Abstract
Linezolid plays an increasingly important role in the treatment of multidrug-resistant tuberculosis (MDR-TB). However, patients should be carefully monitored due to time- and dose-dependent toxicity. Clarithromycin plays a more modest role. Therapeutic drug monitoring may contribute to assessment of treatment regimens, helping to reduce toxicity while maintaining adequate drug exposure. Oral fluid sampling could provide a welcome alternative in cases where conventional plasma sampling is not possible or desirable. The aim of this study was to clinically validate the analysis of linezolid and clarithromycin and its metabolite hydroxyclarithromycin in oral fluid of patients with multidrug-resistant tuberculosis. Serum and oral fluid samples were simultaneously obtained and analyzed by using validated methods, after extensive cross-validation between the two matrices. Passing-Bablok regressions and Bland-Altman analysis showed that oral fluid analysis of linezolid and clarithromycin appeared to be suitable for therapeutic drug monitoring in MDR-TB patients. No correction factor is needed for the interpretation of linezolid oral fluid concentrations with a ratio of the linezolid concentration in serum to that in oral fluid of 0.97 (95% confidence interval [CI], 0.92 to 1.02). However, the clarithromycin concentration serum/clarithromycin concentration in oral fluid ratio is 3.07 (95% CI, 2.45 to 3.69). Analysis of hydroxyclarithromycin in oral fluid was not possible in this study due to a nonlinear relationship between the concentration in serum and that in oral fluid. In conclusion, the analysis of linezolid (no correction factor) and clarithromycin (correction factor of 3) in oral fluid is applicable for therapeutic drug monitoring in cases of multidrug-resistant tuberculosis as an alternative to conventional serum sampling. Easy sampling using a noninvasive technique may facilitate therapeutic drug monitoring for specific patient categories.
INTRODUCTION
Tuberculosis (TB) is a mostly curable and preventable infectious disease caused by Mycobacterium tuberculosis. Approximately 3.7% of new tuberculosis patients and 20% of previously treated patients are infected with multidrug-resistant strains that are resistant to at least rifampin and isoniazid (1). Treatment regimens of multidrug-resistant tuberculosis (MDR-TB) should consist of at least four anti-TB drugs to which the bacterium is susceptible (2).
The oxazolidinone linezolid is effective against M. tuberculosis and is increasingly used as a part of treatment regimens in patients with multidrug-resistant or extensively drug-resistant tuberculosis (3). However, patients should be carefully monitored due to the time- and dose-dependent serious toxicity of linezolid, such as myelosuppression and polyneuropathy (4).
Clarithromycin has a less pronounced place in MDR-TB treatment regimens due to serum concentrations that usually do not reach MICs (5). Nevertheless, sufficiently high local clarithromycin concentrations are reached in epithelial lining fluid and alveolar cells (6, 7). Furthermore, occasionally observed lower MICs (our unpublished data), the synergistic activity of clarithromycin against MDR-TB strains (8), and an absence of severe adverse events (9) contribute to its place in anti-TB therapy.
Serum concentrations of linezolid have shown large interpatient variability (10). Drug-drug interactions might further contribute to the observed variability in linezolid pharmacokinetics. For instance, clarithromycin has been observed to increase linezolid serum concentrations significantly (11). Therapeutic drug monitoring (TDM) could potentially assist in identifying MDR-TB patients with linezolid exposure that is too low or too high. Conventional serum sampling is not always possible or desirable due to a lack of venous access, complicated logistics, or the invasive character of the technique. Previously, we developed a dried-blood-spot analysis of linezolid (12) and clarithromycin (D. H. Vu, R. A. Koster, M. S. Bolhuis, B. Greijdanus, R. van Altena, D. H. Nguyen, J. R. B. J. Brouwers, D. R. A. Uges, and J. W. C. Alffenaar, unpublished data) as an alternative to conventional serum sampling. A clinically validated method could be useful for patients who do not accept or tolerate an indwelling venous catheter or who have difficult venous access. We therefore aimed to clinically validate the analysis of linezolid, clarithromycin, and hydroxyclarithromycin (OH-clarithromycin) concentrations in oral fluid.
