of the same background in this study, we utilized these cells for in vitro study of gluconeogenesis-related genes. While expression levels of G6pc were very low in vehicle-treated or Dex-treated AML12 cells, the level was starkly induced by dbcAMP. We found that the induction was intensely repressed by the simultaneous addition of CDDO-Im, a strong Nrf2 inducer (Fig. 5C). Similarly, Fbp1 and PGC1α genes were also induced by dbcAMP in the AML12 cells, and these induced expressions were attenuated by the addition of CDDO-Im (see Fig. S8B in the supplemental material).
Nrf2 represses transcription of gluconeogenesis-related genes.
While accumulating lines of evidence support the notion that Nrf2 is a potent activator of transcription and this notion is also applicable to the regulation of energy consumption-related genes, our present results strongly argue that Nrf2 acts to negatively regulate transcription of gluconeogenesis-related genes. To address this point, we prepared a firefly Luc-based reporter of the G6pc gene (G6pc-Luc2P reporter harboring −1388 to +82 from the transcription starting site) and examined transactivation by Nrf2 of this gene. We found that CDDO-Im treatment potently repressed the G6pc gene regulatory activity that was induced by dbcAMP in AML12 cells (Fig. 5D). We also employed CREB induction of the G6pc regulatory activity and found that Nrf2 overexpression suppressed the CREB-based transcriptional activity nicely in a dose-dependent manner (Fig. 5E). Importantly, the expression level of CREB protein was decreased in db/db::Keap1flox/− mice to more than half that of db/db::Keap1flox/+ littermates (Fig. 5F). The expression of CREB was also suppressed by CDDO-Im treatment in AML12 cells (see Fig. S8C in the supplemental material). We also determined plasma glucagon levels and found that the Keap1flox/− mutant did not show altered plasma glucagon levels in either the lean or db/db background (Fig. 5G).
We then examined whether Nrf2 attenuates the upregulation of gluconeogenesis-related genes brought about by the cAMP signals in vivo. In this analysis, we focused on the CREB target genes PGC1α and Nr4a2, whose products are well-known regulators of the gluconeogenesis genes (28, 29), and both genes harbored the consensus cAMP response element (CRE) (30). We found that dbcAMP administration to lean mice potently increased expression of both PGC1α and Nr4a2 genes in the liver; these genes were strongly suppressed by genetic Nrf2 induction in Keap1flox/− mice (see Fig. S8D in the supplemental material). We also evaluated another gluconeogenesis-related gene, Pck1, encoding phosphoenolpyruvate carboxykinase (PEPCK), in db/db::Keap1flox/− mouse liver. Both PEPCK protein (see Fig. S8E) and Pck1 gene expression (see Fig. S8F) were decreased in db/db::Keap1flox/− mouse liver. These results demonstrate that Nrf2 decreases cAMP-mediated gluconeogenesis-related gene expression, perhaps through an attenuation of cAMP-CREB signals and/or a decline of CREB levels, and support our contention that the downregulation of gluconeogenesis-related genes by Nrf2 contributes to the decreases of blood glucose and protection against diabetes mellitus.
Nrf2 contributes to expression of cytoprotective genes in pancreatic β cells.
To elucidate whether Nrf2 contributes to the β-cell function, we then examined expression of Nrf2 in pancreatic islets. It has been reported that Nrf2 mRNA is abundantly expressed in the small intestine but less so in the liver (8). We found that Nrf2 mRNA was expressed abundantly in islets of the ICR strain of mice, almost to a level similar to that in the intestine, and this expression was completely abrogated in Nrf2−/− mice (Fig. 6A). Importantly, small islets were frequently observed in the Nrf2−/− mouse pancreas (Fig. 6B). Quantification of islet size revealed that they were substantially smaller in Nrf2−/− mice than in Nrf2+/+ mice. In contrast, staining patterns for glucagon were not affected (see Fig. S9 in the supplemental material). Insulin content was decreased in isolated Nrf2−/− mouse islets (Fig. 6C), and insulin release was decreased from these Nrf2−/− mouse islets under basal (2.8 mmol/liter of glucose) and high-glucose (16.7 mmol/liter) conditions (Fig. 6D).
Fig 6.
