Abstract
The effects of clinically available drugs targeting muscarinic cholinergic, adrenergic, dopaminergic, and serotonergic receptors; intracellular calcium levels and/or the function of calcium-dependent biochemical pathways; ion channels; and cellular pumps were tested against a keratitis isolate of Acanthamoeba castellanii belonging to the T4 genotype. In vitro growth inhibition (amoebistatic) assays were performed by incubating A. castellanii with various concentrations of drugs in the growth medium for 48 h at 30°C. To determine amoebicidal effects, amoebae were incubated with drugs in phosphate-buffered saline for 24 h, and viability was determined using trypan blue exclusion staining. For controls, amoebae were incubated with the solvent alone. Of the eight drugs tested, amlodipine, prochlorperazine, and loperamide showed potent amoebicidal effects, as no viable trophozoites were observed (>95% kill rate), while amiodarone, procyclidine, digoxin, and apomorphine exhibited up to 50% amoebicidal effects. In contrast, haloperidol did not affect viability, but all the drugs tested inhibited A. castellanii growth. Importantly, amlodipine, prochlorperazine, and loperamide showed compelling cysticidal effects. The cysticidal effects were irreversible, as cysts treated with the aforementioned drugs did not reemerge as viable amoebae upon inoculation in the growth medium. Except for apomorphine and haloperidol, all the tested drugs blocked trophozoite differentiation into cysts in encystation assays. Given the limited availability of effective drugs to treat amoebal infections, the clinically available drugs tested in this study represent potential agents for managing keratitis and granulomatous amoebic encephalitis caused by Acanthamoeba spp. and possibly against other meningoencephalitis-causing amoebae, such as Balamuthia mandrillaris and Naegleria fowleri.
INTRODUCTION
Acanthamoeba spp. are causative agents of painful blinding keratitis, frequently associated with contact lens wear, and granulomatous amoebic encephalitis (GAE), which almost always results in death (1–3). The approximate rate of Acanthamoeba encephalitis-associated deaths has been suggested to be 1.57 deaths per 10,000 HIV/AIDS deaths in developed countries, while the number of infections may be much higher in developing countries with warmer climates due to increased ubiquity, increased outdoor activities, and/or limited availability of highly active antiretroviral therapy (HAART) (4). The incidence rate of Acanthamoeba keratitis (AK) varies between different geographical locations. For example, in Hong Kong, an incidence rate of 0.33 per 10,000 contact lens wearers is reported, 0.05 per 10,000 in Holland, 0.01 per 10,000 in the United States, 0.19 per 10,000 in England, and 1.49 per 10,000 in Scotland (reviewed in reference 4). However, these variations do not reflect the geographical distribution of Acanthamoeba and are most likely due to extended wear of contact lenses, lack of awareness of the potential risks associated with wearing contact lenses, enhanced detection, and/or local conditions that promote the growth of pathogenic amoebae only, e.g., water hardness or salinity. Current methods for treatment of AK include application of a mixture of drugs for prolonged periods, and even then, the outcome remains extremely poor (1–4). For example, the treatment of AK includes an hourly topical application of 0.02% polyhexamethylene biguanide or 0.02% chlorhexidine digluconate, together with a diamidine (0.1% propamidine isethionate, also known as Brolene, or 0.1% hexamidine, also known as Desomedine), for 4 to 5 days. Subsequently, application is reduced to every 2 hours during the day for up to a month (5). This is followed by application 6 times a day for the next several months to a year, and even then, the recurrence rate is more than 10%. If bacteria are also associated or suspected with the infection, the addition of an antibiotic, i.e., neomycin or chloramphenicol, is recommended. For encephalitis, the current therapeutic agents include a combination of ketoconazole, fluconazole, sulfadiazine, pentamidine isethionate, amphotericin B, azithromycin, itraconazole, rifampin, voriconazole, and miltefosine, which may be effective against the central nervous system infections due to free-living amoebae (6, 7), but the mortality rate remains high (1–3). Thus, there is an urgent need for improved antimicrobial chemotherapy and/or alternative strategies to develop therapeutic interventions. The majority of human infections due to Acanthamoeba have been associated with isolates of the T4 genotype. For example, more than 90% of AK cases have been linked with the genotype. Similarly, T4 has been the major genotype associated with nonkeratitis infections, such as GAE and cutaneous infections (1–4). Here, using growth inhibition and viability assays, the effects of clinically available drugs targeting various cellular receptors and biochemical pathways were tested against an axenically grown keratitis isolate of Acanthamoeba castellanii belonging to the T4 genotype.
