Abstract
A comprehensive comparative analysis of the structure-antifungal activity relationships for the series of biosynthetically engineered nystatin analogues and their novel semisynthetic derivatives, as well as amphotericin B (AMB) and its semisynthetic derivatives, was performed. The data obtained revealed the significant influence of the structure of the C-7 to C-10 polyol region on the antifungal activity of these polyene antibiotics. Comparison of positions of hydroxyl groups in the antibiotics and in vitro antifungal activity data showed that the most active are the compounds in which hydroxyl groups are in positions C-8 and C-9 or positions C-7 and C-10. Antibiotics with OH groups at both C-7 and C-9 had the lowest activity. The replacement of the C-16 carboxyl with methyl group did not significantly affect the in vitro antifungal activity of antibiotics without modifications at the amino group of mycosamine. In contrast, the activity of the N-modified derivatives was modulated both by the presence of CH3 or COOH group in the position C-16 and by the structure of the modifying substituent. The most active compounds were tested in vivo to determine the maximum tolerated doses and antifungal activity on the model of candidosis sepsis in leukopenic mice (cyclophosphamide-induced). Study of our library of semisynthetic polyene antibiotics led to the discovery of compounds, namely, N-(l-lysyl)-BSG005 (compound 3n) and, especially, l-glutamate of 2-(N,N-dimethylamino)ethyl amide of S44HP (compound 2j), with high antifungal activity that were comparable in in vitro and in vivo tests to AMB and that have better toxicological properties.
INTRODUCTION
Despite the relatively recent introduction of new antifungal drug such as next-generation azoles and echinocandins, polyene macrolides continue to be the most potent broad-spectrum antifungals available for the clinical use. Amphotericin B (AMB; compound 1) (Fig. 1) is the drug of choice for the treatment of mycotic infections caused by a wide range of fungi (1). Clinical use of this drug mostly covers life-threatening fungal infections, particularly in patients who have undergone organ transplantation, received aggressive chemotherapy, and patients with AIDS. However, AMB therapy is limited by considerable toxicity (nephrotoxicity, central nervous system, and liver damage side effects) and very poor aqueous solubility. Reduction of AMB toxicity would minimize adverse side effects in patients and also allow high-dose treatment of infections caused by emerging fungal pathogens that are sensitive only to high concentrations of the drug. Until recently, the search for less toxic polyene antibiotics was carried out in two main directions: chemical modification of the natural antibiotics and investigations of lipid and liposomal formulations of polyenes.
Fig 1.
Structures of the polyene macrolide antibiotics amphotericin B (compound 1) and bioengineered nystatin analogues (compounds 2 to 7).
Chemical modifications of the C-16 carboxyl and/or mycosamine 3′ amino groups of AMB were extensively studied and several semisynthetic derivatives of AMB with improved characteristics have been described (2–5). However, despite some promising initial results obtained for such derivatives, no new AMB-based antifungal agent has appeared on the market, except for the lipid and liposomal formulations (1, 6).
Over the few last years, a novel promising concept for the search of less toxic and highly active polyenes based on genetic engineering of antibiotic-producing microorganisms has been developed (7, 8). The application of this new approach made available polyenes with unique structures, which can hardly be obtained by chemical modifications of natural antibiotics. For example, the strain of Streptomyces noursei producing nystatin A1 (NYT) was modified by biosynthetic engineering to produce a range of novel polyene macrolides which have differences in the position C-16 and in polyhydroxylated (polyol) region C-7 to C-10: S44HP (compound 2), BSG005 (compound 3), BSG022 (compound 4), BSG019 (compound 5), BSG003 (compound 6), and BSG018 (compound 7) (Fig. 1) (9, 10). Some of these novel polyene macrolides obtained by biosynthetic engineering were as active as AMB in the in vitro tests. Moreover, antibiotics S44HP (compound 2) and BSG005 (compound 3) (Fig. 1) demonstrated advantages over AMB in the in vivo tests (11, 12).
The important role of the hydroxyl groups in the C-7 to C-10 region for the antifungal activity of the antibiotic compounds 1 to 7, demonstrated in previous investigations, revealed that the most active were compounds 1 to 5 (the MICs against Candida albicans were 0.11 to 0.20 μg/ml) (9–12). For compounds 6 and 7 the shift of hydroxyl group from C-10 to C-9 led to the dramatic decrease of antifungal activity (MICs against C. albicans were 12 and 9 μg/ml, correspondingly) (10).
The new bioengineered polyene macrolides (compounds 2 to 7) represent a special interest as scaffolds for chemical modifications at the amino and/or carboxyl groups. Series of mono- and di-substituted derivatives of S44HP (compound 2) were recently obtained by chemical modifications of the exocyclic C-16 carboxyl and/or the amino group of mycosamine moiety (13, 14). It has been demonstrated that some of these novel derivatives are superior to AMB in terms of both toxicity and therapeutic efficacy in mice models. Thus, combination of genetic engineering techniques and chemical modification methods appears to be a very promising approach to the search for a new polyene drug candidate. It is also useful for the investigations of the structure-activity relationships and the mechanism of action of antifungal antibiotics of this type, which is still insufficiently understood.
The aim of the present work was the analysis of the structure-antifungal activity relationships for the series of novel semisynthetic derivatives of genetically engineered antibiotics S44HP, BSG005, BSG003, and BSG022 in comparison with AMB, its semisynthetic derivatives, and the bioengineered antibiotics BSG018 (compound 7) and BSG019 (compound 5). The data obtained revealed the influence of the particular positions of the hydroxyl groups in the region C-7 to C-10 and a substituent in position C-16 and 3′-N-mycosamine on the antifungal activity of polyene antibiotics.
