Abstract
Strategies involving new drug combinations, as well as new uses of existing drugs, are urgently needed to reduce the time required to cure patients with drug-sensitive or multidrug-resistant (MDR) tuberculosis (TB). We compared the sterilizing activity of the standard first-line antitubercular regimen, rifampin-isoniazid-pyrazinamide (RHZ), with that of the novel regimen PA-824–moxifloxacin–pyrazinamide (PaMZ), which is currently being studied in clinical trials (NCT01498419), in the guinea pig model of chronic TB infection, in which animals develop necrotic granulomas histologically resembling their human counterparts. Guinea pigs were aerosol infected with ∼2 log10 bacilli of wild-type Mycobacterium tuberculosis H37Rv, and antibiotic treatment was initiated 6 weeks after infection. Separate groups of animals received RHZ, PaMZ, or single or two-drug components of the latter regimen administered at human-equivalent doses 5 days/week for a total of 8 weeks. Relapse rates were assessed 3 months after discontinuation of treatment to determine the sterilizing activity of each combination regimen. PaMZ given at human-equivalent doses was safe and well tolerated for the entire treatment period and rendered guinea pig lungs culture negative more rapidly than RHZ did. After 1 month of treatment, 80% and 50% of animals in the RHZ and PaMZ groups, respectively, had lung culture-positive relapse. Both combination regimens prevented microbiological relapse when administered for a total of 2 months. Our data support the use of PaMZ as a novel isoniazid- and rifamycin-sparing regimen suitable for treatment of both drug-sensitive TB and MDR-TB.
INTRODUCTION
The increasing global burden of drug-resistant tuberculosis (TB) underscores the need for new drugs but also for shorter, better-tolerated regimens to reduce treatment durations from the current 18 to 24 months for multidrug-resistant TB (MDR-TB) (1, 2). Shorter regimens are expected to improve adherence to treatment, thereby reducing transmission and the emergence of new drug resistance, as well as facilitating coadministration with antiretroviral regimens and curbing costs. As a result of continuing efforts to evaluate novel drug combinations with treatment-shortening potential, several new drugs have been shown to contribute to reducing the time required to achieve a relapse-free state in animal models (3–6).
A paradigm shift from “drug development” to “regimen development,” wherein the regimen, not an individual drug, is the unit of development, seems a more feasible approach to deliver sufficiently novel combinations more rapidly (1). The novel regimen PaMZ, comprising PA-824 (Pa), moxifloxacin (M), and pyrazinamide (Z), a combination containing neither rifampin (R) nor isoniazid (H), was found to have a sterilizing activity superior to that of the current first-line regimen, RHZ, in mice (3, 7). In addition, studies in the mouse model have revealed synergism between the agents in the novel combination (3, 7). Recently, a phase II clinical study confirmed the potent initial activity of PaMZ observed in mice, demonstrating that the early bactericidal activity (EBA) of this novel regimen is not inferior to that of the standard antitubercular regimen (8). However, it remains unclear whether the potent sterilizing activity and treatment-shortening potential of PaMZ in mice will also be observed in humans.
Although the mouse model has been used historically in TB chemotherapy studies, the guinea pig model, in which animals develop pathology more closely resembling human TB lesions, may be advantageous for testing the sterilizing activity of novel anti-TB drugs (9–14). Because of the limited data available in the literature (15–17), we first characterized the pharmacokinetic (PK) parameters of Pa and M in guinea pigs to establish human-equivalent doses. We then studied the bactericidal and sterilizing activities of PaMZ against chronic tuberculosis in guinea pigs to validate results from earlier mouse studies and to evaluate the potential of PaMZ as a novel regimen for the treatment of drug-susceptible TB and MDR-TB.
MATERIALS AND METHODS
Mycobacterium tuberculosis strain.