MATERIALS AND METHODS
All chemicals were purchased from Sigma (Poole, Dorset, United Kingdom) unless otherwise stated. Among various drugs tested, amlodipine, apomorphine, and loperamide were purchased from Sigma; procyclidine was purchased from Auden McKenzie Pharma; haloperidol was purchased from Searle Pharma Ltd.; amiodarone and prochlorperazine were purchased from Sanofi-Aventis; and digoxin was purchased from GlaxoSmithKline (Table 1). All drugs tested are available clinically, and some are used to treat nervous system disorders (Table 1).
Table 1.
Clinically available drugs tested in this study and their known modes of action
| Drug | Clinical use | Mode of action |
|---|---|---|
| Amiodarone | Various types of cardiac dysrhythmias, both ventricular and atrial | Class III antiarrhythmic agent; shows beta blocker-like and potassium channel blocker-like actions |
| Binding to the nuclear thyroid receptor | ||
| Apomorphine | Parkinson's disease, erectile dysfunction, Alzheimer's disease | Nonselective (D) dopaminergic receptor agonist |
| An antagonist at 5-HT and α-adrenergic receptors | ||
| Digoxin | Atrial fibrillation and atrial flutter | Binds to sodium/potassium-transporting ATPase alpha-1 chain to inhibit its function at heart muscle cells and other tissues |
| Inhibitor of cholesterol side chain cleavage enzyme, mitochondrial | ||
| Haloperidol | Schizophrenia and acute psychotic states and delirium and neuroleptanalgesia | Dopamine antagonist |
| Muscarinic receptor antagonist | ||
| Histamine antagonist | ||
| Loperamide | Used against diarrhea; inhibits local release of acetylcholine | μ-Opioid receptor agonist |
| Calmodulin binder | ||
| Prochlorperazine | Nausea, vertigo, and migraine headaches | Dopamine (D2) receptor antagonist |
| Muscarinic receptor antagonist | ||
| Histamine antagonist | ||
| Procyclidine | Parkinsonism, akathisia, and acute dystonia | Blocks the neurotransmitter acetylcholine in the central and peripheral nervous system at muscarinic receptors |
| Amlodipine | Antihypertensive and in the treatment of angina pectoris | Calcium antagonist that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle |
| Acts as a functional inhibitor of acid sphingomyelinase (sphingomyelin is involved in signal transduction and apoptosis, or cell death) |
Acanthamoeba cultures.
A keratitis isolate of A. castellanii belonging to the T4 genotype was grown in 10 ml of PYG medium (0.75% [wt/vol] proteose peptone, 0.75% [wt/vol] yeast extract, and 1.5% [wt/vol] glucose) in T-75 tissue culture flasks at 37°C without shaking (8). The media were refreshed 15 to 20 h prior to experiments. A. castellanii cells adhering to flasks represented the trophozoite form and were collected by placing the flasks on ice for 30 min with gentle agitation and used in all experiments.
Amoebistatic and amoebicidal assays.
To determine the amoebistatic activities of drugs, A. castellanii trophozoites (2 × 105 amoebae/ml/well) were incubated in PYG medium with different concentrations (10 μg to 500 μg per ml) of drugs in 24-well plates at 30°C for 48 h. After this incubation, the amoebae were counted using a hemocytometer. The data are represented as the means and standard errors of at least three independent experiments performed in duplicate. To determine the amoebicidal effect of drugs on A. castellanii, amoebicidal assays were performed. Briefly, A. castellanii trophozoites were incubated with different concentrations of drugs (10 μg to 500 μg per ml) in phosphate-buffered saline (PBS) in 24-well plates (5 × 105 amoebae/ml/well). The plates were incubated at 37°C for 24 h. Following this incubation, amoeba viability was determined by adding 0.1% trypan blue and determining the number of live (nonstained) and dead (stained) A. castellanii organisms (between 200 and 300) using a hemocytometer. The counts from A. castellanii incubated with PBS alone were used as controls. Data are represented as the means and standard errors of at least three independent experiments performed in duplicate.
