Abstract
Acinetobacter baumannii is one of the main pathogens that cause ventilator-associated pneumonia (VAP) and is associated with a high rate of mortality. Little is known about the efficacy of macrolides against A. baumannii. In order to confirm the efficacy of azithromycin (AZM) against VAP caused by multidrug-resistant A. baumannii (MDRAB), we used a mouse model that mimics VAP by placement of a plastic tube in the bronchus. AZM (10 and 100 mg/kg of body weight) was administered subcutaneously every 24 h beginning at 3 h after inoculation. Phosphate-buffered saline was administered as the control. Survival was evaluated over 7 days. At 48 h postinfection, mice were sacrificed and the numbers of viable bacteria in lungs and bronchoalveolar lavage fluid were compared. Histopathological analysis of lung specimens was also performed. The treatment groups displayed significantly longer survival than the control group (P < 0.05). AZM did not have an antimicrobial effect. Histopathological examination of lung specimens indicated that the progression of lung inflammation was prevented in the AZM-treated groups. Furthermore, total cell and neutrophil counts, as well as cytokine levels, in bronchoalveolar lavage fluid were significantly decreased (P < 0.05) in the AZM-treated groups. AZM may have a role for the treatment of VAP with MDRAB because of its anti-inflammatory effects.
INTRODUCTION
Acinetobacter baumannii is a worldwide-known nosocomial pathogen that has become increasingly common over the past few decades and is associated with high rates of morbidity and mortality (1–3). A. baumannii causes pulmonary, urinary tract, bloodstream, and surgical wound infections (3, 4). Ventilator-associated pneumonia (VAP) is caused by A. baumannii more often than by other pathogens (5). Recently, increasing numbers of drug-resistant A. baumannii strains have been identified. Among Gram-negative pathogens, Acinetobacter has the highest multidrug resistance rate (6), and among all bacterial pathogens, A. baumannii causes the highest rate of mortality from VAP (5). Furthermore, infections due to multidrug-resistant A. baumannii (MDRAB) strains are associated with a worse prognosis than infections caused by non-MDRAB strains (7, 8). Therefore, it is very difficult to treat VAP caused by MDRAB. Murine models of A. baumannii pneumonia obtained using cyclophosphamide and porcine mucin have been reported (9–11). However, there are no reports of a model of VAP caused by A. baumannii.
It is gradually being accepted that macrolides not only possess antibacterial activity but also exert immunomodulatory effects. Macrolides have been shown to improve the rate of survival from community-acquired pneumonia (12). Furthermore, clarithromycin accelerates the resolution of VAP and weaning from mechanical ventilation (13). However, little is known about the efficacy of azithromycin (AZM) against A. baumannii infections.
AZM is available as an intravenous formulation in many countries and is a major macrolide more often used in intensive care settings than the other macrolides. In this study, we investigated the efficacy of AZM in a murine model of pneumonia that mimics VAP caused by MDRAB.
MATERIALS AND METHODS
Bacterial strain.
Animals were infected with MDRAB strain AMU62852 (MICs, imipenem = 16 μg/ml, amikacin ≧ 32 μg/ml, cinoxacin ≧ 4 μg/ml), which was kindly provided by the Aichi Medical University Graduate School of Medicine. This strain has the blaoxa-51-like gene, which is specific to A. baumannii (14). The bacteria were stored at −80°C in a Microbank system (Pro-Lab Diagnostics, Ontario, Canada) until use.
Laboratory animals.
Male specific-pathogen-free ddY mice (age, 6 weeks; body weight, 30 to 35 g) were purchased from the Shizuoka Agricultural Cooperative Association for Laboratory Animals (Shizuoka, Japan). All animals were housed in a pathogen-free environment and received sterile food and water in the Laboratory Animal Center for Biomedical Science at Nagasaki University. The experimental protocols were approved by the Ethics Review Committee for Animal Experimentation at Nagasaki University.
Preparation of bacteria.