Nrf2 contributes to β-cell protection. (A) Nrf2 mRNA expression in isolated islets of Nrf2+/+ or Nrf2−/− mice normalized with 18S rRNA (n = 3). ***, P < 0.001 versus Nrf2+/+ liver. (B) IHC of insulin in pancreatic sections (low, 5× objective; high, 20× objective) of 10-week-old male Nrf2+/+ or Nrf2−/− mice (left) and the quantified islet size in these sections (right; n = 368 to 406; islets from 10 mice in each group). Scale bars, 500 μm (low) and 100 μm (high). ***, P < 0.001 versus Nrf2+/+. (C) Insulin contents of isolated islets from 10-week-old male Nrf2+/+ or Nrf2−/− mice normalized with total protein contents (n = 8). ***, P < 0.001 versus Nrf2+/+. (D) GSIS from islets isolated from 10-week-old male Nrf2+/+ or Nrf2−/− mice (n = 8). ***, P < 0.001. (E) Insulin and antioxidant gene mRNA expression in islets from Nrf2+/+ and Nrf2−/− mice normalized with 18S rRNA (n = 3). ***, P < 0.001. (F) Antioxidant gene expressions in islets compared with livers of male 10-week-old wild-type mice. Data are normalized with 18S rRNA (n = 4). ***, P < 0.001 versus liver. Error bars, SEM.
Expression levels of antioxidant enzyme genes were also examined in the Nrf2−/− mouse islets. Levels of Nrf2 target gene mRNAs, including Nqo1, Hmox1, Gstp1, Gpx2, and Txnrd1, were all decreased in Nrf2−/− mouse islets compared to littermate Nrf2+/+ mouse islets (Fig. 6E). In order to assess which antioxidant genes contribute to the cytoprotection in islets, we compared expression levels of these genes in islets with those in the liver. We found that Nqo1, Hmox1, Gpx2, and Gpx4 gene expressions in islets were similar to those in liver, while the other genes were less abundantly expressed in islets than in liver (Fig. 6F). Importantly, three of the highly expressed genes in islets (Nqo1, Hmox1, and Gpx2) were strongly reduced in Nrf2−/− mouse islets (Fig. 6E), supporting the contention that Nrf2 contributes to β-cell protection by inducing the expression of these enzymes, and therefore, Nrf2 deficiency causes impairment of β-cell function and insulin secretion.
Insulin secretion levels paradoxically increase in Nrf2-null mutant mice.
To assess whether Nrf2-null mice show a reciprocal phenotype against Keap1 KD mice, we analyzed glucose metabolism in Nrf2−/− mice in vivo. Blood glucose and plasma insulin levels did not change substantially in the Nrf2−/− mice compared to the control mice (see Fig. S10A and B in the supplemental material). Insulin secretion in ipGTT was relatively higher in Nrf2−/− mice than in littermate Nrf2+/+ mice (see Fig. S10C). We also conducted the ITT in Nrf2−/− mice. Blood glucose levels in ITT were also decreased in Nrf2−/− mice (see Fig. S10D). As we previously noted that Nrf2 induction markedly decreased blood glucose levels in vivo and that pancreatic islets were small in Nrf2−/− mice, the observation that blood glucose levels decreased and insulin levels increased, albeit mildly, in Nrf2−/− mice in vivo was rather unexpected.
We found that insulin release-related genes, Mafa, Hnf1a, Gck, and Kir6.2, were all upregulated in the Nrf2−/− mouse islets (see Fig. S10E in the supplemental material). The fold increase in glucose-stimulated insulin secretion (GSIS) upon shifting from low glucose to high glucose was enhanced in Nrf2−/− mouse isolated islets (see Fig. S10F). We surmise that Nrf2 deficiency potentially yielded the lower glucose phenotype in the ipGTT and ITT in vivo under unstressed conditions due to the relative increase of GSIS and that this may explain the paradoxical decreases of blood glucose levels in Nrf2-null mutant mice.
Small molecular inducer of Nrf2 signaling exerts antidiabetic effects.
