Abstract
Conventional MIC testing of amphotericin B results in narrow MIC ranges challenging the detection of resistant strains. In order to discern amphotericin B pharmacodynamics, the in vitro activity of amphotericin B was studied against Aspergillus isolates with the same MICs by using a new in vitro pharmacokinetic/pharmacodynamic (PK/PD) model that simulates amphotericin B human plasma levels. Clinical isolates of Aspergillus fumigatus, A. terreus, and A. flavus with the same Clinical and Laboratory Standards Institute modal MICs of 1 mg/liter were exposed to amphotericin B concentrations following the plasma concentration-time profile after single-bolus administration with Cmax values of 0.6, 1.2, 2.4, and 4.8 mg/liter. Fungal growth was monitored for up to 72 h based on galactomannan production. Complete growth inhibition was observed only against A. fumigatus with amphotericin B with a Cmax of ≥2.4 mg/liter. At the lower Cmax values 0.6 and 1.2 mg/liter, significant growth delays of 34 and 52 h were observed, respectively (P < 0.001). For A. flavus, there was no complete inhibition but a progressive growth delay of 1 to 50 h at an amphotericin B Cmax of 0.6 to 4.8 mg/liter (P < 0.001). For A. terreus, the growth delay was modest (up to 8 h) at all Cmaxs (P < 0.05). The Cmax (95% confidence interval) associated with 50% activity for A. fumigatus was 0.60 (0.49 to 0.72) mg/liter, which was significantly lower than for A. flavus 3.06 (2.46 to 3.80) mg/liter and for A. terreus 7.90 (5.20 to 12.29) mg/liter (P < 0.001). A differential in vitro activity of amphotericin B was found among Aspergillus species despite the same MIC in the order A. fumigatus > A. flavus > A. terreus in the in vitro PK/PD model, possibly reflecting the different concentration- and time-dependent inhibitory/killing activities amphotericin B exerted against these species.
INTRODUCTION
Amphotericin B (AMB) is an antifungal drug of major importance in the treatment of invasive aspergillosis (1). It is a highly lipophilic and amphoteric molecule that interacts with fungal cell membrane, forming pores and disrupting its integrity (2). Due to its unique mechanism of action, it demonstrates a wide range of pharmacodynamic effects and broad spectrum of antifungal activity. However, conventional MIC testing of amphotericin B resulted in narrow MIC ranges within one to two 2-fold dilutions challenging the detection of resistant strains (3–5). Efforts to develop in vitro assays that separate susceptible and resistant strains using richer media or gradient drug concentrations strips were unsuccessful (3, 5). Species-specific epidemiological cutoff values (ECV) have been proposed for amphotericin B and Aspergillus spp. based on Clinical and Laboratory Standards Institute (CLSI) broth microdilution methodology, with the Aspergillus terreus ECV being one dilution higher than the A. fumigatus and A. flavus ECV (6).
In addition to inhibitory activity captured by the MIC, amphotericin B exerts a range of different pharmacodynamic effects such as postantifungal effect and concentration-dependent killing (7). All of these effects are usually determined after fungal exposure to constant drug concentrations (2). However, in vivo, fungus is exposed to nonconstant amphotericin B concentrations as the drug undergoes metabolism, distribution, and excretion. In particular, its plasma levels follow a triphasic time-concentration profile characterized by the alpha-phase observed within the first 4 h after administration with a half-life of <1 h, the beta-phase observed within 4 to 24 h after administration with a half-life of 6 to 10 h, and the gamma phase observed >24 h of administration with a half-life of >120 h (8). Simulating this time-concentration profile in vitro is a challenge because amphotericin B binds to plastic surfaces and degrades over time (9).
We recently developed an in vitro model that simulated human pharmacokinetics of antifungal drugs and enabled to study the pharmacodynamics of decreasing drug concentrations as in human plasma (10). This pharmacokinetic/pharmacodynamic (PK/PD) model showed considerable differences of voriconazole activity against Aspergillus species which had the same MICs indicating that studying the in vitro activity of decreasing drug concentrations provides unique information of pharmacodynamic effects of antifungal drugs (11). With this model, the time- and concentration-dependent pharmacodynamic properties of antifungal drugs can be studied, and PK/PD analysis simulating human pharmacokinetics can be performed.
We therefore studied the activity of amphotericin B against A. fumigatus, A. flavus, and A. terreus strains with similar MICs with the new in vitro PK/PD model simulating single-dose pharmacokinetics of amphotericin B in human plasma and monitoring Aspergillus growth over time with galactomannan production. Despite the same MICs, important pharmacodynamic differences were found among the three species with amphotericin B being less active against A. flavus and A. terreus than against A. fumigatus reflecting differences in inhibitory, killing, and post-drug exposure effects.