MATERIALS AND METHODS
Polyene antibiotics.
Sigma-Aldrich Co. (St. Louis, MO) supplied AMB (compound 1). SINTEF (Trondheim, Norway) supplied biosynthetically engineered nystatin analogues compounds 2 to 7. Commercially available Fungizone was used in the in vivo experiments for comparison.
Synthesis of polyene analogs.
A series of semisynthetic derivatives of AMB (compounds 1a to 1i), BSG005 (compounds 3k to 3n), BSG022 (compound 4j), and BSG003 (compound 6j) were synthesized by the methods that have been described earlier for the synthesis of the corresponding S44HP derivatives (compounds 2a to 2n) (13, 14). Purification of the semisynthetic derivatives was carried out using column chromatography on silica gel (Merck, Whitehouse Station, NJ) or on Sephadex G25 (Sigma-Aldrich Co., St. Louis, MO). The purity of the obtained compounds was confirmed by the thin-layer chromatography and high-pressure liquid chromatography (HPLC) methods. HPLC was carried out on a Shimadzu HPLC instrument (Kyoto, Japan) of the LC 10 series on a Kromasil 100-C18 column (4 by 250 mm; particle size, 6 μm) at an injection volume of 20 μl and a wavelength 408 nm with flow rate 1.0 ml/min. System A consisted of 0.2% ammonium formate (HCOONH4; pH 4.5) and acetonitrile (CH3CN), and the proportion of CH3CN varied from 30 to 70% for 30 min; system B consisted of 0.2% ammonium formate (HCOONH4) (pH 4.5) and acetonitrile (CH3CN), and the proportion of CH3CN varied from 25 to 65% for 40 min; system C consisted of 0.01 M orthophosphoric acid (H3PO4; pH 2.6) and acetonitrile (CH3CN), the proportion of CH3CN varied from 30 to 70% for 30 min. The structure of the obtained compounds was confirmed by physicochemical and spectral methods (Table 1). Mass spectra data were obtained on matrix-assisted laser desorption ionization–time of flight Bruker BIFLEX III instrument.
Table 1.
Properties of the novel compounds 1a to 1i, 3k to 3n, 4j, and 6j
| Compound | TLC, Rf (system)a | HPLC, Rt, min (system) | Brutto formula | Mol wt |
|
|---|---|---|---|---|---|
| Calculated | Determinedb [M+H]1+ | ||||
| 1a | 0.59 (I) | 20.42 (A) | C57H88N2O16 | 1,056.6134 | 1,079.608* |
| 1b | 0.32 (II) | 10.54 (A) | C49H78N2O17 | 966.5300 | 967.538 |
| 1c | 0.31 (I) | 11.14 (A) | C48H76N2O16 | 936.5195 | 959.600* |
| 1d | 0.57 (I) | 19.88 (A) | C51H82N2O18 | 1,010.5563 | 1,011.568 |
| 1e | 0.59 (I), 0.62 (I) | 12.19 (A), 12.76 (A) | C51H80N2O19 | 1,024.5355 | 1,047.560* |
| 1f | 0.55 (I) | 12.99 (C) | C51H80N2O18 | 1,008.5406 | 1,031.532* |
| 1g | 0.47 (I) | 13.66 (C) | C51H80N2O18 | 1,008.5406 | 1,031.550* |
| 1h | 0.55 (II) | 6.06 (C) | C53H85N3O17 | 1,035.5879 | 1,058.656* |
| 1i | 0.15 (I) | 11.65 (A) | C59H93NO27 | 1,247.5935 | 1,248.604 |
| 3k | 0.65 (III) | 10.20 (A) | C53H85NO20 | 1,055.5665 | 1,056.574 |
| 3l | 0.85 (II) | 25.39 (B) | C56H86N2O15 | 1,026.6028 | 1,049.601* |
| 3m | 0.02 (II) | 10.07 (A) | C53H89N3O15 | 1,007.6294 | 1,008.542 |
| 3n | 0.03 (I) | 13.05 (A) | C53H87N3O16 | 1,021.6086 | 1,022.614 |
| 4j | 0.32 (II) | 13.57 (B) | C51H83N3O15 | 977.5824 | 978.600 |
| 6j | 0.31 (II) | 19.73 (B) | C51H83N3O16 | 993.5773 | 994.595 |
System I, CHCl3-methanol-H2O-HCOOH (130:60:10:1); system II, CHCl3-methanol-H2O-NH4OH (130:70:10:1); system III, AcOEt–n-PrOH–NH4OH (15:10:10).
*, [M+Na]+1.
l-Glutamate salt of 2j.
Water soluble form of compound 2j (l-glutamate of compound 2j) was obtained as it has been described previously (14).
Biology: in vitro antifungal activity.
The antifungal activity in vitro was tested against the yeast strains Candida albicans ATCC 14053 and Cryptococcus humicolus ATCC 9949 and the fungus strains Aspergillus niger ATCC 16404 (all received from were received from American Type Culture Collection [ATCC]) and Fusarium oxysporum VKM F-140 (All-Russian Collection of Microorganisms; VKM–Department of the G. K. Skryabin Institute of Biochemistry and Physiology of Microorganisms RAS at the Pushchino Biological Research Center), using the broth microdilution method (2-fold dilution) as described in CLSI documents M27-A and M38-A (15, 16). The medium RPMI 1640 with l-glutamine and phenol red, without sodium bicarbonate, supplemented with 0.2% glucose (ICN Biomedicals, Inc., OH) and buffered with 0.165 M morpholinepropanesulfonic acid (MOPS, pH 7.0; Acros Organics, NJ), was used. Two series (batches) of liquid medium RPMI 1640 were used.