MATERIALS AND METHODS
From December 2011 to October 2012, patients from the Tuberculosis Center Beatrixoord (Haren, The Netherlands) were included. Patients were ≥18 years old, were diagnosed with MDR-TB, and provided written informed consent. The study protocol was approved by the local Medical Ethical Review Committee as part of a previously reported study. The prospective pharmacokinetic study is registered at ClinicalTrials.gov (registration number NCT01521364).
All patients received oral dosages of 300 mg linezolid twice daily and 250 mg clarithromycin once daily. Full pharmacokinetic curves were obtained at steady state, after at least 2 weeks of administration of both drugs, using a practically feasible sampling schedule that resulted in adequate area under the time-concentration curves (AUCs) in previous studies in our center. Blood and oral fluid samples were collected simultaneously before and 1, 2, 3, 4, 8, and 12 h after medication intake. Blood samples were drawn, and after centrifugation, serum samples were stored at −20°C until analysis. Oral fluid samples were collected by using a small cotton roll on which the patients chewed for approximately 2 min (Salivette; Sarstedt, Leicester, United Kingdom). Oral fluid samples were centrifuged and then stored at −20°C until analysis.
Linezolid and clarithromycin concentrations in serum and oral fluid were analyzed by using validated high-performance liquid chromatography tandem mass spectrometry methods (13, 14). Cross-validation between two matrices was performed by comparing calibration samples of pooled serum and nonstimulated, pooled oral fluid from six batches.
Pharmacokinetic parameters such as, most importantly, AUC were calculated by using Kinfit software (MWPharm 3.60; Mediware, Groningen, The Netherlands), as described in a previous study, using noncompartmental, trapezoidal calculations (10). Other pharmacokinetic parameters that were calculated using Kinfit software were maximum concentration of drug in serum (Cmax), Cmin, apparent clearance (CL), elimination rate constant (kel), and half-life (t1/2). Of these parameters, CL, kel, and t1/2 were determined by using log-linear regression of the concentrations in the terminal period.
The method was clinically validated by comparing the linezolid, clarithromycin, and OH-clarithromycin concentrations in serum samples with the concentration in oral fluid by using Passing-Bablok regressions and Bland-Altman analysis (Analyze-It Software, Ltd.). Pearson's correlation and the Wilcoxon signed-rank test were applied to other comparisons.
RESULTS
Baseline patient demographics are presented in Table 1. Seven patients with MDR-TB, four males and three females, were included in the study. The isolates that were obtained from all patients displayed resistance to at least rifampin and isoniazid. The patients had a median age of 31 years (interquartile range [IQR], 25.5 to 33.5 years) and weighed in at a median of 71.5 kg (IQR, 56.0 to 75.3 kg). Five patients were from Somalia, one was from Turkey, and one was from The Netherlands. At the time of sampling, patients were on MDR-TB treatment for a mean of 61.4 days (range, 33 to 149 days). One patient was HIV positive, for which the patient was on combination antiretroviral therapy (cART) with an adequate virological and immunological response (before-admission CD4 count, 380 cells/mm3).
Table 1.
Baseline demographics (n = 7) and results from drug susceptibility testing
| Parameter | Value |
|---|---|
| Mean age (yr) (range) | 31.0 (25.5–33.5) |
| No. (%) of male patients | 4 (57) |
| Median body wt (kg) (IQR) | 71.5 (56.0–75.3) |
| Median ht (m) (IQR) | 1.73 (1.64–1.74) |
| No. (%) of patients of ethnicity | |
| African | 5 (71) |
| Caucasian | 1 (14) |
| Asian | 1 (14) |
| No. (%) of HIV-positive patients | 1 (14) |
| No. of isolates resistant to drug based on DST/total no. of isolates | |
| Rifampin | 7/7 |
| Isoniazid | 7/7 |
| Ethambutol | 5/7 |
| Pyrazinamidea | 4/6 |
| Streptomycin | 6/7 |
| Capreomycin | 2/7 |
| Amikacin | 0/7 |
| Ciprofloxacin | 1/7 |
| Clarithromycina | 3/5 |
| Clofaziminea | 0/4 |
| Linezolid | 0/7 |
| Moxifloxacin | 1/7 |
| Protionamidea | 2/6 |
| Rifabutin | 6/7 |
Drug susceptibility testing (DST) was not available for all isolates of the included patients.