To prepare inocula, A. baumannii was cultured on a Muller-Hinton II agar plate at 37°C for 18 h and grown in Luria-Bertani (LB) broth for 6 h at 37°C. Bacteria were then harvested by centrifugation (3,000 × g, 15 min). The organisms were suspended in sterile saline and adjusted to a concentration of 1 × 107 CFU/ml, as estimated by turbidimetry.
Experimental model of VAP.
Disposable sterile plastic cut-down intravenous catheters with a 3-Fr (outer diameter, 1 mm; Atom Co., Tokyo, Japan) were used for tracheal intubation. The tubes were 5.0 mm in length and had a few slits at the proximal end to prevent clogging by oral secretions.
Mice were treated with cyclophosphamide intraperitoneally at day 4 (150 mg/kg of body weight) and day 1 (100 mg/kg) before infection. The intubation procedure was performed under pentobarbital anesthesia (40 mg/kg delivered by intraperitoneal injection). Intubation was induced as described previously (15). Briefly, the blunt end of the inner needle of an intravenous catheter (Angiocath; Becton, Dickinson Vascular Access, Sandy, UT) was inserted through the oral cavity with the outer sheath and the attached tube at the tip. The tube was advanced through the vocal cords into the trachea. The inner needle was retracted, followed by a final gentle push of the outer sheath to place the tube in the main bronchus. The placed tube remained in the main bronchus during the subsequent experiments. Mice were then inoculated with A. baumannii suspended in saline solution (0.05 ml; 5 × 105 CFU/mouse) through the outer sheath and the tube.
Treatment protocol.
AZM (Pfizer, Groton, CT) was administered subcutaneously every 24 h beginning at 3 h after inoculation. In the control group, phosphate-buffered saline (PBS) instead of AZM was injected into the mice. In humans, 500 mg is the recommended dose, which is almost equal to 10 mg/kg in mice. Furthermore, a higher dosage of AZM is required in mice than humans due to the more rapid liver metabolism in mice, resulting in an elimination half-life of 2.3 h compared to one of 68 h in humans (16, 17) Therefore, 10 mg/kg or 100 mg/kg was chosen as the dose of AZM in this study. Treatment was continued from day 0 (3 h after inoculation) through day 6 postinfection. Mouse survival (n = 11 for each group) was evaluated for 7 days. At 48 h postinfection, each group was analyzed by bacteriological (n = 5 to 6 for each group) and histopathological (n = 5 for each group) examination. Bronchoalveolar lavage (BAL) fluid (BALF) was also analyzed (n = 7 for each group). We performed the survival study and the bacteriological study separately.
Bacteriological and histopathological examination.
Mice were sacrificed by cervical dislocation at 48 h postinfection. The lungs were dissected under aseptic conditions and suspended in 1 ml of saline. The organs were homogenized using an homogenizer (AS One Co., Osaka, Japan), and homogenates were quantitatively inoculated onto Muller-Hinton II agar plates using serial dilutions, followed by incubation at 37°C for 18 h. For histopathological examination, lung specimens were fixed in 10% buffered formalin and stained with hematoxylin-eosin.
BAL and cytokine ELISA.
BAL was performed as described previously (18). Briefly, mice were treated and sacrificed at 48 h after inoculation. The chest was opened to expose the lungs and trachea, and a disposable sterile plastic cut-down intravenous catheter was inserted into the trachea. BAL was sequentially performed three times using 1.0 ml of saline each time. The recovered fluid fractions were pooled for each animal. Total cell counts were performed following Turk staining. For differential cell counts, the cells were centrifuged onto slides by centrifugation at 850 rpm for 2 min, and the slides were then stained with Diff-Quik stain. Differential cell counts were performed by counting 100 cells. The concentrations of interleukin-1β (IL-1β), interleukin-6 (IL-6), and macrophage inflammatory protein 2 (MIP-2) in BALF were assayed using mouse cytokine enzyme-linked immunosorbent assay (ELISA) test kits (R&D Systems, Minneapolis, MN).