The compounds tested were initially solubilized in dimethyl sulfoxide (DMSO) at a starting concentration of 1,600 μg/ml. Series of dilutions (1,600 to 3.13 μg/ml) were prepared from the stock solutions in the same solvent, diluted 50-fold in the test medium and twice when inoculated, that reduced the final solvent concentration to 1%.
MICs were measured as the lowest concentrations of agents that prevent any visible growth. Experiments were made three times for every agent and repeated twice at 4-week intervals. The results of the experiments were definitely reproducible. In cases of full coincidence of the data obtained, the MIC is represented as a single number. In other cases, the MICs obtained are represented in the table as ranges of numbers. The results of MICs obtained in vitro with two series of broth medium RPMI 1640 are presented in Fig. 2 to 4 and Table 2.
Fig 2.
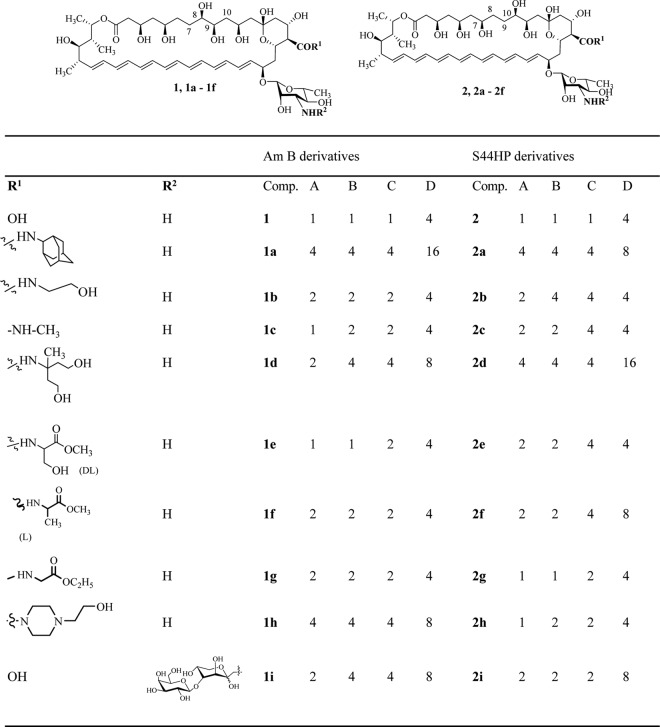
Antifungal activity (MIC, μg/ml) of the concurrent derivatives of AMB (compounds 1a to 1i) and S44HP (compounds 2a to 2i) compared with the parent antibiotics AMB (compound 1) and S44HP (compound 2) against C. albicans ATCC 14053 (A), C. humicolus ATCC 9949 (B), A. niger ATCC 16404 (C), and F. oxysporum VKM F-140 (D).
Fig 4.

Antifungal activity of AMB (compound 1) and DMAE amides of S44HP (compound 2j), BSG003 (compound 4j), and BSG022 (compound 6j).
Table 2.
Antifungal activities of BSG005 derivatives (compounds 3k to 3n) compared to the corresponding S44HP derivatives (compounds 2k to 2n), AMB (compound 1), S44HP (compound 2), and BSG005 (compound 3) against C. albicans ATCC 14053, C. humicolus ATCC 9949, A. niger ATCC 16404, and F. oxysporum VKM F-140a
| Compound | MIC (μg/ml) |
|||
|---|---|---|---|---|
| A | B | C | D | |
| S44HP derivatives | ||||
| 2 | 1 | 1 | 1 | 4 |
| 2k | 1 | 1 | 2 | 8 |
| 2l | 2 | 2 | 4 | >16 |
| 2m | 1 | 1 | 1 | 1 |
| 2n | 8 | 8 | 16 | >16 |
| 1 | 1 | 1 | 1 | 4 |
| BSG-005 derivatives | ||||
| 3 | 1 | 1 | 2 | 2 |
| 3k | 2 | 2 | 2 | 8 |
| 3l | 16 | >16 | >16 | >16 |
| 3m | 4 | 8 | 8 | 8 |
| 3n | 1 | 1 | 1 | 2 |
A, C. albicans ATCC 14053; B, C. humicolus ATCC 9949; C, A. niger ATCC 16404; D, F. oxysporum VKM F-140.
In vivo antifungal activity. (i) Animals.
First-generation hybrids (C57BL/6 × DBA/2)F1 (B6D2F1) of male mice (weight, 20 to 22 g) received from the Central farm “Kryukovo” of Russian Academy of Medical Science (RAMS) were used. Animals were maintained in the vivarium in plastic cages (with hardwood bedding in environmentally controlled conditions: 24 ± 1°C, 12/12-h light/dark cycle) on a standard diet of bricketed forages with easy access to drinking water (ad libitum). After a 2-week quarantine period, healthy animals were used in experimental work. The animal experiments were performed in compliance with the EU and Russian Guidelines for Animal Experiments and Welfare authorized by the Russian Ministry of Health (1045-73 and 52-F3).
(ii) Antifungal agents.