MATERIALS AND METHODS
Strains.
Three clinical strains of A. fumigatus, A. flavus, and A. terreus isolated from patients with invasive pulmonary aspergillosis were studied. The MICs, as determined thrice with the CLSI broth microdilution method, were 1 mg/liter for A. fumigatus, 1 mg/liter for A. flavus, and 1 to 2 mg/liter (mode 1, mg/liter) for A. terreus (12, 13). The A. terreus strain was included because of its known reduced susceptibility to amphotericin B. The strains were maintained at −70°C in 10% glycerol and cultured twice in Sabouraud dextrose agar at 30°C for 5 to 7 days. A conidial suspension was prepared in normal saline with 1% Tween 20. Conidia were counted with a Newbauer chamber in order to obtain a final suspension 105 CFU/ml, and their concentration was confirmed by quantitative cultures on Sabouraud dextrose agar.
Antifungal susceptibility testing.
In order to explore the in vitro susceptibility of the three isolates by using other methodologies, the isolates were also tested with the gradient concentration strip method Liofilchem MIC test strips (MTS) (Varelas SA, Athens, Greece) according to the manufacturer's instructions and the 2,3-bis {2-methoxy-4-nitro-5-[(sulfenylamino)carbonyl]-2H-tetrazolium-hydroxide} (XTT) methodology as previously described (14). Briefly, for MTS method agar plates with RPMI 1640, morpholinepropanesulfonic acid (MOPS), and 2% glucose were inoculated in three directions with a cotton swab dipped into a 0.5 McFarland conidial inoculum, and the MTS was applied and incubated at 35°C for 24 and 48 h. The MIC was determined as the drug concentration at which the border of the elliptical inhibition zone corresponding to 100% inhibition intersected the strip. For the XTT methodology, 2-fold serial dilutions of amphotericin B in RPMI 1640-MOPS in 96-well flat-bottom microtitration plates were inoculated with 1 × 104 to 5 × 104 CFU/ml, incubated for 48 h when 0.1 mg of XTT/ml and 25 μM menadione was added into each well, and further incubated for 2 h at 35°C when the absorbance at 450 nm was measured. Then, the percent growth in each well was calculated compared to growth in the drug-free control. The MIC was determined as the lowest drug concentration with <10% growth. Furthermore, the minimal fungicidal concentration (MFC) was determined by using an XTT methodology as previously described (13). Briefly, after XTT MIC determination, all clear wells were washed with saline and fresh medium was added. After incubation for 24 h at 35°C, XTT-menadione was added, and the percent growth was calculated based on the absorbance at 450 nm. The MFC was determined as the lowest drug concentration showing <10% growth. All tests were performed three times.
Antifungal drug and medium.
Amphotericin B (AMB; Fungizone, Bristol-Myers) was reconstituted at 10,000 mg/liter according to the manufacturer's instructions and stored at −70°C. The medium contained 10.4 g of RPMI 1640/liter with glutamine but without sodium bicarbonate (Sigma-Aldrich, St. Louis, MO) and 0.165 M MOPS buffer (pH 7.0; Invitrogen, Carlsbad, CA), with 100 mg of chloramphenicol (Sigma-Aldrich)/liter.
In vitro pharmacokinetic/pharmacodynamic model.
The in vitro pharmacokinetic simulation model consists of (i) a glass beaker containing 700 ml of medium (external compartment [EC]), in which is placed (ii) a 10-ml dialysis tube (internal compartment [IC]), the wall of which consists of cellulose permeable membrane, allowing the free diffusion of molecules with a molecular mass of <20 kDa, and (iii) a peristaltic pump (Minipuls Evolution, Gilson, France), which removes the content of EC and adds medium at a rate equivalent to drug removal from human serum (10). The conidial suspension is inoculated in the IC within which the growing fungus and its derivative galactomannan (molecular mass, 20 to 60 kDa) remain trapped, whereas nutrients and drug diffuse freely between the IC and the EC. The concentration of the galactomannan increases with fungal growth. The drug is injected into the EC, and its concentration is adjusted by the pump to correspond to the average half-life observed in human plasma after the intravenous administration of amphotericin B. The EC was covered with aluminum foil in order to minimize light exposure and placed on a heated magnetic stirrer (37°C). Before starting each experiment, the temperature and flow rate were controlled. All experiments were repeated twice.
Determination of amphotericin B concentrations.
The drug levels in the IC were determined by a microbiological method using the strain Paecilomyces variotii ATTC 22319, which is susceptible to AMB (15). Specifically, P. variotii conidia at final concentration 5 × 104 CFU/ml were inoculated into prewarmed at 54°C RPMI 1640 medium plus MOPS with 15 g of agar/liter and poured into plastic plates (10 by 10 cm). After solidification of the agar, 1-cm-diameter holes were opened and filled with 100 μl of known drug dilutions (range, 0.25 to 16 mg/liter), as well as 100 μl of IC samples. The plates were incubated at 35°C for 24 h when the diameters of inhibition zones were measured. Unknown drug concentrations in the IC samples were determined using the standard curve constructed from known drug concentrations and corresponding diameters of inhibition zones.