Encystation assays.
For encystation assays, 2 × 106 amoebae were incubated in PBS containing 50 mM MgCl2 and 10% glucose in 24-well plates, without shaking, at 30°C for 72 h. After this incubation, the number of amoebae was determined using a hemocytometer, followed by addition of sodium dodecyl sulfate (SDS) (0.5% final concentration) for 10 min. The SDS-solubilized trophozoites (cysts are resistant to SDS at 0.5% concentration, and they remain intact) and cysts were enumerated using a hemocytometer. The data are represented as the means and standard errors of at least three independent experiments performed in duplicate. To determine the effects of drugs on amoeba differentiation, encystation assays were performed in the presence of various drugs. Briefly, 2 × 106 amoebae were incubated in PBS with various concentrations (10 μg to 500 μg per ml) of drugs in the presence of 50 mM MgCl2 and left at room temperature for 1 h. After this, 10% glucose was added as a trigger for encystation in each well, and the amoebae were incubated at 30°C for 72 h. Finally, amoeba cysts were enumerated using a hemocytometer as described above.
Cysticidal assays.
For cysticidal assays, 2 × 106 amoeba trophozoites were inoculated on nonnutrient agar plates, and the plates were incubated for up to 7 days to allow trophozoite transformation into the cyst stage. After this incubation, each plate was flooded with 10 ml of PBS, and the cysts were scraped off the agar surface using a rubber scraper, yielding more than 99% cysts. The mature cysts (approximately 2 × 106) were incubated in PBS with various concentrations of drug (10 μg to 500 μg per ml) for up to 24 h. After this incubation, the cysts were centrifuged for 10 min at 1,000 × g, and the supernatants were aspirated, followed by the addition of 0.5 ml of PBS. This process was repeated 3 times to remove extracellular drug. Finally, the cysts were resuspended in PYG medium and inoculated in 24-well plates (0.5 ml PYG medium/well). The plates were incubated at 30°C for 48 h, and emergence of trophozoites was considered to indicate viable amoebae while the absence of excystation was considered a cysticidal effect. In some experiments, plates were incubated for up to a week to observe the emergence of viable trophozoites.
RESULTS
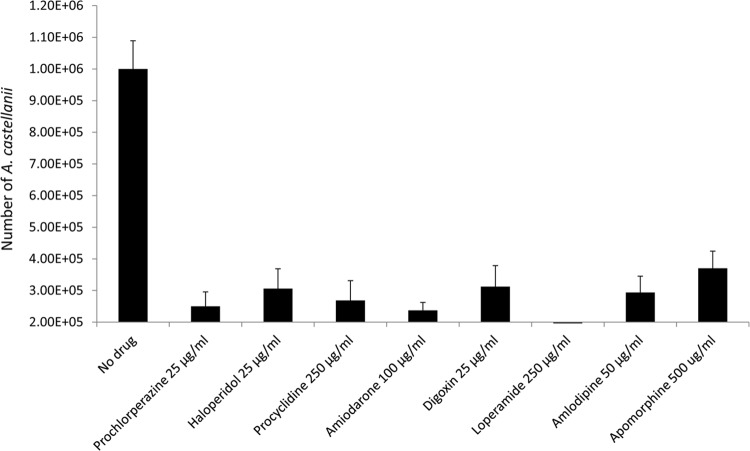
Prochlorperazine, haloperiadol, and digoxin abolish Acanthamoeba growth at 25 μg per ml.
A. castellanii trophozoites were incubated with various concentrations of drugs in growth medium for their amoebistatic effects. In controls, A. castellanii trophozoites were incubated in PBS (nonnutrient) and exhibited no growth (Fig. 1), while A. castellanii incubated in PYG medium (growth medium) exhibited >8-fold increase in amoeba numbers from the original inoculum (Fig. 1). Among various drugs tested, prochlorperazine, haloperidol, digoxin, and amlodipine abolished A. castellanii growth. The minimum concentrations of drugs required to significantly inhibit A. castellanii growth are shown in Fig. 1 (P < 0.01 using a paired t test with one-tail distribution). The results revealed that concentrations of prochlorperazine, haloperidol, and digoxin as low as 25 μg per ml showed more than 90% growth inhibition (Fig. 1). In contrast, the minimum concentration of loperamide or procyclidine required to block A. castellanii growth was 250 μg per ml (Fig. 1), while 100 μg per ml of amiodarone and 50 μg per ml of amlodipine were needed to block A. castellanii growth (Fig. 1).