Solutions of the studied compounds were prepared “ex temporae” and were kept in dark glass vials to avoid ingress of light. The solutions were prepared as following: dry antibiotic substances (5 mg) were mixed with dry sodium deoxycholate (4.1 mg) in a sterile glass vial. Phosphate buffer (10 ml; NaH2PO4, 1.59 g; Na2HPO4, 0.96 g; H2O to 100 ml) was added to the mixture, and the suspension was immediately subjected to vigorous shaking for 10 min until homogeneous solutions were formed. The obtained solutions (2 ml) were placed into the new sterile glass vials, 6 ml of 5% neutral sterile glucose solution was added, and the resulting solutions (0.125 mg/ml) were used for intravenous administration.
The maximum tolerated doses (MTD) of compounds 3n, 5, l-glutamate of compound 2j, and AMB (compound 1) were determined. The antibiotic preparations were singly injected into the mice's tail vein within 1 to 1.5 h after the preparation of solutions. The speed of injection did not exceed 0.5 ml/min. Each antibiotic was used in a range of doses resulting in 0 to 100% lethality and a minimum of three intermediate doses. Animals were randomized into groups, each containing six mice. MTDs were calculated using Litchfield-Wilcoxon probit analysis with the statistical analysis software StatPlus-3.5.0–2005. The results are shown in Table 3.
Table 3.
MTD and ED data for compounds 3n and 5 and l-glutamate of compound 2j compared to AMB (compound 1)a
| Compound | Dose (mg/kg/daily) |
ED/MTD (%) | |
|---|---|---|---|
| MTD | ED | ||
| 1 | 2.01 (1.88 ÷ 2.23) | 1.25 | 62 |
| l-Glutamate of 2j | 14.7 (12.8 ÷ 16.8) | 0.4 | 2.7 |
| 3n | 4.48 (4.23 ÷ 4.75) | 1.25 | 28 |
| 5 | 42.8 (41.2 ÷ 44.5) | 16.0 | 37.3 |
MTD, maximal tolerated dose; ED, effective dose that means elimination of 99% of infectious agent from kidneys.
Study of the specific activity of polyene antibiotics in a model of candidosis sepsis in leukopenic mice.
At first we studied the specific activity of polyene antibiotics by examining the induction of the temporary leucopenia. Cyclophosphamide (Biotex; Saransk) was administered at a dose of 100 mg/kg/day 3 days before and 1 day after infection. Animals were randomly split into experimental and control groups, each containing six mice. Animals were infected intravenously with C. albicans (strain 14053 ATCC; inoculum, 3 × 105 CFU/mouse in volume of 0.1 ml). It should be noted that the C. albicans inoculum remained constant in all experiments. Intravenous introduction of tested compounds in a volume of 0.2 ml (at a speed of 0.2 ml/30 s) was carried out 30 min after infection.
This experiment was planned so that in each experiment one dose (for AMB and for tested preparations) was used; each dose was recorded daily within 4 days, since the day of infection (days 0, 1, 2, and 3). Each experiment was carried out with three groups of animals: an untreated group, a group of animals infected with C. albicans, and a group of infected mice that received cyclophosphamide. There was a also placebo group (intact, uninfected animals, which were intravenously (in the same volume as for the medical preparations) injected with 0.2 ml of diluent (phosphate buffer plus 5% glucose [1:1]). The placebo group did not show any activity. C. albicans was not detected in uninfected animals.
After the last injection of tested preparations, mice were weighed and sacrificed. Then, in sterile conditions, the C. albicans burdens were determined by viable counting of homogenates from the kidneys. The kidneys were aseptically removed, then weighed and ground in porcelain mortars with sterile corundum. Dilutions of the suspensions were seeded into petri dishes with Sabouraud agar and incubated for 48 h at 35°C; the developed colonies of C. albicans were counted, and their quantity on 1 g of kidney tissue was determined. The first dilution was 10−1. A zero result at this cultivation accepted for 5 CFU/g.
Statistical processing was carried out with the help of computer program Microsoft Office Excel 2003. Significant distinctions had a P of ≤0.05 at comparison by using the Student t criterion. The data are presented in Table 3.
RESULTS
Chemistry.
Our analysis of structure-activity relationship for the series of genetically modified and semisynthetic polyene antibiotics of the AMB group is based on the comparison of properties of previously described derivatives of S44HP (13, 14), as well as novel derivatives of antibiotics AMB (compound 1), BSG005 (compound 3), BSG022 (compound 4), and BSG003 (compound 6).
First, a series of semisynthetic derivatives of AMB, namely, N-(2-adamantyl)amide of AMB (compound 1a), N-(2-hydroxyethyl)amide of AMB (compound 1b), N-methylamide of AMB (compound 1c), N-1-[di-(2-hydroxyethyl)ethyl amide of AMB (compound 1d), N-(hydroxymethyl, methoxycarbonyl)-methyl amide of AMB (amide of methyl ester of dl-serine) (compound 1e), N-(methyl, methoxycarbonyl)-methyl amide of AMB (amide of methyl ester of l-alanine) (compound 1f), N-(ethoxycarbonyl)methyl amide of AMB (amide of ethyl ester of glycine) (compound 1g), N-(N′-2-hydroxyethyl)-piperazide of AMB (compound 1 h), and N-[(β-d-(galactosyl-1→4)-O-1-desoxy-d-fructos-1-yl] AMB (compound 1i), were synthesized by methods described earlier for the synthesis of the S44HP derivatives: N-(2-adamantyl) amide of S44HP (compound 2a), N-(2-hydroxyethyl)amide of S44HP (compound 2b), N-methylamide of S44HP (compound 2c), N-1-[di-(2-hydroxyethyl)ethyl amide of S44HP (compound 2d), N-(hydroxymethyl, methoxycarbonyl)-methyl amide of S44HP (amide of methyl ester of DL-serine) (compound 2e), N-(methyl, methoxycarbonyl)-methyl amide of S44HP (amide of methyl ester of l-alanine) (compound 2f), N-(ethoxycarbonyl)methyl amide of S44HP (amide of ethyl ester of glycine) (compound 2g), N-(N′-2-hydroxyethyl)-piperazide of S44HP (compound 2h), and N-[(β-d-(galactosyl-1→4)-O-1-desoxy-d-fructos-1-yl] S44HP (compound 2i), respectively (Fig. 2) (13). Since it has been demonstrated previously using NMR method that the interaction of antibiotic S44HP with an aldohexose carbohydrate led to an Amadori rearrangement product, i.e., an N-carbohydrate containing polyene antibiotic, which exists in three equilibrium forms: linear keto form, cyclic (α+β) pyranose form, and cyclic (α+β) furanose form (13). For the sake of simplicity, only one form, namely, the cyclic (α+β) pyranose form, of compounds 1i or 2i, is presented in Fig. 2.