Since genetic induction of Nrf2 signaling strongly abrogated the onset of diabetes via β-cell protection and improved insulin resistance, we next examined antidiabetic effects of the Nrf2 inducer CDDO-Im in db/db mice. Currently, CDDO-Im represents one of the most potent Nrf2 inducers (31). To clarify the potential of chemical induction of Nrf2 in the form of an oral antidiabetic drug, we carried out long-term oral CDDO-Im administration. Before initiating long-term CDDO-Im administration studies, we tested the influence of a single administration of CDDO-Im on Nqo1 and Nrf2 gene expression in db/db mice (see Fig. S11A in the supplemental material). Nqo1 expression in the liver and SkM of db/db mice was higher than that in lean m/m mice (see Fig. S11B). A single dose of CDDO-Im markedly induced Nqo1 expression in the liver and SkM of db/db mice (see Fig. S11B). In addition, Nrf2 expression was increased in db/db mice compared with lean m/m mice in the liver and SkM (see Fig. S11C), and that in SkM was enhanced by CDDO-Im (see Fig. S11C). These data suggest that Nrf2 signaling is available in db/db mice and is further augmented by CDDO-Im treatment.
We also determined whether CDDO-Im induces Nrf2 target genes in isolated islets from db/db mice (see Fig. S12A in the supplemental material). We found that 10 nmol/liter of CDDO-Im markedly induced Nrf2 target genes, Nqo1, Hmox1, and Gsta4, in db/db islets (see Fig. S12B). To determine whether oral administration of CDDO-Im could induce Nrf2 target genes in islets, we next conducted dose-response analyses. Since large numbers of islets were needed for multiple doses, we changed the mouse model from db/db mice to wild-type mice. We found that CDDO-Im induces Nqo1 expression from 1 nmol/liter, with a peak concentration of 10 nmol/liter (see Fig. S12C in the supplemental material). CDDO-Im also enhanced Hmox1 expression slightly from 0.1 nmol/liter and did significantly at 10 nmol/liter (see Fig. S12C). As lower concentrations of CDDO-Im induced Nrf2 target gene expression in islets, it is expected that oral administration of CDDO-Im affects gene regulation in islets of db/db mice.
We administered CDDO-Im orally 3 times per week to db/db mice (see Fig. S13A in the supplemental material). CDDO-Im administration significantly dampened increases in blood glucose compared with those in vehicle-treated mice beginning at 9 weeks of age (Fig. 7A), without significant effects on body weight (Fig. 7B). Plasma insulin levels declined markedly by 10 weeks in vehicle-treated db/db mice, while CDDO-Im administration significantly ameliorated, but did not eliminate, the decline of insulin secretion after 14 weeks (Fig. 7C). HOMA-IR was decreased by CDDO-Im administration (Fig. 7D), suggesting an improvement in insulin resistance. CDDO-Im markedly improved blood glucose levels in the OGTT (Fig. 7E) and decreased the AUC of the OGTT (see Fig. S13B). An increase in insulin secretion after glucose administration in the OGTT was completely diminished in vehicle-treated db/db mice but restored by the CDDO-Im treatment (Fig. 7F). CDDO-Im also restored β-cell insulin staining and increased the insulin-positive area of the pancreas (Fig. 7G), indicating that Nrf2 induction by CDDO-Im acts to protect β cells.
Fig 7.
Administration of CDDO-Im protects β-cell function and improves insulin resistance in db/db mice. (A to C) Blood glucose levels (A), body weight (B), and plasma insulin levels (C) of vehicle- and CDDO-Im-treated db/db mice fed ad libitum (male, n = 8). P < 0.001 (***), P < 0.01 (**), and P < 0.05 (*) versus vehicle. (D) HOMA-IR of 16-week-old male db/db mice (n = 8). **, P < 0.01 versus vehicle. (E and F) Blood glucose (E) and plasma insulin (F) levels in the OGTT of db/db mice. Glucose (0.5 g/kg of BW) was orally administered to 16-week-old male db/db mice after 14 h of fasting (n = 8). P < 0.001 (***) and P < 0.01 (**) versus vehicle; P < 0.05 (*) versus time zero. (G) IHC of insulin in pancreatic sections (low, 5× objective; high, 20× objective) of 18-week-old db/db mice (left) and quantified insulin-positive areas in these sections (right) (n = 8). Scale bars, 500 μm (low) and 100 μm (high). *, P < 0.05 versus vehicle. Error bars, SEM.