Statistical analysis.
Survival analysis was performed using the log-rank test, and survival rates were calculated using the Kaplan-Meier method. Data are expressed as means ± standard error of the means (SEMs). Statistical significance was determined by using a one-way analysis of variance with Bonferroni's multiple-comparison test. P values below 0.05 were considered to indicate a statistically significant difference.
RESULTS
Pathogenicity of VAP model.
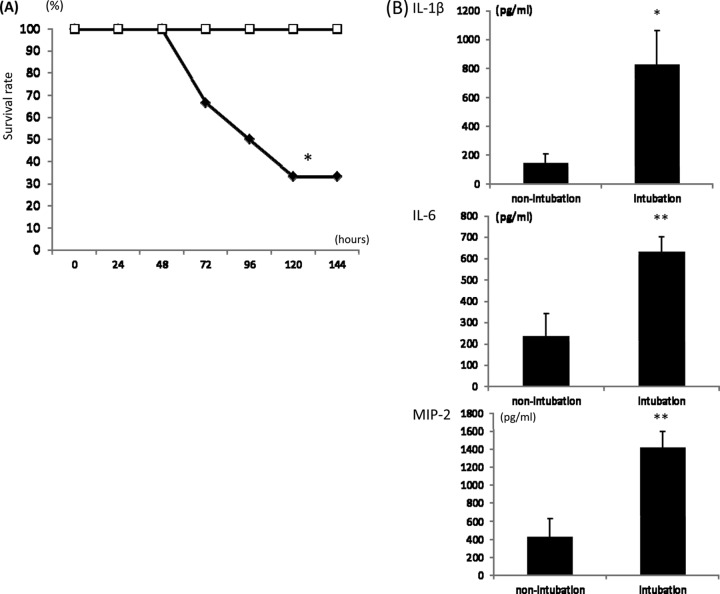
In preliminary experiments, the pathogenicity of A. baumannii was analyzed. After treatment with cyclophosphamide, bacteria were inoculated into mice with or without intubation. The mortality of the intubated group was higher than that of the nonintubated group (Fig. 1A), and the levels of inflammatory cytokines in BALF were higher in the intubated group (Fig. 1B). Thus, we used mice with intubation for the subsequent experiments.
Fig 1.
Effect of intubation in an MDRAB infection model. (A) In the survival study, mice with intubation (filled diamonds) and without intubation (empty squares) (n = 6 each) were inoculated with MDRAB. Survival was estimated at the indicated times, and the results are displayed as a Kaplan-Meier plot. (B) Cytokine levels in BALF were detected using ELISAs. Nonintubation represents mice that were not intubated but inoculated with MDRAB. Intubation indicates mice that were inoculated with MDRAB after intubation. The data are expressed as means ± SEMs (n = 8 in each group). *, P < 0.05 versus the nonintubation group; **, P < 0.01 versus the nonintubation group.
Survival.
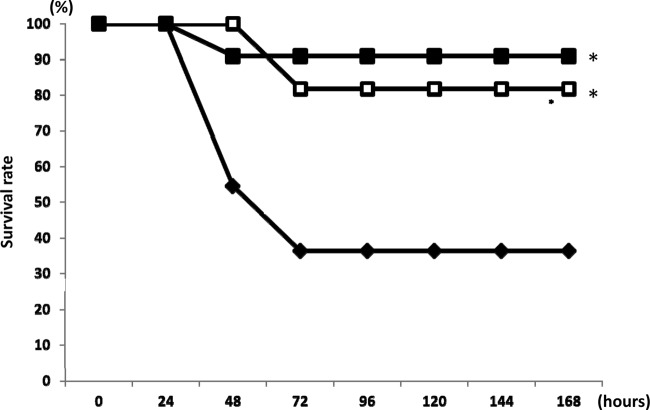
As shown in Fig. 2, the survival of mice over 7 days following infection with MDRAB was significantly longer in mice treated with AZM than mice in the control group (P < 0.05). There was no significant difference in the number of days of survival between the 10-mg/kg and 100-mg/kg AZM treatment groups.