Comparison of oral fluid and serum analysis methods.
Comparison of analyses in two matrices showed that linezolid, clarithromycin, and OH-clarithromycin had no significant differences of intercept and slope in serum and in oral fluid. The calibration curves for oral fluid were analyzed three times, with all coefficients of variation (CVs) being below 15%. All biases in concentration were <12% for linezolid and <8% for clarithromycin and its metabolite.
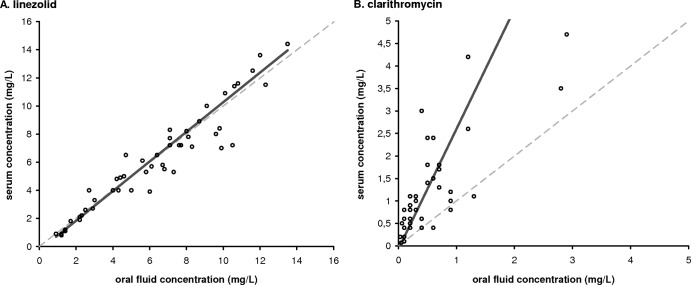
Passing-Bablok regression (n = 49) of the linezolid concentration in serum and oral fluid showed a proportional bias of 1.05 (95% confidence interval [CI], 0.94 to 1.11) and a constant bias of −0.26 (95% CI, −0.52 to 0.05) (Fig. 1A). For clarithromycin, the Passing-Bablok scatter plot (n = 42) showed a proportional bias of 2.67 (95% CI, 1.95 to 3.75) and a constant bias of −0.06 (95% CI, −0.18 to 0.21) (Fig. 1B). There were 7 missing clarithromycin and 8 missing OH-clarithromycin values due to concentrations below the limit of quantitation of the applied method of 0.2 mg/liter. A linear relationship between oral fluid and serum concentrations of both linezolid and clarithromycin was detected by using the Cusum linearity test (P > 0.1). However, the Cusum linearity test detected a nonlinear relationship (0.05 < P < 0.1) between serum and oral fluid OH-clarithromycin concentrations, with Passing-Bablok regression showing a constant bias of 0.02 (95% CI, −0.20 to 0.24) and a proportional bias of 2.00 (95% CI, 1.14 to 3.00).
Fig 1.
Scatter plot with Passing-Bablok fit of serum and oral fluid concentrations in mg/liter. Identity lines are presented as dashed lines, and regression lines are depicted as solid lines. (A) Linezolid (n = 49). The regression line of the linezolid serum/oral fluid concentration ratio has a slope of 1.05 (95% CI, 0.94 to 1.11) and an intercept of −0.26 (95% CI, −0.52 to 0.05). (B) Clarithromycin (n = 42). The regression line of the clarithromycin serum/oral fluid concentration ratio has a slope of 2.67 (95% CI, 1.95 to 3.75) and an intercept of −0.06 (95% CI, −0.18 to 0.21).
Bland-Altman assessment showed good agreement between analyses of linezolid and clarithromycin concentrations in serum and oral fluid, with 4.1% (2/49) of observations for linezolid and 7.1% (3/42) for clarithromycin falling outside 95% limits of agreement (Fig. 2). The observed bias for linezolid (n = 49) was 0.97, with 95% confidence intervals below and above 1 (95% CI, 0.92 to 1.02) (Fig. 2A). For clarithromycin, the observed bias was 3.07 (95% CI, 2.45 to 3.69) (Fig. 2B). Pearson's test revealed that the analyses of linezolid and clarithromycin in serum and in oral fluid were correlated, with r values of 0.95 (P < 0.01) and 0.80 (P < 0.01), respectively.