M. tuberculosis strain H37Rv-JHU (18) was used for these studies. Prior to aerosol infection, cultures were grown in Middlebrook 7H9 broth (Difco Laboratories, Detroit, MI) supplemented with 10% OADC (oleic acid-albumin-dextrose-catalase; Becton, Dickinson), 0.05% Tween, and 0.1% glycerol to mid-log phase (optical density at 600 nm [OD600] of ∼0.6).
Animals.
All animals were maintained under specific-pathogen-free conditions and provided water and standard feed ad libitum. All procedures followed protocols approved by the Institutional Animal Care and Use Committee at Johns Hopkins University. Female outbred Hartley guinea pigs (250 to 300 g) with and without jugular vein vascular catheters were purchased from Charles River Labs (Wilmington, MA).
Pharmacokinetic experiments.
Separate groups of three catheterized guinea pigs each were given (i) a single dose of Pa at 12.5 or 50 mg/kg of body weight; (ii) two doses of Pa at 50 mg/kg, separated by 8 h (q8/16h); or (iii) a single dose of M at 100, 150, 175, or 200 mg/kg. The primary and secondary goals of the dosing studies were to match the human area under the concentration-time curve (AUC) and to approximate human Cmax (maximum concentration of drug in serum) measurements, respectively. To obtain steady-state data (19), four guinea pigs were treated twice daily (q8/16h) for 7 days with Pa at 25 mg/kg or M at 90 mg/kg. Doses were prepared in 40% sucrose water in a final volume of 0.5 ml and delivered to the posterior oropharynx by use of an automatic pipette with a disposable tip. Blood (∼0.3 ml) was drawn serially from guinea pigs through an intravenous catheter at the following time points after antibiotic dosing: 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, and 24 h in the single-dose study and before drug administration on day 0 and day 8 and 0.5, 1, 2, 4, 6, 8, and 24 h after the last dosing in the steady-state study, which is a robust sampling scheme. Briefly, serum was separated, stored at −80°C, and later analyzed for the Pa (20, 21) or M (22–24) concentration by use of validated assays on a Thermo-Finnigan P4000 high-performance liquid chromatography (HPLC) pump (Thermo-Finnigan, San Jose, CA) with a model AS1000 fixed-volume autosampler, a model UV2000 UV detector (Pa) (both from Thermo Electron Corporation, Waltham, MA) or McPherson fluorescence detector (M) (McPherson, Chelmsford, MA), a Gateway E-series computer (Gateway, Poway, CA), and the Chromquest HPLC data management system (Thermo Electron Corporation). The plasma standard concentration curve for Pa ranged from 0.20 to 50 μg/ml (20). The absolute recovery of Pa from plasma was 88.2%. The overall precision of the validation assay across all standards was 0.67 to 5.38%. The six-point standard curve for M ranged from 0.2 to 15 μg/ml, with linearity extending well above this range (23). The recovery of M from plasma was approximately 90%. The overall validation precision for M was 4.19 to 6.62% (23). All of our results represent total drug concentrations. Population values for typical protein binding can be applied to the Cmax and AUC. We performed a noncompartmental analysis using Phoenix WinNonlin 6.3 (Pharsight, Mountain View, CA). This included the Cmax, time to obtain Cmax (Tmax), AUC, and half-life (t1/2).
A one-compartment oral absorption model was used to simulate total and free Pa (assuming 5, 7, 10, or 15% unbound drug) and M (assuming 60% unbound drug) concentrations at steady state for different dosing regimens. We used the steady-state median values obtained from the noncompartmental analysis to determine the volume of distribution and elimination rate constant, while the absorption rate constant was adjusted to match the median serum concentrations measured at steady state. The resultant free, unbound (f) concentrations were used to calculate pharmacodynamic (PD) indices (fAUC/MIC ratio, fCmax/MIC ratio, and fTMIC [portion of a 24-h period that the free, unbound fraction of drug exceeded the MIC under steady-state pharmacokinetic conditions]) over 168 h (7 days), using an Excel sheet. For Pa, we performed the PD analysis over MIC values ranging from 0.03125 to 0.25 μg/ml. For M, we performed the PD analysis over MIC values ranging from 0.25 to 1 μg/ml. Simulations were deterministic, with the objective of comparing data between dosing regimens, not assessing the variability in exposure within each dosing regimen.