Fig 1.
A. castellanii (2 × 105 cells) was incubated with various concentrations of drugs in PYG medium at 30°C for 48 h. After incubation, A. castellanii numbers were monitored by hemocytometer counting. A. castellanii incubated in PBS (nonnutrient) exhibited no growth, while A. castellanii incubated in PYG medium (growth medium) exhibited >8-fold increase in amoeba numbers from the original inoculum. All drugs tested showed significant inhibition of A. castellanii growth at the indicated concentrations (P < 0.01; paired t test; one-tail distribution). Among various drugs tested, prochlorperazine, haloperidol, and digoxin inhibited A. castellanii growth completely at 25 μg per ml. The results are representative of at least three independent experiments performed in duplicate. The data are presented as means and standard errors.
At 500 μg per ml, amlodipine, digoxin, loperamide, and prochlorperazine showed up to 100% A. castellanii killing.
To determine the effects of various drugs tested on A. castellanii viability, trophozoites were incubated with different concentrations of drugs in PBS and cell viability was determined using trypan blue exclusion staining. At 500 μg per ml, amlodipine, digoxin, loperamide, and prochlorperazine produced up to 100% A. castellanii death (Fig. 2), while amiodarone, procyclidine, and apomorphine showed up to 50% A. castellanii death (Fig. 2). In contrast, haloperidol did not affect A. castellanii viability (Fig. 2).
Fig 2.
The amoebicidal effects of various drugs were determined using trypan blue staining. Briefly, A. castellanii (5 × 105 amoebae) was incubated with various drugs (500 μg per ml) at 37°C for 24 h. After this incubation, viability was determined by trypan blue staining, and amoebae were enumerated by hemocytometer counting. At 500 μg per ml, amlodipine, loperamide, and prochlorperazine showed more than 99% A. castellanii killing. The results are representative of at least three independent experiments performed in duplicate. The data are presented as means and standard errors.
Amiodarone, amlodipine, digoxin, and loperamide block encystation of A. castellanii.
Encystation assays were performed in the presence or absence of various drugs. At 500 μg per ml, all drugs tested except apomorphine and haloperidol exhibited more than 90% inhibition of encystation (Fig. 3). Notably, the encystation-inhibitory effects of amlodipine, digoxin, prochloperazine, and loperamide are secondary and are most likely due to their potent (more than 95%) amoebicidal effects.
Fig 3.
Encystation assays were performed in the presence of various drugs. Briefly, 2 × 106 amoebae were incubated with various drugs (500 μg per ml) in the presence of 50 mM MgCl2 for 1 h. After this incubation, 10% glucose was added, and the amoebae were incubated at 30°C for 72 h. After the incubation, 0.5% SDS was added to solubilize trophozoites (cysts are resistant to SDS), and cysts were enumerated using a hemocytometer. At 500 μg per ml, all drugs tested except apomorphine and haloperidol exhibited more than 90% inhibition of A. castellanii encystation. The results are representative of at least three independent experiments performed in duplicate. The data are presented as means and standard errors.
Amlodipine, prochlorperazine, and loperamide showed potent cysticidal effects against A. castellanii cysts.
Cysts were scraped from nonnutrient agar plates and incubated in the presence or absence of various drugs for 24 h. After this incubation, the cysts were centrifuged and reinoculated in fresh PYG medium for up to 48 h. The results revealed that 500 μg per ml of amlodipine, prochlorperazine, and loperamide irreversibly damaged A. castellanii cysts (Fig. 4). Even longer incubations did not allow reemergence of amoeba trophozoites. Viable A. castellanii trophozoites emerged in control wells, as well as with all other drugs tested (Fig. 4). Of note, chlorhexidine, pentamidine isethionate, and ketoconazole were used, as drugs currently in use against Acanthamoeba. Consistent with previous findings, all compounds tested showed potent amoebicidal but limited cysticidal effects (reference 4 and data not shown).
Fig 4.