Similarly, the 2-(N,N-dimethylamino)ethyl amides of BSG022 (DMAE amide-BSG022; compound 4j) and BSG003 (DMAE amide-BSG003; compound 6j), and a series of semisynthetic derivatives of BSG005, namely, N-fructosyl-BSG005 (compound 3k), N-(4-N,N-dimethylaminobenzyl)-BSG005 (compound 3l), N,N-di-(3-aminopropyl)-BSG005 (compound 3m), and N-(l-lysyl)-BSG005 (compound 3n) were obtained by the methods described earlier (13). For the comparison of antifungal activities, corresponding derivatives of S44HP, namely, 2-(N,N-dimethylamino)ethyl amide of S44HP (compound 2j; Table 4), N-fructosyl-S44HP (compound 2k), N-(4-N,N-dimethylaminobenzyl)-S44HP (compound 2l), N,N-di-(3-aminopropyl)-S44HP (compound 2m), and N-(l-lysyl)-S44HP (compound 2n) (Fig. 5) were obtained as previously described (13). As mentioned above, only one the cyclic (α+β) pyranose form of Amadori rearrangement product compounds 2k and 3k is presented in Fig. 5. The properties of the novel compounds 1a to 1i, 3k to 3n, 4j, and 6j are presented in Table 1.
Fig 5.
Structures of S44HP (compound 2) and BSG005 (compound 3) and of their semisynthetic derivatives.
Biological evaluation.
Antifungal activities of biosynthetically engineered nystatin analogues, their novel semisynthetic derivatives, and semisynthetic derivatives of AmB were tested compared to AmB against two strains of yeast, Candida albicans and Cryptococcus humicolus, and two strains of filamentous fungi (molds), Aspergillus niger and Fusarium oxysporum. The results of MICs in vitro are represented in Fig. 2 to 4 and Table 2. Compounds 1, 2, 2j, 3, 5, 6, and 6j were also tested against additional numbers of yeasts (see the supplemental material).
First, we analyzed the series of corresponding derivatives of antibiotics AMB (compounds 1a to 1i) and S44HP (compounds 2a to 2i), which allowed monitoring of the influence of the C-7–C-10 region structure on the antifungal activity (Fig. 2).
AMB (compound 1) bears two OH groups at positions C-8 and C-9, whereas S44HP (compound 2) has two OH groups at positions C-7 and C-10 (Fig. 1). As shown in Fig. 2, the corresponding derivatives of AMB and S44HP have very similar in vitro antifungal activities. This suggests that the differences in the structures of the polyol regions of compounds 1 and 2 have no drastic effect on the in vitro antifungal activity of these compounds. However, these modifications may influence the pharmacological properties of these antibiotics. Earlier in vivo studies clearly demonstrated superior pharmacological properties for the biosynthetically engineered compound S44HP (compound 2) versus AMB (compound 1) (11).
Next, we compared the influence of the structure of the polyol region on the antifungal activity of another group of antibiotics that have differences in the C-9-to-C-10 region. Compound BSG005 (compound 3), possessing an OH group at position C-10, had slightly higher activity than compound BSG019 (compound 5), which lacks an OH group in position C-10 (Fig. 3). The shifting of the OH group from position C-10 (as in compound 3) to position C-9 (BSG018; compound 7) decreased the antifungal activity significantly and resulted in almost complete loss of activity against C. humicolus and F. oxysporum (MICs ≥ 16 μg/ml). The order of decreasing activity could be presented as “BSG005 (compound 3) > BSG019 (compound 5) > BSG018 (compound 7)” (Table 3), which is consistent with the earlier data for C. albicans (10).
Fig 3.
Antifungal activity of AMB (compound 1), BSG005 (compound 3), BSG019 (compound 5), and BSG018 (compound 7). The data represented in Fig. 3 and 4 were obtained with a batch of medium RPMI 1640 different from the one used for Fig. 2 and Table 2.
A similar trend in antifungal activity change was found for the series of the DMAE amides of antibiotics 2j, 4j, and 6j (Fig. 4). Compound 2j was as active as AMB (compound 1) against all yeast and molds tested. Compound 4j, lacking an OH group at the C-10 position, compared to AMB (compound 1) and compound 2j, showed slightly lower antifungal activity against all four strains. Compound 6j, in which the OH group was shifted from position C-10 (as in compound 3) to position C-9, (as in compound 7) demonstrated low activity against all test strains, especially against C. humicolus (MIC > 16 μg/ml) and F. oxysporum (MIC = 8 μg/ml). Thus, the change in positions of the hydroxyl groups in the C-9-to-C-10 region of S44HP led to dramatic changes in antifungal activity. Activity decreased in the following order: DMAE amide-S44HP (compound 2j) > DMAE amide-BSG022 (compound 4j) > DMAE amide-BSG003 (compound 6j) (Fig. 4).