To validate the contribution of Nrf2 to antidiabetic effects of CDDO-Im, we crossed Nrf2-null mice with db/db mice and administered CDDO-Im for 7 consecutive days orally (see Fig. S14A in the supplemental material). Blood glucose of CDDO-Im-treated db/db::Nrf2+/+ mice was lower than that in vehicle-treated mice (see Fig. S14B). In contrast, blood glucose levels of CDDO-Im-treated db/db::Nrf2−/− mice were mildly elevated compared with those of vehicle-treated db/db::Nrf2−/− mice (see Fig. S14C). In addition, we determined Nrf2 target gene expression in isolated islets from lean Nrf2-null mice (see Fig. S15A in the supplemental material) and found that CDDO-Im treatment of Nrf2+/+ mouse islets induced Nrf2 target gene expressions (see Fig. S15B). Importantly, CDDO-mediated responses were lost with Nrf2 depletion (see Fig. S15B). These data indicate that oral administration of CDDO-Im ameliorated diabetes by protecting β cells and improving insulin resistance in db/db mice through the Keap1-Nrf2 system.
DISCUSSION
In this study, we have demonstrated the importance of the Keap1-Nrf2 system in the prevention of diabetes mellitus in multiple murine diabetes models. We found that genetic and pharmacological induction of Nrf2 signaling exerts strong ameliorating effects on diabetic mice through both β-cell protection against oxidative stresses and improvement of insulin resistance in the liver and SkM. As summarized in Fig. 8, the induction of Nrf2 protects pancreatic islets by activating expression of antioxidant genes; it also increases expression of energy consumption-related genes in SkM and decreases that of gluconeogenesis-related genes in liver, thereby leading to the improvement of insulin resistance. These results thus support our contention that the Keap1-Nrf2 system is a critical target for preventing the onset and progression of diabetes mellitus. The potent protective action of CDDO-Im in db/db mice highlights a role for Nrf2 inducers as oral antidiabetic drugs.
Fig 8.
Suggested mechanisms of prevention of diabetes by the Keap1-Nrf2 system. Induction of Nrf2 activity increases expression of antioxidant enzyme genes in pancreatic β cells and energy consumption-related genes in SkM but decreases expression of gluconeogenesis-related genes in the liver. The Keap1-Nrf2 system suppresses diabetes onset via both restoration of sufficient insulin secretion and insulin resistance.
There are several preceding and rather controversial observations for the involvement of Nrf2 in the onset of diabetes mellitus. Importantly, STZ-induced β-cell damage could be ameliorated by sulforaphane (32) or oltipraz (13). CDDO-Me decreased blood glucose levels in diabetic model mice (33) and lowered the body weights of human diabetic patients (34). These broad observations suggest that pharmacological induction of Nrf2 activity may be effective for the protection of β cells. However, blood glucose levels have been shown to be rather decreased in Nrf2-deficient mice in vivo (35), and we have confirmed this observation (data not shown). This result rather contradicts the notion suggested above and has hampered attempts to develop Nrf2 inducers for the suppression of blood glucose levels. One plausible explanation for this discrepancy is that Nrf2 deficiency leads to the relative insulin hypersecretion at the expense of the insulin content in the islets, and our preliminary analysis supports this hypothesis (see Fig. S10C and F in the supplemental material). In addition, both Nrf2 deficiency and Nrf2 inducers showed pleiotropic effects independent of the Keap1-Nrf2 system (36–38). Therefore, the precise contribution of the Keap1-Nrf2 system to the prevention of diabetes has remained elusive. Here, we provide for the first time direct and convincing lines of evidence for the involvement of the Keap1-Nrf2 system in the prevention of the onset and progression of diabetes mellitus obtained using genetic models for constitutive activation as well as loss of function of Nrf2.