Pharmacokinetic analysis.
Several clinically relevant AMB doses were simulated in the in vitro model with Cmax values in human plasma of 0.6, 1.2, 2.4, and 4.8 mg/liter (8, 16, 17). After we took into account any loss of amphotericin B during the experiments due to degradation and surface binding, we adjusted the flow rate in order to approximate the plasma concentration profile of amphotericin B in humans with an alpha phase with a short half-life of <1 h observed within 4 h after drug administration, followed by a beta phase with a longer half-life of 6 to 10 h observed 4 to 24 h after drug administration and a gamma phase with a half-life of >120 h observed >24 h after drug administration (8). Amphotericin B concentrations were determined at 0, 4, 6, 8, 24, 44, 48, and 72 h after the introduction of the drug in the IC using the bioassay. The data were analyzed by nonlinear regression based on a three-compartment model described by the equation C = Cαekαt + Cβekβt + Cγekγt, where kα, kβ, and kγ are the rate constants, Cα, Cβ, and Cγ are the y intercepts for the alpha, beta, and gamma phases, respectively, and C is the concentration at a given time t. The half-lives of the alpha, beta, and gamma phases were calculated for EC and IC separately using the equations t1/2,α = kα/ln(2), t1/2,β = kβ/ln(2), and t1/2,γ = kγ/ln(2), respectively, and were compared to the corresponding values observed in human plasma.
Determination of fungal growth.
Fungal growth in the IC was assessed in samples of 100 μl at regular time intervals by determining galactomannan production using an enzyme-linked immunosorbent assay (Platellia; Bio-Rad, Athens, Greece). Samples were diluted with 200 μl of saline in order to reach a final volume of 300 μl before processing. The results were expressed as a galactomannan index (GI) according to the manufacturer's instructions. Galactomannan levels were also determined in the EC in order to ensure that no galactomannan had escaped from the IC.
Real-time PCR conidial equivalent was used as an alternative biomarker of fungal growth and killing. Aspergillus DNA was extracted from 200-μl samples from the IC of the in vitro PK/PD model after 0 and 72 h with the Qiagen DNA blood minikit (Roche Diagnostics, Athens, Greece) after enzymatic (incubation with proteinase K at 56°C for 10 min) and mechanical (1-min vortexing with glass beads) extraction as previously described (18). Real-time PCR was performed with a previously described assay (2Asp assay) using Aspergillus specific primers (ASF1 and ADR1) and probe (ASP28P) (19). The threshold cycle (CT) of each sample, which identifies the cycle number during PCR when fluorescence exceeds a threshold value determined by the software, was converted to conidial equivalent (CE) A. fumigatus DNA by interpolation from a six-point standard curve of CT values obtained with 103 to 108 Aspergillus CFU/ml. The reduction of the PCR CE after 72 h of incubation compared to 0 h was calculated for each species and amphotericin B dose.
Pharmacodynamic analysis.
The in vitro pharmacodynamics of each amphotericin B dose and Aspergillus species were determined based on the GI-time relationship analyzed with the Emax model: E = Emin + Emax·T γ/(T γ + T50), where E is the GI (dependent variable), Emax and Emin, are the maximum and minimum GIs, respectively, T is the incubation time (independent variable), T50 is the time corresponding to 50% of the Emax, and γ is the slope of the curve. In addition, the area under the GI-time curve (AUCGI) was calculated for each amphotericin B dose. As shown previously, the parameters Emax, γ, and T50 describe the extent, rate, and time of galactomannan production, respectively, whereas the AUCGI is a surrogate marker of fungal growth (10, 11). The higher the AUCGI, the greater is the fungal growth. The percentage of fungal growth at each dose was calculated based on the AUCGI of each dose divided by the AUCGI of the growth control. Based on all of these parameters, the in vitro activity of amphotericin B dose against each Aspergillus species was estimated. Finally, the in vitro PK/PD relationship AUCGI versus Cmax was plotted for each species and analyzed by using the Emax model.
Statistical analysis.
All analysis was performed with the software Prism 5.01 (GraphPad, Inc., La Jolla, CA). All Emax models were globally fitted to the data with Emax and Emin shared among data sets. Comparisons between Emax model parameters of different amphotericin B doses and Aspergillus species were assessed using an extra sum-of-squares F test. A P value of <0.05 was considered statistically significant.
RESULTS
Antifungal susceptibility testing.