Fig 2.
Survival study of mice inoculated with MDRAB after treatment with AZM. Eleven mice in each group were treated with AZM at a dose of 10 mg/kg (filled squares) or 100 mg/kg (empty squares) or were treated with PBS (filled diamonds). Survival was then estimated at the indicated times, and the results are displayed as a Kaplan-Meier plot. The survival times of the AZM-treated groups were significantly longer than those of the controls, as determined by the log-rank test. *, P < 0.05 versus the control group (treated with PBS solution).
Bacteriological examination.
Respiratory infection, which was calculated as the log10 number of CFU/ml, occurred in all inoculated mice. There were no significant differences between the numbers of viable bacteria between the control group and the AZM groups (control, 7.49 ± 0.58 log10 CFU/ml;10 mg/kg AZM, 7.64 ± 0.56 log10 CFU/ml; 50 mg/kg AZM, 7.49 ± 0.72 log10 CFU/ml).
Histopathological examination.
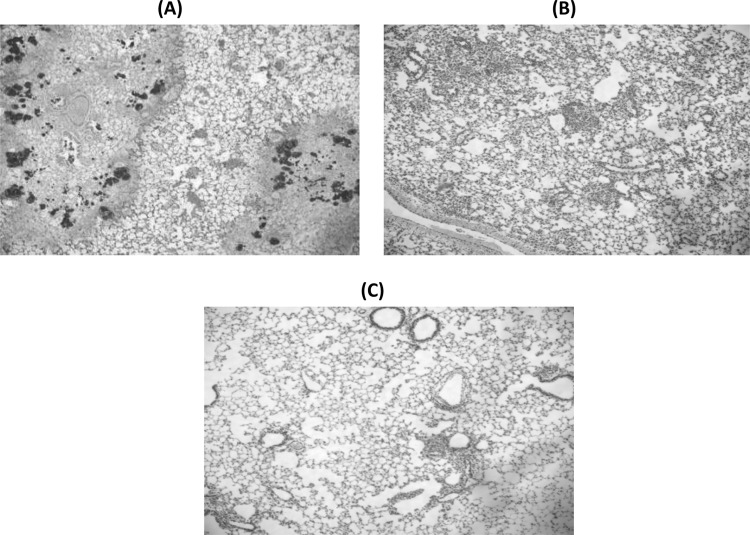
Light microscopic analysis of the hematoxylin-eosin-stained lungs of the control group at 48 h after inoculation revealed large numbers of inflammatory cells infiltrating the alveolar spaces. In the AZM groups, mild inflammatory changes were observed at the 10-mg/kg and the 100-mg/kg doses (Fig. 3B and C).
Fig 3.
Histopathological analysis of the lungs of infected mice following AZM treatment. Shown are sections of the lungs stained with hematoxylin-eosin at 48 h postinfection. (A) The lungs of the control (PBS-treated) group showed large numbers of inflammatory cells infiltrating the alveolar spaces and alveolar hemorrhage. In contrast, AZM at 10 mg/kg (B) and AZM at 100 mg/kg (C) inhibited lung inflammation due to infection (n = 5 in each group). Magnifications, ×40.
Analysis of BALF.