Fig 2.
Bland-Altman plot of serum/oral fluid concentration ratios compared to average serum and oral fluid concentrations. The line representing the bias is presented as a solid line, and the 95% limits of agreement are presented as dashed lines. (A) Linezolid (n = 49). The bias is 0.97 (95% CI, 0.92 to 1.02), and the lower and upper 95% limits of agreement are 0.64 (95% CI, 0.56 to 0.73) and 1.30 (95% CI, 1.22 to 1.38), respectively. (B) Clarithromycin (n = 42). The bias is 3.07 (95% CI, 2.45 to 3.69), and the lower and upper 95% limits of agreement are −0.82 (95% CI, −1.89 to 0.24) and 6.97 (95% CI, 5.90 to 8.03), respectively.
Pharmacokinetic and pharmacodynamic evaluation.
Pharmacokinetic parameters of linezolid and clarithromycin in serum and oral fluid are displayed in Table 2. A paired-sample Wilcoxon signed-rank test showed no statistically significant difference between medians of all pharmacokinetic parameters in serum and oral fluid, except for linezolid kel and t1/2 (P = 0.018). However, the clinical significance of this observed difference is small, since the AUC is the most important pharmacokinetic parameter for therapeutic drug monitoring of linezolid and clarithromycin in patients with multidrug-resistant tuberculosis.
Table 2.
Pharmacokinetic parameters of linezolid and clarithromycin in serum and in oral fluid (n = 7)
| Drug and parameter | Median value (IQR) for sample type |
P valuea | |
|---|---|---|---|
| Serum | Oral fluid | ||
| Linezolid | |||
| AUC0–12 (mg · h/liter) | 63.9 (47.8–83.8) | 62.1 (50.5–89.2) | 0.296 |
| Cmax (mg/liter) | 10.9 (6.8–11.5) | 10.1 (8.2–10.7) | 1.0 |
| Cmin (mg/liter) | 2.2 (1.5–4.2) | 2.3 (1.7–4.2) | 0.084 |
| CL (liters/h) | 3.5 (2.4–5.9) | 3.6 (2.2–5.0) | 0.063 |
| kel (h−1) | 0.14 (0.10–0.17) | 0.13 (0.08–0.16) | 0.018b |
| t1/2 (h) | 4.9 (4.2–7.9) | 5.2 (4.5–9.8) | 0.018b |
| Clarithromycin | |||
| AUC0–12 (mg · h/liter) | 8.2 (6.2–12.2) | 10.7 (9.4–12.1) | 0.091 |
| Cmax (mg/liter) | 1.7 (1.3–2.7) | 2.8 (2.0–3.4) | 0.063 |
| Cmin (mg/liter) | 0.01 (0.01–0.04) | 0.03 (0.03–0.06) | 1.0 |
| CL (liters/h) | 28.5 (19.3–39.1) | 62.2 (52.8–81.0) | 0.237 |
| kel (h−1) | 0.21 (0.19–0.23) | 0.64 (0.49–1.06) | 0.667 |
| t1/2 (h) | 3.3 (3.1–3.6) | 10.2 (6.4–13.5) | 1.0 |
P values comparing pharmacokinetic parameters in serum and in oral fluid.
Statistically significant difference between medians of the parameter in serum and in oral fluid.
Pharmacokinetic and pharmacodynamic parameters of linezolid for all patients are displayed in Table 3. Isolates from all patients were susceptible to linezolid, with a median MIC of 0.25 mg/liter. Patients had median linezolid AUC from 0 to 12 h (AUC0-12) values of 63.9 mg · h/liter (IQR, 47.8 to 83.8 mg · h/liter) in serum and 62.1 mg · h/liter (IQR, 50.5 to 59.2 mg · h/liter) in oral fluid. All patients had an AUC0-24/MIC ratio of >100 in both serum and oral fluid. For patient 2, the AUC0-24/MIC ratio reached approximately 1,000. The median linezolid AUC0-24/MIC ratios were 277 mg · h/liter (IQR, 260 to 517 mg · h/liter) in serum and 288 mg · h/liter (IQR, 262 to 594 mg · h/liter) in oral fluid. The paired-sample Wilcoxon signed-rank test showed no statistically significant difference between the AUC0-12 values or the AUC0-24/MIC ratios in serum and oral fluid (P = 0.296).
