Abstract
Multipurpose technologies that simultaneously protect from sexually transmitted infections and unintended pregnancy are urgently needed. Pod-intravaginal rings (IVRs) formulated with the antiretroviral agents (ARVs) tenofovir, nevirapine, and saquinavir and the contraceptives etonogestrel and estradiol were evaluated in sheep. Steady-state concentrations were maintained for 28 days with controlled, sustained delivery. This proof-of-principle study demonstrates that pod IVRs can deliver three ARVs from different mechanistic classes and a progestin-estrogen combination over the wide range needed for putative preventative efficacy.
INTRODUCTION
Unprotected sex can result in unintended pregnancy as well as sexually transmitted infections (STIs) and represents a major health problem worldwide. Multipurpose technologies (MPTs) (1, 2) for simultaneous protection of individuals from sexual HIV infection and unintended pregnancy may save development time and reduce costs in addressing this global health priority. Topical delivery of one or more antiretroviral (ARV) agents in combination with one or more hormonal contraceptives from intravaginal rings (IVRs) holds significant potential as a female-controlled strategy, especially in resource-limited countries (3, 4). In this approach, the active pharmaceutical ingredients (APIs) are administered in a coitally independent, sustained-release formulation that significantly reduces adherence issues compared to daily-pill, ARV vaginal-gel, and coitally dependent regimens.
Multipurpose IVRs need to simultaneously deliver API combinations at independently controlled rates (5). In some cases, multiple drugs will be needed for a single prevention modality. Conventional IVR technologies—i.e., matrix and reservoir rings (3)—contain the API(s) homogeneously dispersed, either in solution or as a suspension, in the elastomer backbone that makes up the ring. This approach complicates the development of combination IVRs, which partially explains why, to date, all IVRs have delivered one or two APIs (3, 4). With the above parameters in mind, we have developed a novel IVR platform (referred to as “pod IVR”) that can simultaneously deliver multiple drugs in a modular fashion (6). The polymer-coated drug cores, referred to as pods, are embedded in an unmedicated ring. This approach leads to a number of important benefits, discussed in detail elsewhere (6, 7). In the context of combination IVRs as an MPT, the pod IVR design readily enables the delivery of 3 or more drugs from a single device, as described below.
The primary purpose of this proof-of-concept study is to demonstrate that 3 ARV drugs from different mechanistic classes can be delivered in tandem with a progestin-estrogen combination from a novel multipurpose IVR at independently controlled release rates.
MATERIALS AND METHODS
Production of intravaginal rings.
Silicone pod IVRs (Fig. 1) were produced according to published methods (6) and contained two pods of each drug per ring: tenofovir (TFV), nevirapine (NVP), saquinavir (SQV), and estradiol (E2) at 16 mg API per pod (32 mg drug per ring) and etonogestrel (ETG) at 10 mg API per pod (20 mg drug per ring). The pod membrane consisted of poly(vinyl alcohol), and three 2-mm delivery channels per pod were mechanically fashioned in the IVR (6).
Fig 1.
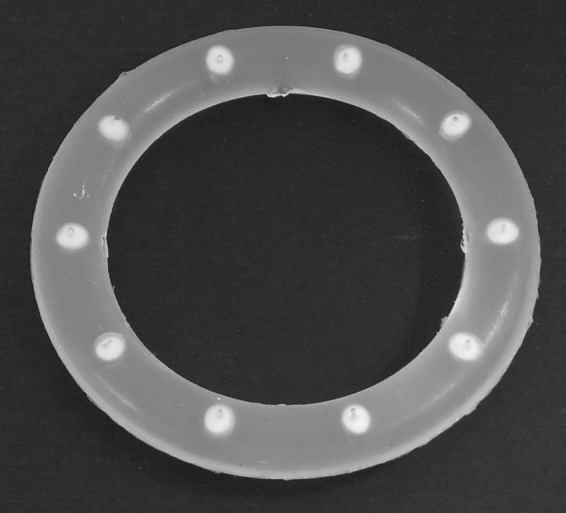
Photograph of a 10-pod IVR sized for use in humans (outer diameter, 56 mm; inner diameter, 40 mm; cross-section, 8 mm).