Aerosol infections.
The basic experimental scheme is shown in Table 1. A total of 132 guinea pigs were aerosol infected with H37Rv-JHU by use of a Madison chamber aerosol generation device (University of Wisconsin, Madison, WI) calibrated to deliver ∼2 log10 CFU to the lungs (10–14, 25).
Table 1.
Basic experimental scheme
| Groupa | No. of animals sacrificed at mob: |
|||||
|---|---|---|---|---|---|---|
| −1.5 | 0 | 0.5 | 1 (+3) | 2 (+3) | Total | |
| Untreated | 4 | 4 | 4 | 4 | 4 | 20 |
| R100H60Z300 | 4 | 4 (10) | 4 (10) | 12 (20) | ||
| Pa25 BID | 4 | 4 | 8 | |||
| M90 BID | 4 | 4 | 8 | |||
| Z300 | 4 | 4 | 8 | |||
| Pa25 BIDM90 BID | 4 | 4 | 8 | |||
| Pa25 BIDZ300 | 4 | 4 | 8 | |||
| M90 BIDZ300 | 4 | 4 | 8 | |||
| Pa25 BIDM90 BIDZ300 | 4 | 4 (10) | 4 (10) | 12 (20) | ||
| Total | 4 | 4 | 32 | 32 (20) | 8 (20) | 132 |
Drug doses (in mg/kg; single or twice-daily dose) are indicated by subscripts. BID, twice daily, every 16 and 8 h. Doses of each drug were determined to be human equivalent based on the AUC and were given orally daily (5 days/week).
Month −1.5, day after infection with M. tuberculosis; month 0, day of treatment initiation; month 0.5, 0.5 month after treatment initiation; and so on. “(+3)” indicates that the number of guinea pigs in parentheses was held for 3 months after treatment completion before sacrifice for relapse assessment.
Antibiotic therapy.
Oral therapy daily (5 days/week) with human-equivalent doses of PaMZ (Pa at 25 mg/kg twice daily, M at 25 mg/kg twice daily, and Z at 300 mg/kg once daily), RHZ (R at 100 mg/kg, H at 60 mg/kg, and Z at 300 mg/kg, all once daily), or single or two-drug (PaM, PaZ, and MZ) components of the PaMZ regimen was initiated 6 weeks after infection and continued for a total of 2 months (Table 1). For each drug given twice daily, the morning dose preceded the afternoon dose by 8 h and the latter preceded the following dose by 16 h (q8/16h). For the RHZ-treated group, the R dose preceded that of the other drugs (H and Z) by at least 1 h to prevent PK antagonism (26, 27). Animals were treated with a formulation consisting of 40% (wt/vol) sucrose, 20% (wt/vol) pumpkin (Libby's 100% pure pumpkin) mixture supplemented with vitamin C (50 mg/kg of mean body weight), and commercial Lactobacillus (BD Lactinex) (all purchased from Walmart, Towson, MD) to help stabilize the enteric flora and prevent gastrointestinal dysbacteriosis or antibiotic-associated enteritis (10).
Study endpoints.
Four animals were sacrificed at month −1.5 and month 0 relative to treatment initiation in order to determine the number of bacilli implanted in each lung on the day after infection and the lung bacillary load at the start of treatment, respectively. In order to determine the bactericidal activity of each regimen, 4 animals from each group were sacrificed at months 0.5 and 1 and, for the three-drug combinations, also at month 2.