The MTS MICs for A. fumigatus, A. flavus, and A. terreus were 0.75 mg/liter, 2 to 3 mg/liter, and 1 to 1.5 mg/liter after 24 h and 2 to 3 mg/liter, >32 mg/liter, and >32 mg/liter after 48 h, respectively. The XTT MICs and MFCs were 1 to 2 mg/liter and 1 to 2 mg/liter for A. fumigatus, 2 to 2 mg/liter and 2 to 4 mg/liter for A. flavus, and 1 to 2 mg/liter and 8 to 16 mg/liter for A. terreus, respectively.
Bioassay for amphotericin B.
The amphotericin B concentrations detected with the bioassay ranged from 0.25 to 16 mg/liter, and the lowest limit of detection was 0.25 mg/liter. Across all experiments performed on the same and different days, the diameter of inhibition zone correlated linearly with the amphotericin B concentration (r2 > 0.77, global r2 = 0.84). The coefficient of variation ranged from 5 to 25% (average, 15%) for all concentrations.
Pharmacokinetic analysis.
The Cmax values in the IC were 0.76 to 0.78, 1.05 to 1.10, 2.5 to 2.7, and 3.9 to 4.4 mg/liter and the AUCs were 4.5 to 5, 8 to 8.6, 31.9 to 33.2, and 64.8 to 67.9 mg · h/liter, respectively, with t1/2,α = 0.2 to 2 h, t1/2,β = 10 to 17 h, and t1/2,γ = 71 h for the simulated amphotericin B doses with Cmax values of 0.6, 1.2, 2.4, and 4.8 mg/liter, respectively. Because of the low detection limit of the bioassay, a gamma phase was observed only for the highest dose of amphotericin B with a Cmax of 4.8 mg/liter. These values were within those observed in human plasma with the largest deviations observed at lower doses (Fig. 1).
Fig 1.
Pharmacokinetic analysis of simulated amphotericin B doses in humans (dashed lines) and in the in vitro pharmacokinetic/pharmacodynamic model (solid lines) with Cmax values of 0.6 mg/liter (light gray), 1.2 mg/liter (medium gray), 2.4 mg/liter (dark gray), and 4.8 mg/liter (black), respectively. Symbols: ▽, IC; ○, expected.
Pharmacodynamic (PD) analysis.
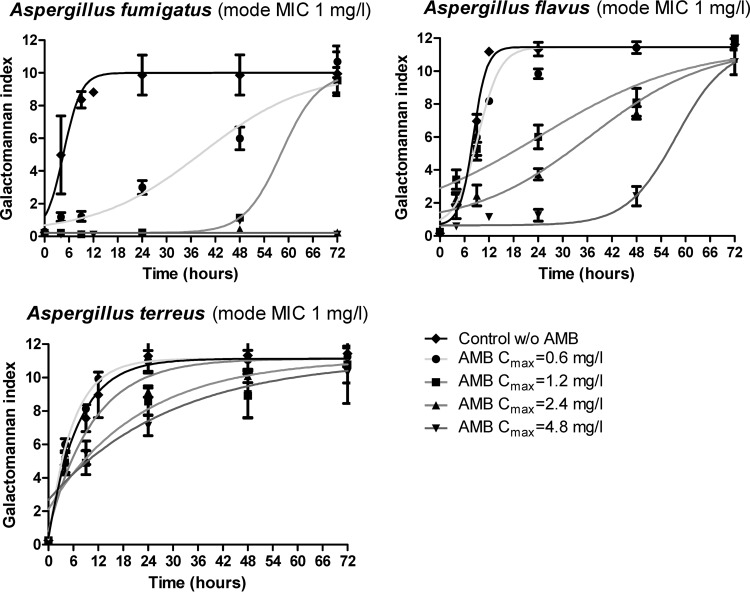
The GI-time curves were described very well with the Emax model (R2 > 0.86), and they were characterized by the same Emax but with different slopes and T50s for the different amphotericin B doses and Aspergillus species. Among all species and doses tested, complete inhibition of galactomannan production was observed only against A. fumigatus with amphotericin B doses corresponding to a Cmax of ≥2.4 mg/liter (Fig. 2). At lower doses, a significant delay in galactomannan production was observed with mean ± the standard error of the mean T50 values of 38.1 ± 2.3 h for Cmax 0.6 mg/liter and 57.9 ± 2.7 h for Cmax 1.2 mg/liter compared to 4.2 ± 0.4 h for the drug-free control (P < 0.001). For A. flavus, there was no complete inhibition but a progressive delay of galactomannan production with increasing amphotericin B doses since the T50 increased from 8.2 ± 0.6 h for the growth control to 9.3 ± 0.6 h at amphotericin B doses with a Cmax of 0.6 mg/liter, 24.3 ± 3.2 h at a Cmax of 1.2 mg/liter, 36.7 ± 3.1 h at a Cmax of 2.4 mg/liter, and 57.8 ± 2.7 h at a Cmax of 4.8 mg/liter (P < 0.001). For A. terreus, the delay in galactomannan production was modest since the T50 of 4 ± 0.4 h for the growth control increased to 12.6 ± 3.3 h at the highest dose of amphotericin B with a Cmax of 4.8 mg/liter (P = 0.013). The differences among the tree species in galactomannan production with the two high doses of amphotericin B with Cmax values of 2.4 and 4.8 mg/liter were confirmed with real-time PCR results with PCR CE at 72 h being reduced by 0.7 and 0.8 log10CE of A. fumigatus, 0.1 and 0.4 log10CE of A. flavus and increased by 1.5 and 0.1 log10CE of A. terreus, respectively (data not shown).