We have delineated the organs that are responsible for the antidiabetic effect of Nrf2. Our tissue-specific Keap1 knockout studies have revealed that SkM is responsible for lowering blood glucose in the ITT. In contrast, the liver participates in the lowering of the blood glucose level in mice fed ad libitum, especially in db/db mice via gluconeogenesis suppression. However, the organs that are responsible for the antiobesity effect are still elusive. Whereas energy consumption-related Cpt1b expression is upregulated in SkM of Keap1flox/− mice and oxygen consumption is indeed increased, a consensus antioxidant response element is not found in the Cpt1b gene regulatory region. In addition, analyses of Keap1 MuKO mice demonstrate that Cpt1b is not augmented by SkM-specific Nrf2 induction, suggesting that Nrf2 indirectly regulates Cpt1b gene expression. These results suggest that while SkM is certainly a main effecter organ for obesity prevention, SkM-specific Nrf2 induction is not sufficient to fully exert the antiobesity effect of Nrf2.
We have found that Nrf2 decreases PGC1α expression in db/db mouse liver. PGC1α plays important roles in gluconeogenesis-related gene induction in liver (29). In contrast, we have also found that Nrf2 increases PGC1α expression in SkM. Since PGC1α contributes to the increase in energy consumption-related gene expression in SkM (39, 40), our results in the liver and SkM regarding PGC1α expression are consistent with the notion that Nrf2 induction prevents onset of diabetes mellitus and obesity.
We have demonstrated that Nrf2 is highly expressed in SkM of juvenile ICR mice. In contrast, Nrf2 is weakly expressed in senile Swiss Webster mice (25). We found that the expression of Nrf2 target genes in the SkM of 8-week-old C57BL/6J mice is lower than that in ICR mice. Of note, there is a single nucleotide polymorphism (SNP) in the Nrf2 upstream promoter region (336 bases upstream from the transcription start site), leading to a markedly differential Nrf2 expression between C57BL/6J and C3H mice (41). A similar SNP has also been found in the human NRF2 gene upstream regulatory element (42), affecting NRF2 gene expression and the morbidity of acute lung injury (43). Nrf2 expression levels in SkM are decreased in sedentary old humans (44). Thus, Nrf2 expression levels in SkM appear to be influenced by age, exercise, and SNP, and these factors may affect intrinsic susceptibility or pharmacological effectiveness of targeting Nrf2 for management of diabetes mellitus.
To clarify the therapeutic effects of the inducer on diabetes mellitus, we designed experiments with a chemical Nrf2 induction model using CDDO-Im. In this analysis, we started CDDO-Im administration to db/db mice at 6 weeks of age, when blood glucose has reached around 450 mg/dl. The results demonstrate successfully that CDDO-Im exerts its antidiabetic effects even after the onset of diabetes, supporting the notion that chemical Nrf2 induction is effective in therapy for diabetes mellitus after onset.
In summary, we found in this study that CDDO-Im administration significantly lowers blood glucose levels in db/db mice and that the genetic activation of Nrf2 signaling in Keap1flox/− mice exerts much more profound antidiabetic effects in db/db and HCD-fed mice, with almost complete prevention of diabetes. While it is unlikely and perhaps undesirable to use pharmacological approaches to fully mimic the constitutive genetic activation of the pathway (45), our genetic models provide a full validation of Nrf2 as a key target for prevention and attenuation of diabetes mellitus.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by Grants-in-Aid for Scientific Research on Innovative Areas and for Scientific Research from the Ministry of Education, Science, Sports and Culture (MEXT) and the Takeda Foundation, Naito Foundation, Japanese Foundation for Applied Enzymology, and Tohoku University Global COE Program for Conquest of Signal Transduction Diseases with Network Medicine from JSPS.
We thank Sayoi Inomata for mouse genotyping, Fumiko Date for immunohistochemistry, and the Biomedical Research Core of Tohoku University Graduate School of Medicine for technical support.
Y.F., H.M., and T.N. are employees of Mochida Pharmaceutical Co. Ltd.
Footnotes
Published ahead of print 28 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00225-13.