Animal body weights and lung and spleen weights were recorded and organs photographed at the time of sacrifice. In order to account for the physiological increase in organ weights in aging animals, organ weights were normalized using the following formula: sacrificed animal organ weight on day of sacrifice × (mean body weight on day after infection/sacrificed animal body weight on day of sacrifice). The lungs and spleen of each animal were examined at necropsy for grossly visible lesions, and small sections from the left lung were dissected, placed into 10% buffered formaldehyde, and paraffin embedded for histopathological staining with hematoxylin and eosin (H&E) and Kinyoun stain for acid-fast bacillus (AFB) detection. At least one entire H&E-stained cross section per animal lung (4 animals/group) was analyzed for degree of inflammation. Histological analysis was performed by two board-certified pathologists (T. J. Gniadek and D. A. Belchis), who were blinded to the identity of each sample. The remainder of each tissue was homogenized and plated as previously described (9–12, 14). Plates were incubated at 37°C for 4 weeks before final CFU counts were determined.
After the completion of 1 month and 2 months of treatment with PaMZ and RHZ, groups of 10 animals were held for an additional 3 months without treatment to determine the sterilizing activity of each combination regimen. Relapse was defined as the presence of mycobacterial colonies upon plating of entire undiluted lung homogenates.
Statistical analysis.
For pharmacokinetic studies, data represent means ± standard deviations (SD). Organ CFU values were log transformed prior to calculating the means and standard deviations for each group. Comparisons of data among experimental groups were performed by Student's t test and analysis of variance followed by Bonferroni multiple-comparison tests, using GraphPad Prism software. P values of <0.05 were considered statistically significant.
RESULTS
Identification of human-equivalent doses of PaMZ in guinea pigs.
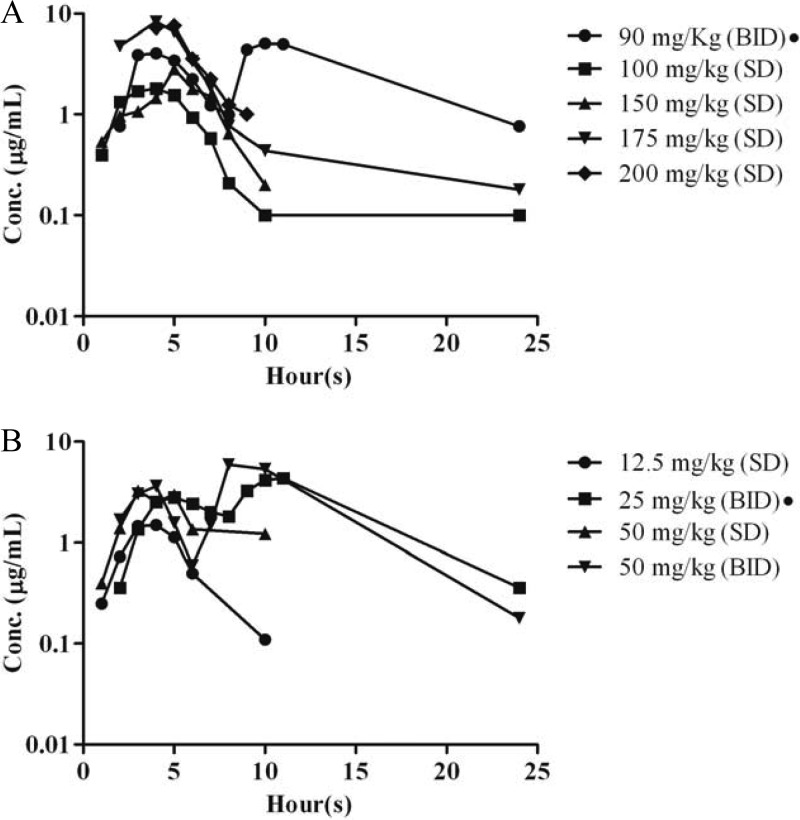
Based on the area under the serum concentration-time curve from 0 h to infinity (AUC0–∞), the human-equivalent doses of R, H, and Z in guinea pigs were determined previously to be 100 mg/kg (R100), 60 mg/kg (H60), and 300 mg/kg (Z300), respectively (9, 10). In the current study, we determined the human-equivalent doses of Pa and M (Fig. 1; Table 2). In a preliminary single-dose study for M, the t1/2 was found to be 3-fold longer in humans. Although M at 150 mg/kg yielded a Cmax in guinea pigs matching that following standard dosing in humans, this dose yielded a relatively low AUC (17.2 mg-h/liter for guinea pigs versus 55 mg-h/liter for humans). We found that M at 175 mg/kg in guinea pigs produced a more human-like AUC (39.70 mg-h/liter), but the resulting Cmax was significantly higher (9.83 μg/ml). On the other hand, splitting this dose into 90 mg/kg twice daily (q8/16h) yielded a Cmax and AUC0–24 closely approximating the corresponding steady-state values following daily dosing with M at 400 mg in humans (Table 2).