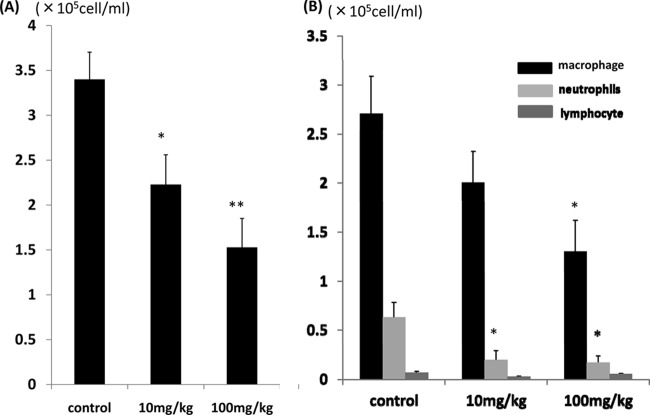
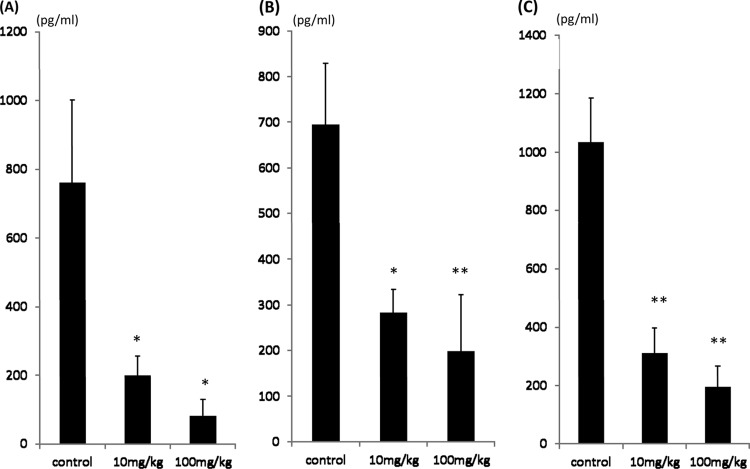
MDRAB induced an increase in the total number of cells and of neutrophils in BALF. The total numbers of cells (Fig. 4A) in BALF were significantly lower in the 10-mg/kg AZM group than the control group (P < 0.05) and even lower in the 100-mg/kg AZM group (P < 0.01). The numbers of neutrophils (Fig. 4B) were significantly lower in the AZM treatment groups (P < 0.05). To further examine the effects of AZM, inflammatory cytokine levels in BALF were analyzed. IL-1β (Fig. 5A), IL-6 (Fig. 5B), and MIP-2 (Fig. 5C) were all detected in the BALF of the control and the AZM groups. The AZM therapies significantly decreased the levels of IL-1β, IL-6, and MIP-2 compared to the levels in the control (P < 0.05 for each comparison). There were not any significant differences in cytokine levels between the 10- and 100-mg/kg dose groups.
Fig 4.
Effect of AZM on the numbers of inflammatory cells in infected mice. The total numbers of cells (A) and differential cell counts (B) in BALF in the control group and the AZM-treated (10 or 100 mg/kg) groups were compared. The total cell numbers were determined using a hemocytometer with Turk stain, and differential cell counts were done on slides stained with Diff-Quik. The data are expressed as means ± SEMs. *, P < 0.05 versus the control group (n = 7 in each group); **, P < 0.01 versus the control group (n = 7 in each group).
Fig 5.
Effect of AZM on inflammatory cytokines in the BALF of infected mice. The cytokine levels in BALF of the control group and the AZM groups (10 mg and 100 mg/kg) were detected using ELISAs: IL-1β (A), IL-6 (B), and MIP-2 (C). The data are expressed as means ± SEMs. *, P < 0.05 versus the control group (n = 7 in each group); **, P < 0.01 versus the control group (n = 7 in each group).
DISCUSSION
VAP is a leading cause of nosocomial infection-related mortality. VAP is difficult to treat because patients usually have serious concomitant diseases and sometimes cannot undergo an invasive examination. A. baumannii has become one of the leading causes of VAP. Carbapenems are recommended as empirical treatments for VAP associated with A. baumannii. However, carbapenem-resistant A. baumannii is becoming a serious problem (2, 19). Furthermore, A. baumannii easily acquires resistance to antibiotics. Thus, treatment of VAP caused by A. baumannii is becoming more challenging.