REFERENCES
- 1. Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. 1995. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell. Biol. 15: 4184– 4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moi P, Chan K, Asunis I, Cao A, Kan YW. 1994. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. U. S. A. 91: 9926– 9930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13: 76– 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. 2004. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 24: 8477– 8486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Furukawa M, Xiong Y. 2005. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the cullin 3-Roc1 ligase. Mol. Cell. Biol. 25: 162– 171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. 2004. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 24: 7130– 7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. 2000. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 275: 16023– 16029 [DOI] [PubMed] [Google Scholar]
- 8. Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. 1997. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236: 313– 322 [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. 2009. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 29: 493– 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uruno A, Motohashi H. 2011. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide 25: 153– 160 [DOI] [PubMed] [Google Scholar]
- 11. Taguchi K, Motohashi H, Yamamoto M. 2011. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 16: 123– 140 [DOI] [PubMed] [Google Scholar]
- 12. Yoh K, Hirayama A, Ishizaki K, Yamada A, Takeuchi M, Yamagishi S, Morito N, Nakano T, Ojima M, Shimohata H, Itoh K, Takahashi S, Yamamoto M. 2008. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells 13: 1159– 1170 [DOI] [PubMed] [Google Scholar]
- 13. Aleksunes LM, Reisman SA, Yeager RL, Goedken MJ, Klaassen CD. 2010. Nuclear factor erythroid 2-related factor 2 deletion impairs glucose tolerance and exacerbates hyperglycemia in type 1 diabetic mice. J. Pharmacol. Exp. Ther. 333: 140– 151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, Liby KT, Sporn MB, Yamamoto M, Kensler TW. 2009. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur. J. Pharmacol. 620: 138– 144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. 2007. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol. Cell. Biol. 27: 7188– 7197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. 2012. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22: 66– 79 [DOI] [PubMed] [Google Scholar]
- 17. Taguchi K, Maher JM, Suzuki T, Kawatani Y, Motohashi H, Yamamoto M. 2010. Genetic analysis of cytoprotective functions supported by graded expression of Keap1. Mol. Cell. Biol. 30: 3016– 3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. 2003. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 35: 238– 245 [DOI] [PubMed] [Google Scholar]
- 19. Azhar M, Wang PY, Frugier T, Koishi K, Deng C, Noakes PG, McLennan IS. 2010. Myocardial deletion of Smad4 using a novel α skeletal muscle actin Cre recombinase transgenic mouse causes misalignment of the cardiac outflow tract. Int. J. Biol. Sci. 6: 546– 555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noguchi N, Yoshikawa T, Ikeda T, Takahashi I, Shervani NJ, Uruno A, Yamauchi A, Nata K, Takasawa S, Okamoto H, Sugawara A. 2008. FKBP12.6 disruption impairs glucose-induced insulin secretion. Biochem. Biophys. Res. Commun. 371: 735– 740 [DOI] [PubMed] [Google Scholar]
- 21. Abramoff M, Magalhaes P, Ram S. 2004. Image processing with ImageJ. Biophotonics Int. 11: 36– 42 [Google Scholar]
- 22. Uruno A, Matsuda K, Noguchi N, Yoshikawa T, Kudo M, Satoh F, Rainey WE, Hui XG, Akahira J, Nakamura Y, Sasano H, Okamoto H, Ito S, Sugawara A. 2011. Peroxisome proliferator-activated receptor-γ suppresses CYP11B2 expression and aldosterone production. J. Mol. Endocrinol. 46: 37– 49 [DOI] [PubMed] [Google Scholar]
- 23. Uruno A, Noguchi N, Matsuda K, Nata K, Yoshikawa T, Chikamatsu Y, Kagechika H, Harigae H, Ito S, Okamoto H, Sugawara A. 2011. All-trans retinoic acid and a novel synthetic retinoid tamibarotene (Am80) differentially regulate CD38 expression in human leukemia HL-60 cells: possible involvement of protein kinase C-δ. J. Leukoc. Biol. 90: 235– 247 [DOI] [PubMed] [Google Scholar]
- 24. Uruno A, Sugawara A, Kanatsuka H, Kagechika H, Saito A, Sato K, Kudo M, Takeuchi K, Ito S. 2005. Upregulation of nitric oxide production in vascular endothelial cells by all-trans retinoic acid through the phosphoinositide 3-kinase/Akt pathway. Circulation 112: 727– 736 [DOI] [PubMed] [Google Scholar]