Table 3.
Pharmacokinetic and pharmacodynamic parameters of linezolid
| Patient | MIC (mg/liter) | AUC0–12 (mg · h/liter) |
AUC0–24/MIC ratio |
||
|---|---|---|---|---|---|
| Serum | Oral fluid | Serum | Oral fluid | ||
| 1 | 0.25 | 34.6 | 42.1 | 277 | 337 |
| 2 | 0.25 | 120.1 | 126.4 | 961 | 1,011 |
| 3 | 0.5 | 61.0 | 62.1 | 244 | 248 |
| 4 | 0.5 | 63.9 | 58.8 | 256 | 235 |
| 5 | 0.25 | 33.0 | 34.5 | 264 | 276 |
| 6 | 0.5 | 76.6 | 72.0 | 306 | 288 |
| 7 | 0.25 | 90.9 | 106.3 | 727 | 850 |
| Totala | 63.9 (47.8–83.8) | 62.1 (50.5–89.2)b | 277 (260–517) | 288 (262–594)b | |
Values are medians (interquartile ranges).
No statistically significant difference between serum and oral fluid (P = 0.296).
Patients had median clarithromycin AUC0-12 values of 8.2 mg · h/liter (IQR, 6.2 to 12.2 mg · h/liter) in serum and 3.5 mg · h/liter (IQR, 3.1 to 4.0 mg · h/liter) in oral fluid. One patient was inadvertently administered 500 mg instead of 250 mg clarithromycin on the day of sampling. The samples obtained from this patient were included in the evaluation. The clarithromycin AUC0-12 values after administration of 500 mg clarithromycin were 29.1 mg · h/liter in serum and 15.7 mg · h/liter in oral fluid, well above the AUCs of the patients receiving 250 mg clarithromycin. After applying a correction factor of 3.07, as determined by using the Bland-Altman assessment, patients had an adjusted median clarithromycin AUC0-12 of 10.7 mg · h/liter (IQR, 9.4 to 12.1 mg · h/liter) in oral fluid. The paired-sample Wilcoxon signed-rank test showed no statistically significant difference but did show a trend toward a difference between clarithromycin AUC0-12 values in serum and in oral fluid after applying a correction factor of 3.07 (P = 0.091).
DISCUSSION
The clinical validation performed in this study showed that oral fluid analyses of linezolid and clarithromycin are suitable for TDM in MDR-TB patients. No correction factor is needed for the interpretation of the linezolid oral fluid concentration. However, the clarithromycin oral fluid concentration should be corrected by multiplying by 3 to enable comparison to clarithromycin serum levels. After applying a correction factor of 3 in the case of clarithromycin or no correction factor in the case of linezolid, the pharmacokinetic parameter AUC0–12 calculated from oral fluid samples is applicable for TDM and could assist in identifying patients with exposures that are too high or too low.
Unfortunately, analysis of OH-clarithromycin in oral fluid is not possible due to a nonlinear relationship with concentrations in serum. Nevertheless, the analysis of OH-clarithromycin shows good linearity over a range of 0.2 to 10 mg/liter in both serum and oral fluid. A possible explanation for the observed nonlinear relationship between analysis of OH-clarithromycin in serum and in oral fluid might be the low OH-clarithromycin concentrations that were observed. In this concentration range, around the limit of quantitation, CVs are relatively high although within acceptable limits of <20%. This could explain the nonlinear relationship between analyses of OH-clarithromycin in serum and oral fluid in this low concentration range. Possibly, analysis of a larger cohort or higher clarithromycin doses with corresponding higher OH-clarithromycin concentrations would reveal a linear relationship. Furthermore, this could confirm that there is no significant difference between clarithromycin exposures in serum and oral fluid (after correction), despite a trend toward statistical significance that was observed in our cohort.