Fig 1.
Plasma concentration profiles of moxifloxacin (A) and PA-824 (B) in guinea pigs. The graphs present median values (n = 3 or 4 per time point). SD, single dose; BID, twice a day (q8/16h). A black circle next to the drug dose indicates repeated doses.
Table 2.
Moxifloxacin and PA-824 pharmacokinetics in guinea pigs, mice, and humansa
| Regimen | Test species | Drug dosage (frequency) | Sampling dose | Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUC0–∞ (mg-h/liter) |
|---|---|---|---|---|---|---|---|
| Moxifloxacin | Guinea pig | 90 mg/kg (BID) | Weekly | 4.55 ± 2.04 | 1.4 ± 1.47 | 3.14 ± 0.95 | 41.47 ± 12.04 |
| Guinea pig | 100 mg/kg (QD) | Single | 1.91 ± 0.36 | 1.68 ± 0.59 | 2.35 ± 0.39 | 11.1 ± 2.3 | |
| Guinea pig | 150 mg/kg (QD) | Single | 5.08 ± 1.97 | 1.25 ± 0.64 | 2.37 ± 0.45 | 17.2 ± 2.4 | |
| Guinea pig | 175 mg/kg (QD) | Single | 9.83 ± 3.02 | 1.0 ± 0 | 2.23 ± 0.76 | 39.70 ± 6.13 | |
| Guinea pig | 200 mg/kg* (QD) | Single | 6.67 ± 2.34 | 2.47 ± 0.33 | 2.63 ± 0.26 | 29.4 ± 6.7 | |
| Mouseb | 100 mg/kg (QD) | Single | 14.2 ± 3.9 | 0.25 | 2.32 ± 0.14 | 23.6 | |
| Humanc | 400mg (QD) | SS | 6.11 ± 1.41 | 1.22 ± 0.44 | 7.55 ± 1.88 | 55.84 ± 13.19 | |
| Humand | 400mg (QD) | 5th | 6.1 (4.5–9.0) | 1 (1.0–2.0) | 8.1 (5.1–9.9) | 55.3 (36.0–79.1) | |
| PA-824 | Guinea pig | 12.5 mg/kg (D1) | Single | 1.68 ± 0.34 | 2.65 ± 1.17 | 1.94 ± 0.38 | 11.19 ± 2.04 |
| Guinea pig | 25 mg/kg (BID) | Weekly | 2.99 ± 0.70 | 2.25 ± 1.25 | 4.7 ± 2.27 | 42.19 ± 21.04 | |
| Guinea pig | 50 mg/kg (D1) | Single | 5.84 ± 4.08 | 2.66 ± 1.15 | 3.16 ± 1.11 | 39.79 ± 27.88 | |
| Guinea pig | 50 mg/kg (BID) | Single | 5.79 ± 1.46 | 7.0 ± 4.14 | 2.16 ± 1.41 | 70.95 ± 9.61 | |
| Mousee | 100 mg/kg (D1) | Single | 21.4 ± 5.7 | 4.7 ± 3.1 | 12.8 ± 1.0 | 327.6 ± 77.1 | |
| Humanf | 200 mg/kg (QD) | SS | 1.7 ± 0.3 | 4.5 (2–8) | 16.0 ± 1.6 | 30.2 ± 3.7 |
Cmax, maximum concentration; Tmax, time to obtain Cmax; AUC0–∞, area under the concentration-time curve from 0 h to infinity; t1/2, half-life; QD, once daily; BID, twice a day (the morning dose was separated from the afternoon dose by 8 h); SS, steady state. *, dose given as part of an RMZ combination. In each case, R was given 60 min prior to administration of the other 2 companion drugs. Data represent means ± SD for 3 or 4 animals. Data with parentheses are medians (ranges).