Essentially, the trend of the change of the activities of the derivatives compounds 2j, 4j, and 6j confirmed the results that have been obtained earlier against C. albicans, which can be presented in the following order of decreasing activity: S44HP (compound 2; C-7–OH and C-10–OH) > BSG022 (compound 4; C-7–OH) > BSG003 (compound 6; C-7–OH and C-9–OH) (10). Thus, the same trend of change in the antifungal activity depending on the position of hydroxyls in the C-9-to-C-10 region was found in series of antibiotics bearing carboxylic (compounds 2, 4, and 6) or methyl (compounds 3, 5, and 7) groups at position C-16, as well as in the series of DMAE amides, compounds 2j, 4j, and 6j.
Similar results have been obtained for antibiotics of the AMB series: the elimination of one OH group in the C-7-to-C-10 region of AMB by biosynthetic engineering yielded 8-deoxyamphotericin B, which demonstrated slightly decreased activity against Saccharomyces cerevisiae (17).
The next part of our investigation was aimed at the determination of the influence of the substituents in the positions C-16 and 3′-amino on the antifungal activity of polyenes. The antifungal activities of the new 3′-N-substituted derivatives of BSG005 (compounds 3k to 3n) compared to the corresponding derivatives of S44HP (compounds 2k to 2n) were evaluated (Fig. 5 and Table 2). These series of genetically engineered polyene macrolides have the same configuration in the C-7-to-C-10 region; compound S44HP (compound 2) bears carboxylic group at the C-16 position, while BSG005 (compound 3) has a methyl group at this position (Fig. 5).
Earlier, it has been shown that the replacement of the C-16 carboxyl group in S44HP (compound 2) with methyl group (BSG005, compound 3) does not decrease antifungal activity in vitro (12). Similarly, it has been demonstrated that the replacement of COOH in AMB for CH3 group did not affect the antifungal activity of the resulting 16-decarboxy-16-methyl analogue of AMB (18). However, chemical modifications of the amino group of BSG005 (compounds 3k to 3n), and comparison of compounds 3k to 3n to the corresponding derivatives of S44HP (compounds 2k to 2n) showed different trends in changes of the antifungal activity (Table 2).
The N-fructosyl derivatives compounds 2k and 3k had equal activity against two investigated filamentous fungi: A. niger and F. oxysporum (MICs = 2 and 8 μg/ml, respectively). The activity of compound 2k against strains of yeasts C. albicans and C. humicolus was 2-fold higher than the activity of compound 3k (MICs = 1 and 2 μg/ml, respectively). Thus, the influence of the C-16–COOH or –CH3 in the case of semisynthetic derivatives bearing hydrophilic neutral sugar substituent on the amino group (compound 2k or 3k) on the antifungal activity was not strongly pronounced.
In contrast to this, three other modifications of the mycosamine amino group of the polyene macrolide compounds 2 and 3 yielded different results. The antifungal activities of the N-substituted derivative compounds 2l to 2n and compounds 3l to 3n bearing an amino group in the substituent showed marked difference. The 4-N,N-dimethylaminobenzyl derivative compound 3l was inactive against all four strains (MICs ≥ 16 μg/ml). The derivative compound 2l bearing the same moiety in the mycosamine amino group was inactive against F. oxysporum (MIC > 16 μg/ml) but had better activity (MIC = 2 to 4 μg/ml) against C. albicans, C. humicolus and A. niger. This suggests that the antifungal activity of polyene macrolides can be differentiated against certain fungi via specific combinations of modifications at C-16 and the amino group of mycosamine.
A similar trend in the antifungal activity change was shown for the pair of derivative compounds 2m and 3m. The compound N,N-di-(3-aminopropyl)-BSG005 (compound 3m) was 4- to 8-fold less active than one of the most active derivatives N,N-di-(3-aminopropyl)-S44HP (compound 2m) against all four strains tested. In contrast to this, the activity of N-(l-lysyl)-BSG005 (compound 3n) was much higher (up to eight times) than the activity of N-(l-lysyl)-S44HP (compound 2n) against all four strains. It should be noted that a similar semisynthetic analog of AMB, N,N-di-(3-aminopropyl)-AMB, also showed higher antifungal activity compared to the parental antibiotic AMB (19).
It can thus be concluded that the activity of the discussed derivatives modified at the amino group depends on the structure of the starting compound, i.e., on the presence of a C-16–CH3 or –COOH group and on the structure of the modifying substituent at the amino group of mycosamine.
Some of the studied compounds that showed high antifungal activities in in vitro experiments, namely, compound 3n, compound 5, and a water-soluble form of compound 2j (l-glutamate salt of compound 2j), were chosen for in vivo investigations.
MTDs were calculated according to the Litchfield-Wilcoxon probit analysis method using StatPlus-3.5.0–2005 software (20). To investigate the in vivo efficacy of the tested compounds, a model of candidosis sepsis in leukopenic mice (cyclophosphamide induced) was used (14, 21).