The kel and t1/2 of linezolid showed a statistically significant difference of medians in oral fluid compared to serum. The parameters kel and t1/2 were calculated from concentrations obtained in the terminal period, i.e., in the last 3 to 4 samples, in a relatively small cohort. The relevance should be confirmed in a larger cohort or using curves with more samples in the terminal period. In clinical practice, not the parameter kel or t1/2 but the pharmacokinetic/pharmacodynamic parameter AUC/MIC ratio of linezolid is used for therapeutic drug monitoring (10, 11).
To date, there has been no comparison in the literature of the analysis of clarithromycin and linezolid concentrations in serum and in oral fluid of patients with MDR-TB. However, there are several studies describing pharmacokinetics of clarithromycin and OH-clarithromycin in saliva (15, 16) but none describing pharmacokinetics of linezolid in oral fluid. Clarithromycin administered to 12 healthy volunteers in a dose of 500 mg twice daily resulted in an AUC0–12 of 18.0 ± 5.0 mg · h/liter (15). We observed a similar AUC0–12 of 15.7 mg · h/liter for the one patient that was administered 500 mg clarithromycin. Saliva/serum ratios of around 2 were reported, lower than the ratio of 3 observed in our study. However, no data were presented comparing results from the analysis of clarithromycin concentrations in serum and in oral fluid, since that study aimed to describe kinetics and not to clinically validate the analysis of oral fluid (15). Another study described pharmacokinetics of clarithromycin in saliva and serum after a single dose of 500 mg clarithromycin (16). However, the aim was to describe the penetration of clarithromycin into saliva and not to clinically validate the analysis of clarithromycin in saliva. The summary of product characteristics (SmPC) of linezolid describes a linezolid oral fluid concentration/plasma concentration ratio of 1.2, comparable to the bias of 0.97 that was observed in the Bland-Altman assessment (17). Our current study describes the method of analysis of clarithromycin and linezolid, cross-validation between serum and oral fluid, and, most importantly, clinical validation in MDR-TB patients. No statistically significant differences were found between the AUC0–12 values or AUC0–24/MIC ratios of serum and oral fluid.
TDM could potentially assist in identifying MDR-TB patients with linezolid exposure that is too low or too high. Analysis of anti-TB drugs in oral fluid may be advantageous for patients with difficult venous access, hindering blood sampling. Furthermore, the noninvasive sampling could be suitable for children with MDR-TB, for whom indwelling intravenous catheters are not an option and for whom no study data on pharmacokinetics are available to guide therapy. Oral fluid sampling for pediatric patients is preferred over conventional serum sampling by a majority of children and their parents (18). Oral fluid sampling might even reduce costs due to the higher level of training of personnel needed for blood sampling and because less time is needed (18). Oral fluid sampling might even take place at home. No children were included in this study. A clinical validation of oral fluid sampling of pediatric MDR-TB patients is urgently needed. Furthermore, the applicability of saliva and/or collection devices other than the Salivette (Sarstedt, Leicester, United Kingdom) for pharmacokinetic analysis and therapeutic drug monitoring in MDR-TB patients should be clinically validated.
In conclusion, the clinically validated analysis of clarithromycin and linezolid in oral fluid could provide a helpful alternative if conventional blood sampling is not possible or desirable. The use of a correction factor of 3.07 for clarithromycin oral fluid concentrations and no correction factor for linezolid makes the oral fluid sampling readily applicable in clinical practice and allows for easy interpretation of results.