Data are from reference 24.
Data are from reference 23.
Data are from reference 22. For this group, the AUC0–24 was determined.
Data are from reference 21. For this group, the AUC0–24 was determined.
Data are from reference 28.
The AUC0–α in guinea pigs receiving a single dose of Pa at 12.5 mg/kg (11.19 mg-h/liter) was somewhat lower than that in humans, though the Cmax was similar to steady-state values in humans receiving 200 mg. At a 50-mg/kg single dose, the Cmax was higher, with a variable AUC. The 50-mg/kg twice-daily (q8/16h) dose yielded an AUC approximately twice that of the target human AUC. The half-life of Pa is markedly longer in humans than in guinea pigs, resulting in significant accumulation of the drug in the former. However, the median AUC following repeated dosing of Pa at 25 mg/kg q8/16h closely matches human exposures at steady state (28).
Pharmacodynamic simulations.
Pa at the dose used in this study (25 mg/kg q8/16h) showed favorable PD parameters (fTMIC and fAUC/MIC ratio) at the low MICs (0.03125 and 0.0625 μg/ml). At the higher MICs, its activity was dependent on the unbound fraction. Also, the twice-daily dosing was more consistent in maintaining concentrations above the MIC. For M, the dose used in this study showed good PD activity (fAUC/MIC and fCmax/MIC) at the low MIC (0.25 μg/ml), but less so at the high MIC (1.0 μg/ml) (Table 3).
Table 3.
Pharmacodynamic parameters of PA-824 and moxifloxacin in guinea pigsa
| Drug | Dose (mg/kg; given BID) | MIC (μg/ml) | % free, unbound drug | %fTMIC | fAUC/MIC | fCmax/MIC |
|---|---|---|---|---|---|---|
| PA-824 | 25 | 0.03 | 15 | 100.00 | 223.60 | 14.13 |
| 25 | 0.03 | 10 | 100.00 | 149.07 | 9.42 | |
| 25 | 0.03 | 7.50 | 100.00 | 111.80 | 7.07 | |
| 25 | 0.03 | 5 | 94.64 | 74.53 | 4.71 | |
| 25 | 0.06 | 15 | 100.00 | 111.80 | 7.07 | |
| 25 | 0.06 | 10 | 94.64 | 74.53 | 4.71 | |
| 25 | 0.06 | 7.50 | 85.71 | 55.90 | 3.54 | |
| 25 | 0.06 | 5 | 73.21 | 37.27 | 2.36 | |
| 25 | 0.13 | 15 | 85.71 | 55.90 | 3.53 | |
| 25 | 0.13 | 10 | 73.21 | 37.27 | 2.36 | |
| 25 | 0.13 | 7.50 | 56.55 | 27.95 | 1.77 | |
| 25 | 0.13 | 5 | 24.40 | 18.63 | 1.18 | |
| 25 | 0.25 | 15 | 56.55 | 27.95 | 1.77 | |
| 25 | 0.25 | 10 | 24.40 | 18.63 | 1.18 | |
| 25 | 0.25 | 7.50 | 2.98 | 13.98 | 0.88 | |
| 25 | 0.25 | 5 | 0 | 9.32 | 0.59 | |
| Moxifloxacin | 90 | 0.25 | 60 | 99.40 | 117.76 | 11.20 |
| 90 | 0.5 | 60 | 74.40 | 58.88 | 5.60 | |
| 90 | 1 | 60 | 49.40 | 29.44 | 2.80 |
AUC, area under the serum concentration-time curve; %fTMIC, cumulative percentage of a 24-h period that the free, unbound fraction of drug exceeded the MIC under steady-state pharmacokinetic conditions; BID, twice daily (the morning dose was separated from the afternoon dose by 8 h). Data were obtained by simulation of the dosing regimens on the basis of the pharmacokinetic profiles for PA-824 and moxifloxacin in guinea pigs.