Fig 2.
Single-dose pharmacodynamic analysis of simulated amphotericin B doses with Cmax values of 0.6, 1.2, 2.4, and 4.8 mg/liter against A. fumigatus, A. flavus, and A. terreus isolates with a CLSI mode MIC of 1 mg/liter, as determined by using the GI in the in vitro PK/PD model.
Finally, the in vitro activity of amphotericin B against the three Aspergillus species was compared by constructing PK/PD curves. In order to quantify the effect of each amphotericin B dose at the entire 72-h period of incubation, the AUCGI was calculated for each dose and species as a surrogate marker of fungal growth and plotted against the corresponding Cmax values (Fig. 3). The in vitro PK/PD relationship followed a sigmoid pattern (global R2 = 0.99). The Cmax (95% confidence interval) associated with 50% activity for A. fumigatus was 0.60 (0.49 to 0.72), which was statistically significant lower than the corresponding Cmax values against A. flavus (3.06 [2.46 to 3.80]) and A. terreus (7.90 [5.20 to 12.29]) (P < 0.001).
Fig 3.
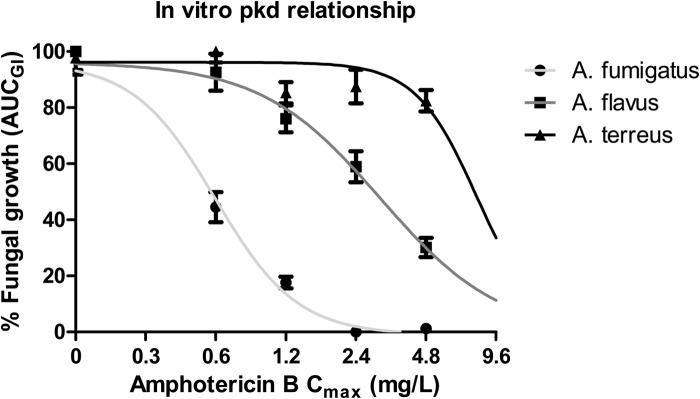
Single-dose exposure-efficacy relationship of amphotericin B against each Aspergillus species with CLSI mode MICs of 1 mg/liter for A. fumigatus, A. flavus, and A. terreus in the in vitro PK/PD model simulating amphotericin B human plasma levels based on the increasing amphotericin B Cmax values (maximum concentration) and the GI as a marker of fungal growth. Symbols: ●, A. fumigatus; ■, A. flavus; ▲, A. terreus.
DISCUSSION
Important pharmacodynamic differences of amphotericin B against the three Aspergillus species were found in the recently developed in vitro PK/PD model where conidia were exposed to decreasing drug concentrations simulating the plasma concentration-time profile of amphotericin B. Despite the same MICs, the strongest in vitro activity of amphotericin B was found against A. fumigatus, followed by A. flavus and A. terreus. The XTT and gradient concentration strip methods showed minor differences in the MICs for the three isolates which clustered within one to two 2-fold dilutions, emphasizing the problem of narrow amphotericin B MIC ranges with these techniques. However, the 48-h MTS MICs were similar for A. flavus and A. terreus and lower for A. fumigatus, whereas XTT MFCs were similar for A. fumigatus and A. flavus and higher for A. terreus advocating for the different pharmacodynamic effects amphotericin B possessed against different Aspergillus species.
Studying the effects of decreasing concentrations of antifungal drugs may provide information about pharmacodynamic properties related to the sub-MIC effect, the postantifungal effect, and time- and concentration-dependent activities. These effects can be quantified by a surrogate marker of fungal growth based on galactomannan production kinetics that captures any difference regarding the antifungal effects described above. Differential antifungal activity was also previously found with the present in vitro PK/PD model for voriconazole against the three Aspergillus species with identical MICs emphasizing the importance of studying nonconstant drug concentrations (10, 11).