The highest in vitro activity of S44HP derivative compound 2j (l-glutamate salt) was confirmed by an in vivo study in the conditions of model candidosis in a leukopenic background. l-Glutamate of compound 2j was almost 10 times less toxic compared to AMB, and the effective dose (ED) was equal to 2.7% of the MTD, whereas AMB had an ED equal to 62% of the MTD (Table 3). The l-glutamate of compound 2j retained high activity at doses from 0.4 mg/kg/day up to 4.0 mg/kg/day, despite of the leukopenic conditions induced by cyclophosphamide. Judging from the ED/MTD ratio, compound 3n also had a considerably better therapeutic index compared to AMB (Table 3). Compound 5 had the lowest toxicity (MTD = 42.4 mg/kg/daily) and the lowest efficacy (ED = 16.0 mg/kg/day) among the tested compounds, although the ED/MTD ratio for compound 5 was better than that for AMB (compound 1) (Table 3). Thus, the activity of BSG019 (compound 5) in the in vitro tests did not correlate well with the data obtained in the in vivo experiments. Most likely, the suboptimal pharmacokinetic properties of this compound can explain the low activity in the animal model.
DISCUSSION
Natural (amphotericin B; compound 1) and bioengineered (compounds 2 to 7) polyene macrolide antibiotics and series of their semisynthetic derivatives represent a unique basis for the study of their structure-antifungal activity relationship and understanding the roles of positions of hydroxyls in the C-7-to-C-10 region, the amino group in mycosamine, and the C-16 substituent (COOH or CH3) in the antifungal properties of polyene antibiotics. Analysis of the structure-activity relationships for new polyenes may provide important information for the design of novel highly active and less toxic antifungal polyenes.
The mechanism of action of polyene macrolides is believed to be associated with their ability to interact with sterol-containing membranes and form pores (channels) through which ions are leaking out of cells, resulting in their death (6, 22). Detailed mechanisms of these interactions, however, remain to be investigated. Recently, a critical role of mycosamine and the C-35–OH group of the AMB molecule for its antifungal activity was shown, and it was stated that “amphotericin primarily kills yeast by simply binding ergosterol” (23). However, only compounds 1 to 3 and most of their semisynthetic derivatives have antifungal properties, while compounds 4 to 7 that also have the C-35–OH group and mycosamine moiety demonstrated low activity. This demonstrates the critical role of the hydroxyl group positions in the region from C-7 to C-10 in biological activity (functional groups on the macrolide cycle can influence the conformation of the macrolide cycle, the formation of hydrogen bonds network, intermolecular interactions, and ion channel formation).
The hypothesis that sterol binding is paramount to the antifungal activity of AMB has been supported by the testing a derivative of AMB without mycosamine moiety that possessed the ability to bind ergosterol but lacked the capacity to form ion channels. It was concluded that a mycosamine-mediated direct binding interaction between AMB and ergosterol is required for both forming ion channels and killing yeast cells (24).
It is interesting that the derivatives of our antibiotics compounds 1 to 3 (with beneficial positions of the hydroxyl groups in the region from C-7 to C-10) with various substituents at the amino group of mycosamine, including rather bulky hydrophobic or hydrophilic groups (Table 1 and Fig. 2 to 4) retained high antifungal activity. Although all of the studied derivatives have substituents that can be protonated (14, 19), the bulky substituents at the amino group might be barriers for the supposed interaction with ergosterol. This suggests that the amino group of mycosamine does not play a critical role in the interaction with ergosterol.
Studies of the antibiotic compounds 2 and 3 showed that the replacement of the C-16 carboxyl group for C-16 methyl group does not decrease antifungal activity of polyene antibiotics in the in vitro tests if the 3′N-amino group of the antibiotic is unmodified. Our results are in agreement with previously found data for AMB and its analog, 16-decarboxy-16-methyl-AMB (18). However, the nature of the C-16 substituent (COOH or CH3, antibiotic compound 2 or 3) greatly influences the antifungal activity of the N-modified semisynthetic derivatives of the polyene macrolides.
Study of our library of semisynthetic polyene antibiotics led to the discovery of compounds, namely, N-(l-lysyl)-BSG005 (compound 3n) and, especially, l-glutamate salt of 2-(N,N-dimethylamino)ethyl amide of S44HP (compound 2j) with high antifungal activity that are comparable in the in vitro and in vivo tests to AMB and have better toxicological properties.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Biosergen AS, the Research Council of Norway, and the Russian Foundation for Basic Research (grant 10-03-00210-a) for partial support of this research.
We are grateful to Natalia M. Malyutina (Gause Institute of New Antibiotics, Moscow) for HPLC analysis.
Footnotes
Published ahead of print 28 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00270-13.