Giamarellos-Bourboulis et al. reported that clarithromycin (CLR) accelerates the resolution of VAP and weaning from mechanical ventilation (13). In their study, A. baumannii was one of the pathogens that most frequently caused VAP. Because macrolides do not exhibit antibiotic effects against A. baumannii, it is thought that they act via mechanisms apart from the antibiotic effect.
In the present study, we investigated the in vivo activity of AZM against MDRAB using a murine model of VAP. In general, MDRAB infections tend to occur in immunosuppressed patients (20). In our previous study, the use of immunosuppressive drugs tended to be the factor responsible for poor clinical outcomes after the acquisition of A. baumannii infections (21). Thus, we employed a neutropenic mouse model treated with cyclophosphamide, as was done in previous studies (22, 23). Furthermore, because the insertion of an endotracheal tube is a risk factor for infection in patients on mechanical ventilation, we intubated the mice with a sterile tube through which MDRAB suspended in saline was delivered (24). This model could reflect the clinical background of A. baumannii infection.
In preliminary experiments, the mortality of the intubated group was higher than that of the nonintubated group (Fig. 1A) and the levels of inflammatory cytokines were higher in the intubated group (Fig. 1B). These results indicate that intubation can accelerate the pneumonia induced by MDRAB. It is difficult to explain the reason for this clearly. However, a possible explanation may be that impairment of the bronchial surface and the delay of bacterial clearance easily cause pathological invasion by MDRAB. In this study, AZM therapy significantly prolonged survival compared to that for the controls (Fig. 2). Furthermore, the survival rate of the low-dose-AZM-treated group (10 mg/kg) was equal to that of the high-dose group (100 mg/kg). These findings suggest that AZM is effective at treating VAP caused by MDRAB. In vitro, some reports mentioned that AZM in addition to other antibiotics has a synergistic effect against MDRAB (25–27). To our knowledge, there have been no reports about the efficacy of AZM against MDRAB in vivo. Surprisingly, AZM treatment alone, without use in combination with other antibiotics, prolonged survival. In our study, there were no significant differences between the level of respiratory infection in the control and in the AZM-treated groups following bacteriological examination. This result indicates that AZM has no antibiotic effect against MDRAB. Furthermore, the AZM-treated groups showed improved histopathological findings (Fig. 3) and decreased numbers of inflammatory cells in BALF (Fig. 4). In addition, AZM treatment significantly decreased the levels of inflammatory cytokines in BALF (Fig. 5). Terao et al. reported that AZM and CLR suppressed the production of inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor alpha by lipopolysaccharide stimulation in mice (28). It has been reported that AZM attenuated acute airway inflammation in the mouse model of paramyxoviral bronchiolitis (29). These data support our results. It is considered that AZM suppresses the excessive inflammatory cytokines and that this suppression prevents the progression to acute lung injury and multiple-organ failure. This may lead to the improvement in the period of survival in the mouse model of MDRAB VAP. Immunomodulatory therapies such as macrolides in immunocompromised hosts can be a beneficial strategy for the treatment of Acinetobacter infections. However, the development of such therapies is dependent on a better understanding of the host immune response to A. baumannii (30). Further studies should investigate the detailed mechanism of action of AZM on the virulence factors exhibited by A. baumannii and host signal pathways. Future studies will need to examine the efficacy of AZM in combination with antibiotics to which MDRAB is sensitive.
In conclusion, AZM attenuates lung inflammation in a murine model of VAP caused by MDRAB. Our results suggest that AZM is useful for the treatment of VAP caused by MDRAB because of its anti-inflammatory effects.
ACKNOWLEDGMENTS
This research was supported by Meiji Seika Pharma Co., Ltd., Tokyo, Japan, by a Grant-in-Aid for Scientific Research (no. 21591294 to K. Yanagihara) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and by a grant from the Global Centers of Excellence Program, Nagasaki University.