The findings of the present study are in agreement with previous time-kill assays wherein supra-MIC (i.e., 4× and 20× the MIC) concentrations of amphotericin B killed A. fumigatus but not A. terreus (20). MFCs of amphotericin B were similar against A. fumigatus and A. flavus and much higher against A. terreus (13). However, MFC/MIC ratios for A. fumigatus were 1 twofold dilution lower than those for A. flavus, differentiating the in vitro activity of amphotericin B against these two species (13). In addition, the three species were previously found to differ also in the post-drug exposure effects since 4× and 1× the MIC of amphotericin B demonstrated a postantifungal effect of >4 h against A. fumigatus and of <4 h against A. flavus and A. terreus (21). The time-dependent activity of amphotericin B inhibition also differed among the three Aspergillus species (22). Exposure of Aspergillus conidia to supra-MICs for 8 h resulted in a significant amount of metabolic activity for A. terreus (16%), less so for A. flavus (8%), and even less for A. fumigatus (5%). Furthermore, despite the same concentration-effect curves of amphotericin B for A. fumigatus and A. flavus at 48 h, the inhibitory concentration-effect curve after 8 h of exposure to amphotericin B were shifted to the left for A. fumigatus but not for A. flavus, indicating that amphotericin B activity is faster against A. fumigatus than A. flavus species (22).
Taking into account all of these different effects exerted by amphotericin B, the order of amphotericin B in vitro activity demonstrated by the present model (A. fumigatus > A. flavus > A. terreus) could be explained by a fast inhibitory action and increased killing rate against A. fumigatus, a slower inhibitory action and reduced killing efficiency against A. flavus, and the slowest inhibitory action and no killing against A. terreus, as was also found with real-time PCR results. In particular, the delayed galactomannan production of A. fumigatus but not A. flavus at a lower-than-the-MIC Cmax of 0.6 mg/liter indicates a strong sub-MIC effect of amphotericin B against the former species. Although there are no data on sub-MIC effects of amphotericin B against Aspergillus spp., such effects were described against Candida spp. (23). At a Cmax of 1.2 mg/liter, galactomannan was detected after 48 h of incubation for A. fumigatus, reflecting the minimal fungicidal action at this concentration (usually observed at 2× the MIC) (13) and the long postantifungal effect observed at 1× the MIC (21), together with a sub-MIC effect, which possibly occurred after amphotericin B concentrations fell below the MIC. The absence of galactomannan production at concentrations >2× the MIC reflects the fungicidal activity amphotericin B demonstrated at time-kill assays (20). For A. flavus, galactomannan was detected at Cmax values of 4.8, 2.4, and 1.2 mg/liter after 48, 24, and 6 h as soon as the concentration fell below the MIC, reflecting the minimal killing and absence of postantifungal effects at ≤4× MIC as previously described (21). Of note, at all three doses, galactomannan production was detected after 4 h despite amphotericin B concentrations being higher than the MIC reflecting the slow inhibitory action of amphotericin B against this species, as previously found (22). Finally, the modest delay in galactomannan production of A. terreus at all doses reflects the lack of killing, postantifungal and possibly sub-MIC effect and the slow inhibitory action against this species. Thus, the single-dose pharmacodynamics in the present in vitro PK/PD model, where amphotericin B concentrations decrease over time, may reflect concentration- and time-dependent inhibitory and killing activities described by MFC, time-kill, and postantifungal effect assays.
Amphotericin B was for decades the treatment of choice for aspergillosis. Clinical and animal data indicated different drug efficacy against infections caused by various Aspergillus species (24). Lack of in vivo efficacy, however, was not associated with significantly increased MIC values (3, 25, 26), which remained similar for all three species examined in the present study (13, 27). The results obtained by the new in vitro model revealed striking differences in the efficacy of amphotericin B against the three Aspergillus species despite their similar MICs with the following order: A. fumigatus > A. flavus > A. terreus. These findings are in agreement with previous comparative animal studies where treatment with amphotericin B was more effective against experimental infection caused by A. fumigatus than against infection with A. flavus and less effective against infection with A. terreus (4, 20). In particular, amphotericin B treatment of guinea pigs infected with an A. flavus or an A. fumigatus strain (each with an MIC of 1 mg/liter), resulted in 0 and 80% survivals, respectively, at the highest dosage of 2.5 mg/kg (4, 20). Furthermore, the in vivo PKPD parameter Cmax/MIC ratio associated with near-maximum survival in an animal model of experimental aspergillosis by A. fumigatus was previously found to be 2.4, similar to the Cmax/MIC ratio found in the present study to be associated with the maximum suppressive effect of amphotericin B against A. fumigatus (28). However, differences in pathogenesis and virulence among these species may confound in vitro-in vivo correlation (29, 30). Clinical studies also demonstrated a higher mortality rate of infections by A. terreus compared to those by A. fumigatus despite amphotericin B therapy (31, 32). It seems that the new in vitro model, described here, may better characterize the pharmacodynamic characteristics of amphotericin B against the most clinically significant Aspergillus species than conventional in vitro susceptibility systems.