- 25. McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. 2001. The Cap‘n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 61: 3299– 3307 [PubMed] [Google Scholar]
- 26. Proulx K, Cota D, Castañeda TR, Tschöp MH, D'Alessio DA, Tso P, Woods SC, Seeley RJ. 2005. Mechanisms of oleoylethanolamide-induced changes in feeding behavior and motor activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289: R729– R737 [DOI] [PubMed] [Google Scholar]
- 27. Yadav R, Gupta SC, Hillman BG, Bhatt JM, Stairs DJ, Dravid SM. 2012. Deletion of glutamate delta-1 receptor in mouse leads to aberrant emotional and social behaviors. PLoS One 7: e32969. 10.1371/journal.pone.0032969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P. 2006. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat. Med. 12:1048– 1055 [DOI] [PubMed] [Google Scholar]
- 29. Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. 2001. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413: 131– 138 [DOI] [PubMed] [Google Scholar]
- 30. Conkright MD, Guzmán E, Flechner L, Su AI, Hogenesch JB, Montminy M. 2003. Genome-wide analysis of CREB target genes reveals a core promoter requirement for cAMP responsiveness. Mol. Cell 11: 1101– 1108 [DOI] [PubMed] [Google Scholar]
- 31. Liby KT, Sporn MB. 2012. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol. Rev. 64: 972– 1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song MY, Kim EK, Moon WS, Park JW, Kim HJ, So HS, Park R, Kwon KB, Park BH. 2009. Sulforaphane protects against cytokine- and streptozotocin-induced β-cell damage by suppressing the NF-κB pathway. Toxicol. Appl. Pharmacol. 235: 57– 67 [DOI] [PubMed] [Google Scholar]
- 33. Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L. 2010. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice. J. Biol. Chem. 285: 40581– 40592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG, BEAM Study Investigators 2011. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365: 327– 336 [DOI] [PubMed] [Google Scholar]
- 35. Meher AK, Sharma PR, Lira VA, Yamamoto M, Kensler TW, Yan Z, Leitinger N. 2012. Nrf2 deficiency in myeloid cells is not sufficient to protect mice from high-fat diet-induced adipose tissue inflammation and insulin resistance. Free Radic. Biol. Med. 52: 1708– 1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. 2006. Triterpenoid CDDO-Me blocks the NF-κB pathway by direct inhibition of IKKβ on Cys-179. J. Biol. Chem. 281: 35764– 35769 [DOI] [PubMed] [Google Scholar]
- 37. Ahmad R, Raina D, Meyer C, Kufe D. 2008. Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)→signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res. 68: 2920– 2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Porter WW, Suh N, Honda T, Gribble GW, Leesnitzer LM, Plunket KD, Mangelsdorf DJ, Blanchard SG, Willson TM, Sporn MB. 2000. A synthetic triterpenoid, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO), is a ligand for the peroxisome proliferator-activated receptor γ. Mol. Endocrinol. 14: 1550– 1556 [DOI] [PubMed] [Google Scholar]
- 39. Handschin C, Spiegelman BM. 2008. The role of exercise and PGC1α in inflammation and chronic disease. Nature 454: 463– 469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin J, Handschin C, Spiegelman BM. 2005. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1: 361– 370 [DOI] [PubMed] [Google Scholar]
- 41. Cho HY, Jedlicka AE, Reddy SP, Zhang LY, Kensler TW, Kleeberger SR. 2002. Linkage analysis of susceptibility to hyperoxia. Nrf2 is a candidate gene. Am. J. Respir. Cell Mol. Biol. 26: 42– 51 [DOI] [PubMed] [Google Scholar]
- 42. Yamamoto T, Yoh K, Kobayashi A, Ishii Y, Kure S, Koyama A, Sakamoto T, Sekizawa K, Motohashi H, Yamamoto M. 2004. Identification of polymorphisms in the promoter region of the human NRF2 gene. Biochem. Biophys. Res. Commun. 321: 72– 79 [DOI] [PubMed] [Google Scholar]
- 43. Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, Kleeberger SR. 2007. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 21: 2237– 2246 [DOI] [PubMed] [Google Scholar]
- 44. Safdar A, deBeer J, Tarnopolsky MA. 2010. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic. Biol. Med. 49: 1487– 1493 [DOI] [PubMed] [Google Scholar]
- 45. Kensler TW, Wakabayashi N. 2010. Nrf2: friend or foe for chemoprevention? Carcinogenesis 31: 90– 99 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.