Footnotes
Published ahead of print 3 June 2013
REFERENCES
- 1. Young LS, Sabel AL, Price CS. 2006. Epidemiologic, clinical, and economic evaluation of an outbreak of clonal multi-resistant Acinetobacter baumannii infection in a surgical intensive care unit. Infect. Control Hosp. Epidemiol. 28:1247–1254 [DOI] [PubMed] [Google Scholar]
- 2. Munoz-Price LS, Weinstein RA. 2008. Acinetobacter infection. N. Engl. J. Med. 358:1271–1281 [DOI] [PubMed] [Google Scholar]
- 3. Maragakis LL, Perl TM. 2008. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 46:1254–1263 [DOI] [PubMed] [Google Scholar]
- 4. Fournier PE, Richet H. 2006. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 42:692–699 [DOI] [PubMed] [Google Scholar]
- 5. Rello J, Ulldemolins M, Lisboa T, Koulenti D, Manez R, Martin-Loeches I, De Waele JJ, Putensen C, Guven M, Deja M, Diaz E. 2011. Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur. Respir. J. 37:1332–1339 [DOI] [PubMed] [Google Scholar]
- 6. Lockhart SR, Abramson MA, Beekmann SE, Gallagher G, Riedel S, Diekema DJ, Quinn JP, Doern GV. 2007. Antimicrobial resistance among gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J. Clin. Microbiol. 45:3352–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reddy T, Chopra T, Macchaim D, Pogue JM, Alangaden G, Salimnia H, Boikov D, Navon-Venezia S, Akins R, Selman P, Dhar S, Kaye KS. 2010. Trends in antimicrobial resistance of Acinetobacter baumannii isolates from a metropolitan Detroit health system. Antimicrob. Agents Chemother. 54:2235–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giske CG, Monnet DL, Cars O, Carmeli Y. 2008. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob. Agents Chemother. 52:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joly-Guillou ML, Wolff M, Pocidalo JJ, Walker F, Carbon C. 1997. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob. Agents Chemother. 41:345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodríguez-Hernández MJ, Pachón J, Pichardo C, Cuberos L, Ibáñez-Martínez J, García-Curiel A, Caballero FJ, Moreno I, Jiménez-Mejías ME. 2000. Imipenem, doxycycline and amikacin in monotherapy and in combination in Acinetobacter baumannii experimental pneumonia. J. Antimicrob. Chemother. 45:493–501 [DOI] [PubMed] [Google Scholar]
- 11. Montero A, Ariza J, Corbella X, Doménech A, Cabellos C, Ayats J, Tubau F, Ardanuy C, Gudiol F. 2002. Efficacy of colistin versus beta-lactams, aminoglycosides, and rifampin as monotherapy in a mouse model of pneumonia caused by multiresistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 46:1946–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnold FW, Summersgill JT, Lajoie AS, Peyrani P, Marrie TJ, Rossi P, Blasi F, Fernandez P, File TM, Jr, Rello J, Menendez R, Marzoratti L, Luna CM, Ramirez JA. 2007. A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 175:1086–1093 [DOI] [PubMed] [Google Scholar]
- 13. Giamarellos-Bourboulis EJ, Pechère JC, Routsi C, Plachouras D, Kollias S, Raftogiannis M, Zervakis D, Baziaka F, Koronaios A, Antonopoulou A, Markaki V, Koutoukas P, Papadomichelakis E, Tsaganos T, Armaganidis A, Koussoulas V, Kotanidou A, Roussos C, Giamarellou H. 2008. Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin. Infect. Dis. 46:1157–1164 [DOI] [PubMed] [Google Scholar]