In summary, the in vitro model simulated well amphotericin B human pharmacokinetics and demonstrated a differential in vitro activity against the three Aspergillus species that was not reflected by their respective MICs. The effects observed in the in vitro PK/PD model may be the sum of concentration- and time-dependent inhibitory/killing activities exerted by amphotericin B with the greatest activity found against A. fumigatus and the lowest against A. terreus. Future studies should focus on testing larger collections of isolates in order to describe the distribution of this new pharmacodynamic effect and taking into account protein binding and amphotericin B disposition in human body in order to obtain clinically relevant drug exposures that were not obtained with the current model. A composite pharmacodynamic effect that describes the different in vitro activities of amphotericin B may overcome the MIC clustering, assess better antifungal activity, and help distinguish susceptible and from resistant strains.
ACKNOWLEDGMENT
This study was supported by the Marie Curie Reintegration Grant MIRG-CT-2007-208796 from the European Commission.
Footnotes
Published ahead of print 28 May 2013
REFERENCES
- 1. Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 2. Moore CB, Sayers N, Mosquera J, Slaven J, Denning DW. 2000. Antifungal drug resistance in Aspergillus. J. Infect. 41:203–220 [DOI] [PubMed] [Google Scholar]
- 3. Johnson EM, Oakley KL, Radford SA, Moore CB, Warn P, Warnock DW, Denning DW. 2000. Lack of correlation of in vitro amphotericin B susceptibility testing with outcome in a murine model of Aspergillus infection. J. Antimicrob. Chemother. 45:85–93 [DOI] [PubMed] [Google Scholar]
- 4. Odds FC, Van Gerven F, Espinel-Ingroff A, Bartlett MS, Ghannoum MA, Lancaster MV, Pfaller MA, Rex JH, Rinaldi MG, Walsh TJ. 1998. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob. Agents Chemother. 42:282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verweij PE, Oakley KL, Morrissey J, Morrissey G, Denning DW. 1998. Efficacy of LY303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob. Agents Chemother. 42:873–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Espinel-Ingroff A, Cuenca-Estrella M, Fothergill A, Fuller J, Ghannoum M, Johnson E, Pelaez T, Pfaller MA, Turnidge J. 2011. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B and Aspergillus spp. for the CLSI broth microdilution method. Document M38-A2. Antimicrob. Agents Chemother. 55:5150–5154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dodds ES, Drew RH, Perfect JR. 2000. Antifungal pharmacodynamics: review of the literature and clinical applications. Pharmacotherapy 20:1335–1355 [DOI] [PubMed] [Google Scholar]
- 8. Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lewis RE, Wiederhold NP, Prince RA, Kontoyiannis DP. 2006. In vitro pharmacodynamics of rapid versus continuous infusion of amphotericin B deoxycholate against Candida species in the presence of human serum albumin. J. Antimicrob. Chemother. 57:288–293 [DOI] [PubMed] [Google Scholar]
- 10. Meletiadis J, Al-Saigh R, Velegraki A, Walsh TJ, Roilides E, Zerva L. 2012. Pharmacodynamic effects of simulated standard doses of antifungal drugs against Aspergillus species in a new in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 56:403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Saigh R, Elefanti A, Velegraki A, Zerva L, Meletiadis J. 2012. In vitro pharmacokinetic/pharmacodynamic modeling of voriconazole activity against Aspergillus species in a new in vitro dynamic model. Antimicrob. Agents Chemother. 56:5321–5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. CLSI 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed Document M38-A2.28. CLSI, Wayne, PA [Google Scholar]
- 13. Meletiadis J, Antachopoulos C, Stergiopoulou T, Pournaras S, Roilides E, Walsh TJ. 2007. Differential fungicidal activities of amphotericin B and voriconazole against Aspergillus species determined by microbroth methodology. Antimicrob. Agents Chemother. 51:3329–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meletiadis J, Mouton JW, Meis JF, Bouman BA, Donnelly JP, Verweij PE. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shadomy S, McCay JA, Schwartz SI. 1969. Bioassay for hamycin and amphotericin B in serum and other biological fluids. Appl. Microbiol. 17:497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ayestaran A, Lopez RM, Montoro JB, Estibalez A, Pou L, Julia A, Lopez A, Pascual B. 1996. Pharmacokinetics of conventional formulation versus fat emulsion formulation of amphotericin B in a group of patients with neutropenia. Antimicrob. Agents Chemother. 40:609–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanders SW, Buchi KN, Goddard MS, Lang JK, Tolman KG. 1991. Single-dose pharmacokinetics and tolerance of a cholesteryl sulfate complex of amphotericin B administered to healthy volunteers. Antimicrob. Agents Chemother. 35:1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Griffiths LJ, Anyim M, Doffman SR, Wilks M, Millar MR, Agrawal SG. 2006. Comparison of DNA extraction methods for Aspergillus fumigatus using real-time PCR. J. Med. Microbiol. 55:1187–1191 [DOI] [PubMed] [Google Scholar]