Study design.
In vivo studies were performed with 3 sheep according to methods described previously (5, 8) at the University of Texas Medical Branch (UTMB) in Galveston, TX, with approval from the Institutional Animal Care and Use Committee. Note that the sheep estrus cycle likely was suppressed by the simultaneous administration of E2 and ETG from the IVR. No significant changes in the weight of the animals or unusual vaginal discharges were observed over the course of the study.
Sample collection, processing, and analysis.
Vaginal rings were inserted on day 0, and cervicovaginal lavage (CVL) samples were collected at predetermined time points (days 7, 14, 21, and 28) (Fig. 2) using published methods (5). Briefly, CVL was collected by gently infusing phosphate-buffered saline solution (10 ml) into the vaginal vault via a sterile 10-ml syringe attached to a sterile pediatric Foley catheter (size, 5 or 8 French units) of adjusted length. The resulting CVL fluid was drawn out with the same device. Vaginal tissue samples were obtained by biopsy on day 14. Samples were processed, stored, and transported as described previously (5). Bioanalysis was performed by liquid chromatography-mass spectrometry (LC-MS), and detailed methods are given in the supplemental material. For CVL, the lower limits of quantitation (LLOQs) were as follows: TFV, 17 nM (5 ng ml−1); NVP, 9 nM (2.5 ng ml−1); SQV, 8 nM (5 ng ml−1); ETG, 31 nM (10 ng ml−1); and E2, 2 nM (0.5 ng ml−1). For tissue, the LLOQs were as follows: TFV, 2 nM (0.5 ng g−1); NVP, 4 nM (1 ng g−1); SQV, 8 nM (5 ng g−1); ETG, 3 nM (1 ng g−1); and E2, 2 nM (0.5 ng g−1). The run-to-run coefficient of variation (9) for all methods was below 10%. A total of 75 measurements were made (3 sheep × 5 samples per sheep × 5 drug levels).
Fig 2.
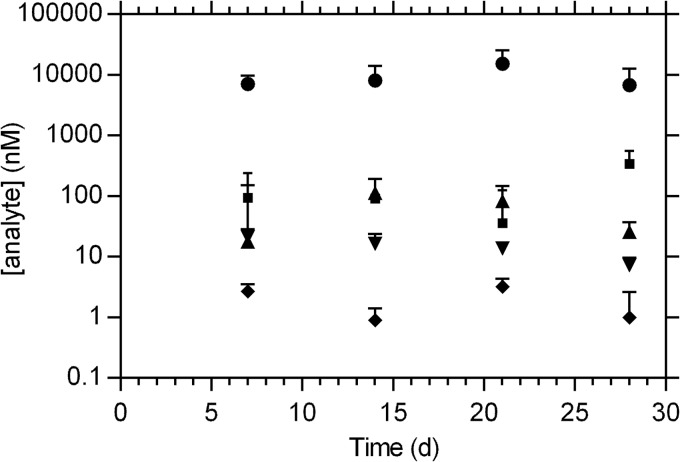
Cervicovaginal lavage concentrations (median + SD) of 5 drugs delivered simultaneously via combination pod IVRs over 28 days in sheep (n = 3). Circles, TFV; squares, NVP; triangles, SQV; inverted triangles, ETG; diamonds, E2.
RESULTS
Cervicovaginal lavage (CVL) concentrations (Fig. 2) were constant over the 28 days, indicating that steady state had been maintained and that sustained release of all five drugs was achieved in a controlled fashion over the length of the study. Tenofovir (TFV) CVL levels (7.7 × 103 ± 6.1 × 103 nM [median ± standard deviation]) were the highest of the five drugs, as expected based on their aqueous solubilities. Nevirapine (NVP) and saquinavir (SQV) CVL concentrations (93 ± 141 nM and 36 ± 79 nM, respectively; 28-day median ± standard deviation) were qualitatively similar for the duration of the study (Fig. 2). Median etonogestrel (ETG) CVL levels (13 ± 6.9 nM) were 8 times higher than the corresponding estradiol (E2) levels (1.6 ± 1.2 nM). These steady-state drug levels span nearly four orders of magnitude.