- 14. Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL. 2006. Identification of Acinetobacter baumannii by detection of the blaoxa-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 44:2974–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaneko Y, Yanagihara K, Kuroki M, Ohi H, Kakeya H, Miyazaki Y, Higashiyama Y, Hirakata Y, Tomono K, Kadota J, Kohno S. 2001. Effects of parenterally administered ciprofloxacin in a murine model of pulmonary Pseudomonas aeruginosa infection mimicking ventilator-associated pneumonia. Chemotherapy 47:421–429 [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann N, Lee B, Hentzer M, Rasmussen TB, Song Z, Johansen HK, Givskov M, Høiby N. 2007. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(−/−) mice. Antimicrob. Agents Chemother. 51:3677–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conte JE, Jr, Golden J, Duncan S, McKenna E, Lin E, Zurlinden E. 1996. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob. Agents Chemother. 40:1617–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yanagihara K, Seki M, Cheng PW. 2001. Lipopolysaccharide induces mucus cell metaplasia in mouse lung. Am. J. Respir. Cell Mol. Biol. 24:66–73 [DOI] [PubMed] [Google Scholar]
- 19. Carry RB, Banerjee SN, Srinivasan A. 2006. Multidrug-resistant Acinetobacter infections, 1995–2004. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., p 27–30 American Society for Microbiology, Washington, DC [Google Scholar]
- 20. McConnell MJ, Actis L, Pachón J. 2013. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol. Rev. 37:130–155 [DOI] [PubMed] [Google Scholar]
- 21. Yamada K, Yanagihara K, Araki N, Harada Y, Morinaga Y, Akamatsu N, Matsuda J, Izumikawa K, Kakeya H, Yamamoto Y, Hasegawa H, Kohno S, Kamihira S. 2012. Clinical characteristics of tertiary hospital patients from whom Acinetobacter calcoaceticus-Acinetobacter baumannii complex strains were isolated. Intern. Med. 51:51–57 [DOI] [PubMed] [Google Scholar]
- 22. Eveillard M, Soltner C, Kempf M, Saint-André JP, Lemarié C, Randrianarivelo C, Seifert H, Wolff M, Joly-Guillou ML. 2010. The virulence variability of different Acinetobacter baumannii strains in experimental pneumonia. J. Infect. 60:154–161 [DOI] [PubMed] [Google Scholar]
- 23. Yuan Z, Ledesma KR, Singh R, Hou J, Prince RA, Tam VH. 2010. Quantitative assessment of combination antimicrobial therapy against multidrug-resistant bacteria in a murine pneumonia model. J. Infect. Dis. 201:889–897 [DOI] [PubMed] [Google Scholar]
- 24. Byers J, Sole M. 2000. Analysis of factors related to the development of ventilator-associated pneumonia: use of existing databases. Am. J. Crit. Care 9:344–349 [PubMed] [Google Scholar]
- 25. Fernández-Cuenca F, Martínez-Martínez L, Pascual A, Perea EJ. 2003. In vitro activity of azithromycin in combination with amikacin, ceftazidime, ciprofloxacin or imipenem against clinical isolates of Acinetobacter baumannii. Chemotherapy 49:24–26 [DOI] [PubMed] [Google Scholar]
- 26. Wareham DW, Bean DC. 2006. In-vitro activity of polymyxin B in combination with imipenem, rifampicin and azithromycin versus multidrug resistant strains of Acinetobacter baumannii producing OXA-23 carbapenemases. Ann. Clin. Microbiol. Antimicrob. 5:10. 10.1186/1476-0711-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Timurkaynak F, Can F, Azap OK, Demirbilek M, Arslan H, Karaman SO. 2006. In vitro activities of non-traditional antimicrobials alone or in combination against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii isolated from intensive care units. Int. J. Antimicrob. Agents 27:224–228 [DOI] [PubMed] [Google Scholar]
- 28. Terao H, Asano K, Kanai K, Kyo Y, Watanabe S, Hisamitsu T, Suzaki H. 2003. Suppressive activity of macrolide antibiotics on nitric oxide production by lipopolysaccharide stimulation in mice. Mediators Inflamm. 12:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beigelman A, Mikols CL, Gunsten SP, Cannon CL, Brody SL, Walter MJ. 2010. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir. Res. 11:90. 10.1186/1465-9921-11-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mortensen BL, Skaar EP. 2012. Host-microbe interactions that shape the pathogenesis of Acinetobacter baumannii infection. Cell. Microbiol. 14:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]