- 19. White PL, Barton R, Guiver M, Linton CJ, Wilson S, Smith M, Gomez BL, Carr MJ, Kimmitt PT, Seaton S, Rajakumar K, Holyoake T, Kibbler CC, Johnson E, Hobson RP, Jones B, Barnes RA. 2006. A consensus on fungal polymerase chain reaction diagnosis?: a United Kingdom-Ireland evaluation of polymerase chain reaction methods for detection of systemic fungal infections. J. Mol. Diagn. 8:376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walsh TJ, Petraitis V, Petraitiene R, Field-Ridley A, Sutton D, Ghannoum M, Sein T, Schaufele R, Peter J, Bacher J, Casler H, Armstrong D, Espinel-Ingroff A, Rinaldi MG, Lyman CA. 2003. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J. Infect. Dis. 188:305–319 [DOI] [PubMed] [Google Scholar]
- 21. Vitale RG, Mouton JW, Afeltra J, Meis JF, Verweij PE. 2002. Method for measuring postantifungal effect in Aspergillus species. Antimicrob. Agents Chemother. 46:1960–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Antachopoulos C, Meletiadis J, Sein T, Roilides E, Walsh TJ. 2007. Use of high inoculum for early metabolic signaling and rapid susceptibility testing of Aspergillus species. J. Antimicrob. Chemother. 59:230–237 [DOI] [PubMed] [Google Scholar]
- 23. Garcia MT, Llorente MT, Minguez F, Prieto J. 2002. Postantifungal effect and effects of sub-MIC concentrations on previously treated Candida sp. influence of growth phase. J. Infect. 45:263–267 [DOI] [PubMed] [Google Scholar]
- 24. Denning DW, Marinus A, Cohen J, Spence D, Herbrecht R, Pagano L, Kibbler C, Kcrmery V, Offner F, Cordonnier C, Jehn U, Ellis M, Collette L, Sylvester R. 1998. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. EORTC Invasive Fungal Infections Cooperative Group. J. Infect. 37:173–180 [DOI] [PubMed] [Google Scholar]
- 25. Lionakis MS, Lewis RE, Chamilos G, Kontoyiannis DP. 2005. Aspergillus susceptibility testing in patients with cancer and invasive aspergillosis: difficulties in establishing correlation between in vitro susceptibility data and the outcome of initial amphotericin B therapy. Pharmacotherapy 25:1174–1180 [DOI] [PubMed] [Google Scholar]
- 26. Mosquera J, Warn PA, Morrissey J, Moore CB, Gil-Lamaignere C, Denning DW. 2001. Susceptibility testing of Aspergillus flavus: inoculum dependence with itraconazole and lack of correlation between susceptibility to amphotericin B in vitro and outcome in vivo. Antimicrob. Agents Chemother. 45:1456–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baddley JW, Marr KA, Andes DR, Walsh TJ, Kauffman CA, Kontoyiannis DP, Ito JI, Balajee SA, Pappas PG, Moser SA. 2009. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the Transplant-Associated Infection Surveillance Network. J. Clin. Microbiol. 47:3271–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wiederhold NP, Tam VH, Chi J, Prince RA, Kontoyiannis DP, Lewis RE. 2006. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:469–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Slesiona S, Gressler M, Mihlan M, Zaehle C, Schaller M, Barz D, Hube B, Jacobsen ID, Brock M. 2012. Persistence versus escape: Aspergillus terreus and Aspergillus fumigatus employ different strategies during interactions with macrophages. PLoS One 7:e31223. 10.1371/journal.pone.0031223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blum G, Perkhofer S, Haas H, Schrettl M, Wurzner R, Dierich MP, Lass-Florl C. 2008. Potential basis for amphotericin B resistance in Aspergillus terreus. Antimicrob. Agents Chemother. 52:1553–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lass-Florl C, Kofler G, Kropshofer G, Hermans J, Kreczy A, Dierich MP, Niederwieser D. 1998. In vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J. Antimicrob. Chemother. 42:497–502 [DOI] [PubMed] [Google Scholar]
- 32. Steinbach WJ, Benjamin DK, Jr, Kontoyiannis DP, Perfect JR, Lutsar I, Marr KA, Lionakis MS, Torres HA, Jafri H, Walsh TJ. 2004. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin. Infect. Dis. 39:192–198 [DOI] [PubMed] [Google Scholar]