Representative effects of various drugs against A. castellanii cysts. A. castellanii cysts were scraped from nonnutrient agar plates and incubated with various drugs (500 μg per ml) for 24 h. After this incubation, the cysts were washed three times with PBS and reinoculated in fresh PYG medium at 30°C for up to 48 h. When treated with 500 μg per ml of amlodipine, prochlorperazine, and loperamide, A. castellanii cysts did not reemerge as viable A. castellanii trophozoites. Viable A. castellanii trophozoites emerged in control wells, as well as with all other drugs. The results are representative of three independent experiments.
DISCUSSION
Keratitis and encephalitis caused by Acanthamoeba are serious human infections. The current treatment regimen involves a mixture of drugs to provide additive/synergistic effects, but the prognosis remains poor (1–5). This may be due to difficulties in diagnosis resulting in delay in initiation of chemotherapy, poor penetration of antimicrobial compounds across the blood brain barrier, and perhaps the ability of amoebae to switch phenotype into the resistant cyst form. Here, the effects of various clinically approved compounds on growth, viability, and encystation of A. castellanii were studied. The results demonstrated antiacanthamoebal properties through inhibition of growth and proliferation and by affecting the viability of the amoebae. Most of the drugs tested in the present study are FDA approved for use against noncommunicable diseases, with known mechanisms of action on target cells.
Of the eight drugs tested, three (amlodipine, loperamide, and prochlorperazine) showed more than 99% amoebicidal, as well as cysticidal, effects. Amlodipine is a dihydropyridine calcium channel blocker used in the treatment of hypertension and angina pectoris (9, 10). Its distinctive pharmacokinetic characteristics are attributed to a high degree of ionization. Following oral administration of 5 to 10 mg, the plasma concentration rises gradually to peak within 6 to 8 h, and its half-life is 40 to 50 h. It is metabolized by the liver into pyridine metabolites, while 60% of the administered dose is excreted in urine as inactive metabolites; oral toxicity (50% lethal dose [LD50]) in mice is 37 mg per kg of body weight. Loperamide is a widely used antidiarrheal that acts primarily through activation of opioid receptors in the gastrointestinal tract (9, 10). Its maximum daily oral dose is 16 mg per kg of body weight in adults, and its half-life is 9.1 to 14.4 h, with peak plasma levels within 5 h after oral administration. The unchanged compound and its metabolite, N-demethylated loperamide, are excreted through feces, while oral toxicity (LD50) in mice is 105 mg per kg. The maximum daily oral dose for prochlorperazine is 50 mg per day in adults and 12.5 mg intravenously (i.v.), and the half-life is 8 to 9 h, with peak plasma levels within 6 h, but it may exhibit a wide range of side effects, such as acute dystonic reactions, pseudoparkinsonism, or akathisia. The N-desmethyl metabolite is detected, while oral toxicity (LD50) in mice is 400 mg per kg (10). All three agents mentioned above broadly affect intracellular calcium levels and/or the function of calcium-dependent biochemical pathways, which may explain their amoebicidal and cysticidal effects in vitro. Acanthamoeba viability is heavily dependent on its ability to crawl, approach food particles, phagocytose, encyst and excyst, and divide to reproduce, and thus, drugs affecting these functions have deleterious effects on its viability. Among other functions, calcium effects cytoskeletal changes by stimulating myosin contractility (11), activation of actin filament severin (12), inhibition of actin cross-linking by alpha-actinin (13), or binding to calmodulin (14) or other low-molecular-weight calcium-binding proteins, including CBP (15) and calpain (16). Several actin-associated proteins, such as actophorin (17); actobindin (18); and 23,000-, 28,000-, 32,000-, and 38,000-Da gelation proteins (19) and calcium-sensitive actin gelation protein (20, 21), have been purified and characterized. A cDNA clone encoding an actin-bundling protein, AhABP, was recently isolated from Acanthamoeba healyi, a causative agent of granulomatous amoebic encephalitis (22). Additionally, several Acanthamoeba extracellular proteases act in a calcium-dependent manner (23, 24).