Vaginal tissue drug levels measured on day 14 are compared with the corresponding CVL drug concentrations in Table 1. The drugs partitioned from the lumen into the vaginal tissues according to the following trend (i.e., tissue-to-CVL ratios): E2 ∼ NVP > SQV ∼ ETG > TFV.
Table 1.
Day 14 drug distribution and concentrations across key pharmacokinetic compartments in sheep (n = 3) following simultaneous administration from combination 5-drug pod IVRs
| Drug | Concn (nM)a |
Tissue-to-CVL ratio | |
|---|---|---|---|
| Cervicovaginal lavage | Vaginal tissueb | ||
| Tenofovir (TFV) | 8.2 × 103 ± 5.9 × 103 | 20 × 103 ± 9.4 × 103 | 2.4 |
| Nevirapine (NVP) | 92 ± 14 | 92 × 103 ± 20 × 103 | 1.0 × 103 |
| Saquinavir (SQV) | 0.11 × 103 ± 78 | 8.1 × 103 ± 4.1 × 103 | 71 |
| Etonogestrel (ETG) | 17 ± 7.2 | 1.1 × 103± 1.6 × 103 | 65 |
| Estradiol (E2) | 0.87 ± 0.55 | 1.1 × 103 ± 6.5 × 103 | 1.2 × 103 |
Median ± standard deviation.
Based on the assumption that vaginal tissue has a density of 1 g ml−1 (24).
DISCUSSION
One of the primary challenges associated with developing an MPT for the prevention of both sexual HIV infection and unintended pregnancy is that multiple APIs need to be incorporated into a single product. The challenge is further compounded if multiple APIs per prevention modality are required for it to be efficacious. In the case of contraception using IVRs, progestin-estrogen combinations have demonstrated superior clinical efficacy over devices delivering only progestin (10). Several large multicenter trials evaluating a matrix IVR delivering the progestin levonorgestrel found pregnancy rates at 12 months between 3.7% (11) and 5.1% (12). In contrast, the NuvaRing, which delivers a combination of ETG and ethinyl estradiol, demonstrated clinical efficacy in excess of 99% in Europe and in the United States (13–15).
A combination of multiple ARV agents, preferably targeting different phases in the HIV infection cycle, is likely to provide more efficient protection than a single drug (16, 17). As a result, an efficacious multipurpose IVR for female-controlled prevention of HIV infection and unintended pregnancy requires delivery of at least four APIs.
Matrix and reservoir IVR technologies have demonstrated that up to two APIs can be delivered from a single device (3, 4). The technical hurdles in developing and manufacturing IVRs based on these technologies, including segmented designs (4), that deliver five APIs simultaneously and at individually controlled rates appear daunting. To this end, we have developed a novel pod IVR technology where solid API drug cores are coated with biocompatible polymer membranes to afford the so-called pods, which are subsequently embedded an inert elastomer ring (6). An IVR sized for human use can support up to 10 pods, and each pod can theoretically contain a different API being released at an independently controlled rate.
In this proof-of-principle study, we developed a 5-drug pod IVR containing two pods of each API and evaluated the device in sheep. Three ARV agents from different mechanistic classes were used in combination, including TFV, a nucleoside reverse transcriptase inhibitor (NRTI) under clinical evaluation in a vaginal gel for HIV prevention (18, 19); NVP, a non-NRTI in the same class as dapivirine, which is being evaluated as an IVR formulation in a large multinational clinical trial (http://www.mtnstopshiv.org/news/studies/mtn020); and SQV, a protease inhibitor that has not been used previously in a topical vaginal formulation but that has shown promise as a microbicide by blocking viral maturation and transmission of HIV-1 at mucosal surfaces (20). The remaining two APIs in our novel IVR were ETG and E2 for contraception.