REFERENCES
- 1. Demain AL, Sanchez S. 2009. Microbial drug discovery: 80 years of progress. J. Antibiot. 62:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borowski E. 2000. Novel approaches in the rational design of antifungal agents of low toxicity. Farmaco 55:206–208 [DOI] [PubMed] [Google Scholar]
- 3. Jarzebskyi A, Falkowski L, Borowski E. 1987. Synthesis and structure-activity relationships of amides of amphotericin B. J. Antibiot. 40:1023–1027 [DOI] [PubMed] [Google Scholar]
- 4. Paquet V, Volmer AA, Carreira EM. 2008. Synthesis and in vitro biological properties of novel cationic derivatives of amphotericin. B. Chemistry 14:2465–2481 [DOI] [PubMed] [Google Scholar]
- 5. Belakhov V, Shenin Y. 2007. Synthesis and antifungal activity of N-benzyl derivatives of amphotericin. B. Pharm. Chem. J. 41:362–366 [Google Scholar]
- 6. Cereghetti DM, Carreira EM. 2006. Amphotericin B: 50 years of chemistry and biochemistry. Synthesis 6:914–942 [Google Scholar]
- 7. Solovieva SE, Olsufyeva EN, Preobrazhenskaya MN. 2011. Chemical modification of antifungal polyene macrolide antibiotics. Russ. Chem. Rev. 80:103–126 [Google Scholar]
- 8. Caffery P, Aparicio JF, Malpartida F, Zotchev SB. 2008. Biosynthetic engineering of polyene macrolides towards generation of improved antifungal and antiparasitic agents. Curr. Top. Med. Chem. 8:639. [DOI] [PubMed] [Google Scholar]
- 9. Bruheim P, Borgos SEF, Tsan P, Sletta H, Ellingsen TE, Lancelin JM, Zotchev SB. 2004. Chemical diversity of polyene macrolides produced by Streptomyces noursei ATCC 11455 and recombinant strain ERD44 with genetically altered polyketide synthase NysC. Antimicrob. Agents Chemother. 48:4120–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brautaset T, Sletta H, Degnes KF, Sekurova ON, Bakke I, Volokhan O, Andreassen T, Ellingsen TE, Zotchev SB. 2011. New nystatin-related antifungal polyene macrolides with altered polyol region generated via biosynthetic engineering of Streptomyces noursei. Appl. Environ. Microbiol. 77:6636–6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Treshchalin ID, Sletta H, Borgos SE, Pereverzeva EP, Voeikova TA, Ellingsen TE, Zotchev SB. 2005. Comparative analysis of antifungal activities in vitro and acute toxicity in vivo of S44HP, an analogue of nystatin obtained by genetic engineering. Antibiot. Khimioter. 50:18–22 (In Russian.) [PubMed] [Google Scholar]
- 12. Brautest T, Sletta H, Nedal A, Borgos SEF, Degnes KF, Baffe I, Volokhan O, Sekuriva ON, Treshalin ID, Mirchink EP, Dikiy A, Ellingsen TE, Zotchev SB. 2008. Improved antifungal polyene macrolides via engineering of the nystatin biosynthetic genes in Streptomyces noursei. Chem. Biol. 15:1198–1206 [DOI] [PubMed] [Google Scholar]
- 13. Preobrazhenskaya MN, Olsufyeva EN, Solovieva SE, Tevyashova AN, Reznikova MI, Luzikov YN, Terekhova LP, Trenin AS, Galatenko OA, Treshalin ID, Mirchink EP, Bukhman VM, Sletta H, Zotchev SB. 2009. Chemical modification and biological evaluation of new semisynthetic derivatives of 28,29-didehydronystatin A1 (S44HP), a genetically engineered antifungal polyene macrolide antibiotic. J. Med. Chem. 52:189–196 [DOI] [PubMed] [Google Scholar]
- 14. Preobrazhenskaya MN, Olsufyeva EN, Tevyashova AN, Printsevskaya SS, Solovieva SE, Reznikova MI, Trenin AS, Galatenko OA, Treshalin ID, Pereverzeva ER, Mirchink EP, Zotchev SB. 2010. Synthesis and study of the antifungal activity of new mono- and di-substituted derivatives of a genetically engineered polyene antibiotic 28,29-didehydro-nystatin A1 (S44HP). Novel water-soluble derivative active in the model of the candidosis sepsis in leucopenic mice. J. Antibiot. 63:55–64 [DOI] [PubMed] [Google Scholar]
- 15. Clinical and Laboratory Standards Institute 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. Document M27-A. CLSI, Wayne, PA [Google Scholar]
- 16. Clinical and Laboratory Standards Institute 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. Document M38-A. CLSI, Wayne, PA [Google Scholar]
- 17. Byrne B, Carmody M, Gibson E, Rawlings B, Caffrey P. 2003. Biosynthesis of deoxyamphotericins and deoxyamphoteronolides by engineered strains of Streptomyces nodus. Chem. Biol. 10:1215–1224 [DOI] [PubMed] [Google Scholar]
- 18. Carmody M, Murphy B, Byrne B, Power P, Rai D, Rawlings B, Caffrey P. 2005. Biosynthesis of amphotericin derivatives lacing exocyclic carboxyl groups. J. Biol. Chem. 280:34420–34426 [DOI] [PubMed] [Google Scholar]
- 19. Paquet V, Carreira EM. 2006. Significant improvement of antifungal activity of polyene macrolides by bisalkylation of the mycosamine. Org. Lett. 8:1807–1809 [DOI] [PubMed] [Google Scholar]
- 20. Litchfield JT, Wilcoxon FJ. 1949. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 96:99–113 [PubMed] [Google Scholar]
- 21. Takemoto K, Yamamoto Y, Ueda Y. 2006. Evaluation of antifungial pharmacodinamic characteristics of AMBisome against Candida albicans. Microbiol. Immunol. 50:579–586 [DOI] [PubMed] [Google Scholar]
- 22. Kruijff De BDemel RA. 1974. Polyene antibiotic-sterol interaction in membranes of Acholoplasma laidlawii cells and lecithin liposomes. III. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim. Biophys. Acta 339:57–70 [DOI] [PubMed] [Google Scholar]
- 23. Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, Burke MD. 2011. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. U. S. A. 109:2234–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palacios DS, Dailey I, Siebert DM, Wilcock BC, Burke MD. 2011. Synthesis-enabled functional group deletions reveal key underpinnings of amphotericin B ion channel and antifungal activities. Proc. Natl. Acad. Sci. U. S. A. 108:6733–6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.