At a high concentration, loperamide affected A. castellanii viability. Loperamide is an opiate that reduces calcium influx across cell membranes and/or decreases cellular availability of calcium (25) and is used in the treatment of diarrhea (26). At higher concentrations, loperamide blocks calmodulin activity, calcium channels, N-methyl-d-aspartate receptor channels, and maitotoxin-elicited calcium influx. At micromolar concentrations, it has a remarkable stimulatory effect on the capacitative calcium influx that is triggered in many cells by depletion of the inositol-triphosphate-sensitive stores of calcium in the endoplasmic reticulum (27). In our assays, loperamide showed potent amoebicidal and cysticidal effects at high concentrations, probably by affecting regulatory intermediates and/or some yet-unidentified cardinal biochemical pathways in A. castellanii.
An indirect role of calcium in inhibition of transformation due to a calcium-regulatory protein and its control of cyclic nucleotide levels in the cell has been proposed based on inhibition by trifluoperazine, a calmodulin antagonist (28). In our study, prochlorparazine, a drug of the same class as trifluoperazine, produced potent amoebicidal and cysticidal effects. Haloperidol and prochlorperazine act primarily as dopamine receptor blockers and have been used as antipsychotic drugs. It was interesting that prochlorperazine showed potent amoebicidal, as well as cysticidal, effects, while haloperidol was effective against trophozoites but not cysts. This is consistent with previous studies, which showed that chlorpromazine is effective against trophozoites and cysts of A. castellanii (29). Notably, haloperidol has bioavailabilities of 100% via the i.v. route and 60 to 65% by the oral route and has rapid onset of action, within a few hours. The concentration of haloperidol in brain tissue is about 20-fold higher than blood levels. It is slowly eliminated from the brain tissue. The elimination half-life is about 3 weeks, and excretion is via biliary and renal routes. Previous studies have shown that a combination of chlorpromazine and rokitamycin exhibited synergistic amoebistatic, amoebicidal, and cysticidal activities against A. castellanii, suggesting their usefulness as chemotherapeutic agents against Acanthamoeba infections (29). The precise mode of action of prochlorparazine against Acanthamoeba is unclear, but it may involve inhibition of amoeba calcium-regulatory proteins or lipophilic action on the amoeba plasma membrane (10, 29). Prochlorperazine is thought to exert its antipsychotic effects by blocking dopamine receptors but also has moderate anticholinergic and alpha-adrenergic receptor-blocking activity compared to haloperidol, which is a weak anticholinergic (muscarinic) M1 (silent antagonist) at 10,000 nM (30). Consistent with our findings and the differential affinities of prochlorperazine and haloperidol for cholinergic receptors in particular, it is tempting to speculate about the presence of a cholinergic receptor (M1) subtype on A. castellanii, and studies are in progress to address this issue.
Another anticholinergic agent included in our study was procyclidine, which is widely used as an antiparkinsonian agent because of its anticholinergic action (31). Procyclidine primarily antagonizes muscarinic receptors M1, M2, and M4, of which M1 and M4 are diffusely distributed throughout the brain. Procyclidine showed substantial effects as an amoebicidal drug in our assays. Subcutaneous doses of 300 mg per kg are not toxic, and oral absorption of procyclidine is 100%. The plasma half-life is 12.6 h, while the intravenous LD50 in mice is about 60 mg per kg of body weight (10). Among other drugs tested, apomorphine is a nonselective dopamine agonist that activates dopamine receptors, D1- and D2-receptors, with preference for the latter subtype (32). Apomorphine also possesses agonistic affinity for receptors like 5-HT1A, 5-HT2A, 5-HT2B, and 5-HT2C (32). As prochlorperazine, chlorpromazine, and haloperidol are D1 and D2 receptor antagonists, it is logical to conclude that either they act on A. castellanii via receptors other than the D-receptor subtype or apomorphine exhibited its effects via 5HT or some other receptor subtype (10), and this will be the subject of future studies. Of note, subcutaneous and/or intravenous administration exhibits 