Here we have demonstrated that our pod IVR released five drugs at the approximate relative rates hypothesized for an effective device, affording CVL levels in the following decreasing order: TFV > NVP ∼ SQV > ETG > E2. High intraluminal TFV levels are known to be required for effective HIV prevention (21). Median TFV CVL levels over the course of this study were 7.7 × 103 nM (2.2 × 103 ng ml−1). A previous study in the same breed of sheep using an identical CVL collection method found that drug concentration measured in CVL is diluted approximately 10× to 50× relative to cervicovaginal fluid (CVF) levels (5). Based on this dilution range, we estimate that the median 2.2 × 103 ng ml−1 CVL levels measured in sheep are comparable to the 105 ng ml−1 median 24-h CVF concentrations observed in women following exposure to 4 ml 1% TFV gel (22), shown to be protective clinically (18). Median 24-h TFV tissue levels in a 1% TFV gel clinical study were 24 × 103 nM (7 × 103 ng ml−1) (22), while the median day 14 vaginal tissue TFV concentration in our sheep study was 20 × 103 nM (5.6 × 103 ng ml−1). The 50% inhibitory concentrations (IC50s) of NVP (10 to 100 nM) (http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/20636s025,20933s014lbl.pdf) and SQV (1 to 30 nM) (http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/20828s015ppi.pdf) are similar and several orders of magnitude lower than those of TFV (0.04 to 8.5 × 103 nM) (23), suggesting that the observed tissue levels for all three ARV agents are an appropriate starting point in developing a safe and effective MPT product. Vaginal lumen levels of ETG were 8 times higher than the corresponding E2 concentrations, in agreement with the relative daily release rates of ETG and EE from the NuvaRing (120 μg day−1 and 15 μg day−1, respectively) (14).
The flexible design of the pod IVR platform has been discussed in detail in terms of accelerated development as well as ease and cost effectiveness of manufacturing (6, 7). Multiple degrees of freedom in the control of delivery (i.e., pod polymer membrane, number of pods, delivery channel number, and cross-sectional area) allow release rates to be rapidly titrated to target levels, and established in vitro-in vivo correlation models (5, 7, 8) minimize the number of required animal studies in preclinical development. Here, proof of principle for an MPT based on a 5-drug IVR was demonstrated. Future studies will need to rationally select the most appropriate drug combinations and target pharmacokinetic parameters (target drug levels in key anatomic compartments) that will lead to favorable health outcomes.
Supplementary Material
ACKNOWLEDGMENTS
Partial support for the project described was provided by award R21AI076136 and 5R21AI079791/4R33AI079791 (TFV IVR development) and award 5R33AI07606205 (ovine model development) from the National Institute of Allergy And Infectious Diseases.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy And Infectious Diseases or the National Institutes of Health.
Footnotes
Published ahead of print 10 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00547-13.
REFERENCES
- 1. Friend DR, Doncel GF. 2010. Combining prevention of HIV-1, other sexually transmitted infections and unintended pregnancies: development of dual-protection technologies. Antiviral Res. 88:S47–S54 [DOI] [PubMed] [Google Scholar]
- 2. Friend DR. 2012. Drug delivery in multiple indication (multipurpose) prevention technologies: systems to prevent HIV-1 transmission and unintended pregnancies or HSV-2 transmission. Expert Opin. Drug Deliv. 9:417–427 [DOI] [PubMed] [Google Scholar]
- 3. Malcolm RK, Edwards KL, Kiser P, Romano J, Smith TJ. 2010. Advances in microbicide vaginal rings. Antiviral Res. 88:S30–S39 [DOI] [PubMed] [Google Scholar]
- 4. Kiser PF, Johnson TJ, Clark JT. 2012. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 14:62–77 [PubMed] [Google Scholar]