Footnotes
Published ahead of print 20 May 2013
REFERENCES
- 1. World Health Organization 2012. Global tuberculosis report 2012. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2. World Health Organization (ed). 2011. Guidelines for the programmatic management of drug-resistant tuberculosis. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 3. Lee M, Lee J, Carroll MW, Choi H, Min S, Song T, Via LE, Goldfeder LC, Kang E, Jin B, Park H, Kwak H, Kim H, Jeon H, Jeong I, Joh JS, Chen RY, Olivier KN, Shaw PA, Follmann D, Song SD, Lee J, Lee D, Kim CT, Dartois V, Park S, Cho S, Barry CE. 2012. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N. Engl. J. Med. 367:1508–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu HB, Jiang RH, Li L, Xiao HP. 2012. Linezolid in the treatment of MDR-TB: a retrospective clinical study. Int. J. Tuberc. Lung Dis. 16:358–363 [DOI] [PubMed] [Google Scholar]
- 5. Truffot-Pernot C, Lounis N, Grosset JH, Ji B. 1995. Clarithromycin is inactive against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 39:2827–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conte JE, Jr, Golden JA, Duncan S, McKenna E, Zurlinden E. 1995. Intrapulmonary pharmacokinetics of clarithromycin and of erythromycin. Antimicrob. Agents Chemother. 39:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodvold KA, Gotfried MH, Danziger LH, Servi RJ. 1997. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob. Agents Chemother. 41:1399–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cavalieri SJ, Biehle JR, Sanders WE., Jr 1995. Synergistic activities of clarithromycin and antituberculous drugs against multidrug-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 39:1542–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chu SY, Sennello LT, Bunnell ST, Varga LL, Wilson DS, Sonders RC. 1992. Pharmacokinetics of clarithromycin, a new macrolide, after single ascending oral doses. Antimicrob. Agents Chemother. 36:2447–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alffenaar JW, van Altena R, Harmelink IM, Filguera P, Molenaar E, Wessels AM, van Soolingen D, Kosterink JG, Uges DR, van der Werf TS. 2010. Comparison of the pharmacokinetics of two dosage regimens of linezolid in multidrug-resistant and extensively drug-resistant tuberculosis patients. Clin. Pharmacokinet. 49:559–565 [DOI] [PubMed] [Google Scholar]
- 11. Bolhuis MS, van Altena R, Uges DR, van der Werf TS, Kosterink JG, Alffenaar JW. 2010. Clarithromycin significantly increases linezolid serum concentrations. Antimicrob. Agents Chemother. 54:5418–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vu DH, Bolhuis MS, Koster RA, Greijdanus B, de Lange WC, van Altena R, Brouwers JR, Uges DR, Alffenaar JW. 2012. Dried blood spot analysis for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Antimicrob. Agents Chemother. 56:5758–5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harmelink IM, Alffenaar JW, Wessels AM, Greijdanus B, Uges DR. 2008. A rapid and simple liquid chromatography-tandem mass spectrometry method for the determination of linezolid in human serum. EJHP Science 14:3–7 [Google Scholar]
- 14. de Velde F, Alffenaar JW, Wessels AM, Greijdanus B, Uges DR. 2009. Simultaneous determination of clarithromycin, rifampicin and their main metabolites in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877:1771–1777 [DOI] [PubMed] [Google Scholar]
- 15. Burkhardt O, Borner K, Stass H, Beyer G, Allewelt M, Nord CE, Lode H. 2002. Single- and multiple-dose pharmacokinetics of oral moxifloxacin and clarithromycin, and concentrations in serum, saliva and faeces. Scand. J. Infect. Dis. 34:898–903 [DOI] [PubMed] [Google Scholar]
- 16. Wust J, Hardegger U. 1993. Penetration of clarithromycin into human saliva. Chemotherapy 39:293–296 [DOI] [PubMed] [Google Scholar]
- 17. Pfizer 2005. Zyvoxid. Product information. Pfizer, New York, NY [Google Scholar]
- 18. Gorodischer R, Burtin P, Hwang P, Levine M, Koren G. 1994. Saliva versus blood sampling for therapeutic drug monitoring in children: patient and parental preferences and an economic analysis. Ther. Drug Monit. 16:437–443 [DOI] [PubMed] [Google Scholar]