100% bioavailability, with a half-life of 40 min. Potential routes of metabolism in humans include sulfation, N-demethylation, glucuronidation, and oxidation. The maximum concentration in cerebrospinal fluid (CSF) occurs 10 to 20 min after administration. Digoxin is a potent inhibitor of the active transport of sodium and potassium across cell membranes. This biological effect is accomplished by binding to Na and K-ATPase, resulting in the inhibition of the cellular ion pump in a reversible manner (33). Although the mechanism of action as an antiamoeba agent remains unidentified, a combination of lytic and apoptotic signaling induction may contribute to its amoebicidal effects observed in the present study. Digoxin crosses both the blood brain barrier and the placenta. Following intravenous administration to healthy volunteers, 50% to 70% of the digoxin dose is excreted unchanged in the urine, and only a small percentage (∼16%) of the given dose is metabolized, with a half-life of about 36 h. The end metabolites include 3-β-digoxigenin, 3-keto-digoxigenin, and their glucuronide and sulfate conjugates (10). Amiodarone effects may be mediated by perturbation of the lipid environment of the ion channels. Amiodarone resembles thyroxin (thyroid hormone) chemically, and its binding to the nuclear thyroid receptor might contribute to its pharmacological properties. It has a bioavailability of approximately 50%, with the maximum plasma concentration attained within 3 to 7 h, and a half-life of 58 days. Amiodarone is metabolized to desethylamiodarone by the cytochrome P450 (CYP450) enzyme group in the liver, and excretion is primarily via hepatic and biliary routes. In conclusion, for the first time we have shown that a diverse group of drugs affecting the cellular availability of calcium, sodium, and potassium ions through surface and metabotropic receptors exert a possible electrophysiological effect on A. castellanii, inhibiting growth, viability, and encystation. Future studies will dissect the molecular mechanisms to validate the mode of action of these drugs and/or identify novel biochemical pathways and receptors.
ACKNOWLEDGMENT
This work was partially supported by Aga Khan University.
Footnotes
Published ahead of print 13 May 2013
REFERENCES
- 1. Marciano-Cabral F, Cabral G. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siddiqui R, Khan NA. 2012. Biology and pathogenesis of Acanthamoeba. Parasit. Vectors 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Visvesvara GS, Moura H, Schuster FL. 2007. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 50:1–26 [DOI] [PubMed] [Google Scholar]
- 4. Khan NA. 2006. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol. Rev. 30:564–595 [DOI] [PubMed] [Google Scholar]
- 5. Perez-Santonja JJ, Kilvington S, Hughes R, Tufail A, Metheson M, Dart JKG. 2003. Persistently culture positive Acanthamoeba keratitis; in vivo resistance and in vitro sensitivity. Ophthalmology 110:1593–1600 [DOI] [PubMed] [Google Scholar]
- 6. Maritschnegg P, Sovinz P, Lackner H, Benesch M, Nebl A, Schwinger W, Walochnik J, Urban C. 2011. Granulomatous amebic encephalitis in a child with acute lymphoblastic leukemia successfully treated with multimodal antimicrobial therapy and hyperbaric oxygen. J. Clin. Microbiol. 49:446–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Webster D, Umar I, Kolyvas G, Bilbao J, Guiot MC, Duplisea K, Qvarnstrom Y, Visvesvara GS. 2012. Treatment of granulomatous amoebic encephalitis with voriconazole and miltefosine in an immunocompetent soldier. Am. J. Trop. Med. Hyg. 87:715–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan NA, Siddiqui R. 2009. Acanthamoeba affects the integrity of human brain microvascular endothelial cells and degrades the tight junction proteins. Int. J. Parasitol. 39:1611–1616 [DOI] [PubMed] [Google Scholar]
- 9. Sweetman SC. 2011. Martindale: the complete drug reference, 37th ed Pharmaceutical Press, London, United Kingdom [Google Scholar]
- 10. Brunton LL, Chabner BA, Knollman BC. 2011. Goodman and Gilman's The pharmacological basis of therapeutics, 12th ed McGraw-Hill, New York, NY [Google Scholar]