- 5. Moss JA, Malone AM, Smith TJ, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Vincent KL, Motamedi M, Friend DR, Clark MR, Baum MM. 2012. Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob. Agents Chemother. 56:875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baum MM, Butkyavichene I, Gilman J, Kennedy S, Kopin E, Malone AM, Nguyen C, Smith TJ, Friend DR, Clark MR, Moss JA. 2012. An intravaginal ring for the simultaneous delivery of multiple drugs. J. Pharm. Sci. 101:2833–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moss JA, Malone AM, Smith TJ, Butkyavichene I, Cortez C, Gilman J, Kennedy S, Kopin E, Nguyen C, Sinha P, Hendry RM, Guenthner P, Holder A, Martin A, McNicholl J, Mitchell J, Pau C-P, Srinivasan P, Smith JM, Baum MM. 2012. Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques. Antimicrob. Agents Chemother. 56:5952–5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moss JA, Baum MM, Malone AM, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Willis R, Vincent KL, Motamedi M, Smith TJ. 2012. Tenofovir and tenofovir disoproxil pharmacokinetics from intravaginal rings. AIDS 26:707–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Snyder LR, Kirkland JJ, Glajch JL. 1997. Practical HPLC method development, 2nd ed John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 10. Brache V, Faundes A. 2010. Contraceptive vaginal rings: a review. Contraception 82:418–427 [DOI] [PubMed] [Google Scholar]
- 11. Koetsawang S, Ji G, Krishna U, Cuadros A, Dhall GI, Wyss R, Rodriquex la Puenta J, Andrade ATL, Khan T, Kononova ES, Lawson JP, Parekh U, Elstein M, Hingorani V, Na-ning W, Zhong-beng Y, Landgren B-M, Boukhris R, Li-lan L, Boccard S, Machin D, Pinol A, Rowe PJ. 1990. Microdose intravaginal levonorgestrel contraception: a multicenter clinical trial. 1. Contraceptive efficacy and side-effects. Contraception 41:105–124 [DOI] [PubMed] [Google Scholar]
- 12. Sahota J, Barnes PMF, Mansfield E, Bradley JL, Kirkman RJE. 1999. Initial UK Experience of the levonorgestrel-releasing contraceptive intravaginal ring. Adv. Contracept. 15:313–324 [DOI] [PubMed] [Google Scholar]
- 13. Roumen F. 2002. Contraceptive efficacy and tolerability with a novel combined contraceptive vaginal ring, NuvaRing. Eur. J. Contracept. Reprod. Health Care 7:19–24 [PubMed] [Google Scholar]
- 14. Oddsson K, Leifels-Fischer B, de Melo NR, Wiel-Masson D, Benedetto C, Verhoeven CHJ, Dieben TOM. 2005. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception 71:176–182 [DOI] [PubMed] [Google Scholar]
- 15. Madden T, Blumenthal P. 2007. Contraceptive vaginal ring. Clin. Obstet. Gynecol. 50:878–885 [DOI] [PubMed] [Google Scholar]
- 16. Garcia-Lerma JG, Otten RA, Qari SH, Jackson E, Cong ME, Masciotra S, Luo W, Kim C, Adams DR, Monsour M, Lipscomb J, Johnson JA, Delinsky D, Schinazi RF, Janssen R, Folks TM, Heneine W. 2008. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 5:291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Balzarini J, Van Damme L. 2007. Microbicide drug candidates to prevent HIV infection. Lancet 369:787–797 [DOI] [PubMed] [Google Scholar]
- 18. Karim QA, Karim SSA, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karim SSA, Karim QA. 2011. Antiretroviral prophylaxis: a defining moment in HIV control. Lancet 378:e23–e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stefanidou M, Herrera C, Armanasco N, Shattock RJ. 2012. Saquinavir inhibits early events associated with establishment of HIV-1 infection: potential role for protease inhibitors in prevention. Antimicrob. Agents Chemother. 56:4381–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karim SSA, Kashuba ADM, Werner L, Karim QA. 2011. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet 378:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwartz JL, Rountree W, Kashuba ADM, Brache V, Creinin MD, Poindexter A, Kearney BP. 2011. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One 6:e25974. 10.1371/journal.pone.0025974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gilead Sciences, Inc 2012. Product monograph. PrViread® (tenofovir disoproxil fumarate tablets) 300 mg antiretroviral agent. Gilead Sciences, Inc., Foster City, CA [Google Scholar]
- 24. Nuttall J, Kashuba A, Wang R, White N, Allen P, Roberts J, Romano J. 2012. The pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques. Antimicrob. Agents Chemother. 56:103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.