- 11. Tan Z, Boss WF. 1992. Association of phosphatidylinositol kinase, phosphatidylinositol monophosphate kinase, and diacylglycerol kinase with the cytoskeleton and F-actin fractions of carrot (Daucus carota L.) cells grown in suspension culture. Plant Physiol. 100:2116–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto K, Pardee JD, Reidler J, Stryer L, Spudich JA. 1982. Mechanism of interaction of Dictyostelium severin with actin filaments. J. Cell Biol. 95:711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Witke W, Hofmann A, Köppel B, Schleicher M, Noegel AA. 1993. The Ca2+-binding domains in nonmuscle type alpha-actinin: biochemical and genetic analysis. J. Cell Biol. 121:599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu Q, Liu T, Clarke M. 1993. Calmodulin and the contractile vacuole complex in mitotic cells of Dictyostelium discoideum. J. Cell Sci. 104:1119–1127 [DOI] [PubMed] [Google Scholar]
- 15. Dharamsi A, Tessarolo D, Coukell B, Pun J. 2000. CBP1 associates with the Dictyostelium cytoskeleton and is important for normal cell aggregation under certain developmental conditions. Exp. Cell Res. 258:298–309 [DOI] [PubMed] [Google Scholar]
- 16. Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. 1997. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 272:32719–32722 [DOI] [PubMed] [Google Scholar]
- 17. Maciver SK, Wachsstock DH, Schwarz WH, Pollard TD. 1991. The actin filament severin protein actophorin promotes the formation of rigid bundles of actin filaments crosslinked with alpha-actinin. J. Cell Biol. 115:1621–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lambooy PK, Korn ED. 1986. Purification and characterization of actobindin, a new actin monomer-binding protein from Acanthamoeba castellanii. J. Biol. Chem. 261:17150–17155 [PubMed] [Google Scholar]
- 19. Maruta H, Korn ED. 1977. Purification from Acanthamoeba castellanii of proteins that induce gelation and syneresis of F-actin. J. Biol. Chem. 252:399–402 [PubMed] [Google Scholar]
- 20. Pollard TD. 1976. The role of actin in the temperature-dependent gelation and contraction of extracts of Acanthamoeba. J. Cell Biol. 68:579–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pollard TD. 1981. Purification of a calcium-sensitive actin gelation protein from Acanthamoeba. J. Biol. Chem. 256:7666–7670 [PubMed] [Google Scholar]
- 22. Alafag JI, Moon EK, Hong YC, Chung DI, Kong HH. 2006. Molecular and biochemical characterization of a novel actin bundling protein in Acanthamoeba. Korean J. Parasitol. 44:331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mitro K, Bhagavathiammai A, Zhou OM, Bobbett G, McKerrow JH, Chokshi R, Chokshi B, James ER. 1994. Partial characterization of the proteolytic secretions of Acanthamoeba polyphaga. Exp. Parasitol. 78:377–385 [DOI] [PubMed] [Google Scholar]
- 24. Taylor WM, Pidherney MS, Alizadeh H, Niederkorn JY. 1995. In vitro characterization of Acanthamoeba castellanii cytopathic effect. J. Parasitol. 81:603–609 [PubMed] [Google Scholar]
- 25. Harris RA, Loh HH, Way EL. 1975. Effects of divalent cations, cation chelators and an ionophore on morphine analgesia and tolerance. J. Pharmacol. Exp. Ther. 195:448–498 [PubMed] [Google Scholar]
- 26. Hanauer SB. 2008. The role of loperamide in gastrointestinal disorders. Rev. Gastroenterol. Disord. 8:15–20 [PubMed] [Google Scholar]
- 27. Daly JW, Harper J. 2000. Loperamide: novel effects on capacitative calcium influx. Cell. Mol. Life Sci. 57:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schuster FL, Twomey R. 1983. Calcium regulation of flagellation in Naegleria gruberi. J. Cell Sci. 63:311–326 [DOI] [PubMed] [Google Scholar]
- 29. Mattana A, Biancu G, Alberti L, Accardo A, Delogu G, Fiori PL, Cappuccinelli P. 2004. In vitro evaluation of the effectiveness of the macrolide rokitamycin and chlorpromazine against Acanthamoeba castellanii. Antimicrob. Agents Chemother. 48:4520–4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. 2003. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 28:519–526 [DOI] [PubMed] [Google Scholar]
- 31. Jevtovic-Todorovic V, Meyenburg AP, Olney JW, Wozniak DF. 2003. Anti-Parkinsonian agents procyclidine and ethopropazine alleviate thermal hyperalgesia in neuropathic rats. Neuropharmacology 44:739–748 [DOI] [PubMed] [Google Scholar]
- 32. Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. 2002. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J. Pharmacol. Exp. Ther. 303:791–804 [DOI] [PubMed] [Google Scholar]
- 33. Eichhorn EJ, Gheorghiade M. 2002. Digoxin. Prog. Cardiovasc. Dis. 44:251–266 [DOI] [PubMed] [Google Scholar]