Morbidity and mortality during treatment.
No deaths were observed in either the treated or untreated groups of guinea pigs during the course of the experiment. After 2 weeks of treatment, treated guinea pigs were found to have lower mean body weights than those of the untreated control group, possibly due to gavage-induced stress (see Table S1 in the supplemental material). The combination groups had greater weight loss than the monotherapy groups. However, mean body weights of treated guinea pigs started to increase thereafter and continued to increase even after completion of treatment in animals kept for relapse studies.
Organ weights, gross pathology, and histology during treatment.
On the day after infection, the normalized mean guinea pig lung weight was 2.2 ± 0.1 g, increasing to 2.4 ± 0.4 g at the onset of treatment (see Table S2 in the supplemental material). The normalized mean lung weight of untreated guinea pigs continued to increase, reaching 2.9 ± 0.3 g at month 1 of treatment. In contrast, normalized mean lung weights of treated guinea pigs showed decreasing trends over time, ranging from 1.83 ± 0.28 to 2.8 ± 0.6 g at month 0.5 of treatment, with further declines at month 1 (ranging from 1.65 ± 0.1 to 2.7 ± 0.6 g). However, lung weights increased in animals kept for assessment of relapse rates (see Fig. S1). Similar trends were observed for normalized mean spleen weights of treated and untreated guinea pigs (see Table S2).
PaMZ and RHZ showed equivalent reductions in the number and size of grossly visible lung tubercles, as rapidly as within 2 weeks of treatment initiation (see Fig. S1 in the supplemental material). Histological evaluation revealed diffuse inflammation, nodular aggregates, and multiple necrotic and nonnecrotizing granulomas, which were present at treatment onset and increased in number over the subsequent month in untreated animals. Treatment with PaMZ and RHZ reduced the number and size of granulomas, as well as the number of extracellular AFB within granulomas, to similar degrees 2, 4 (see Fig. S2), and 8 weeks after initiation of therapy.
Bactericidal activity of PaMZ and RHZ for chronic TB infection in guinea pigs.
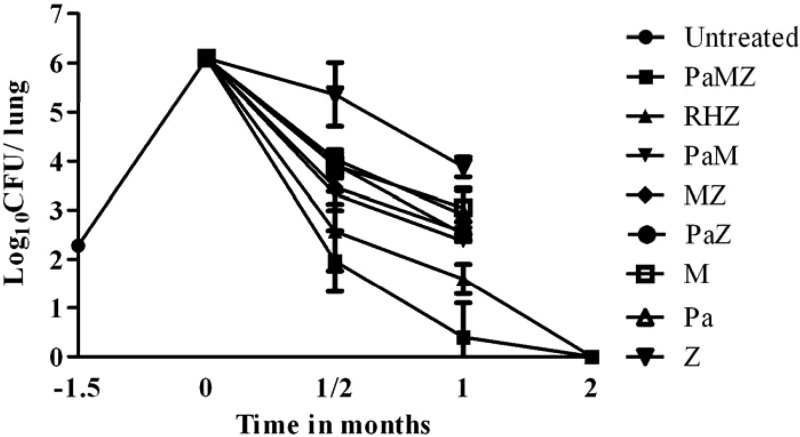
On the day after aerosol infection (month −1.5 of treatment), 2.2 ± 0.08 log10 CFU were recovered from guinea pig lungs. The bacilli grew exponentially, to a peak lung burden of 6.1 ± 0.1 log10 CFU at the time of treatment initiation (month 0). At month 0.5, all combination (two- or three-drug) regimens displayed bactericidal activity, but the three-drug regimens (PaMZ and RHZ) were more potent than the two-drug regimens (PaM, PaZ, and MZ) (Fig. 2). However, the difference was not statistically significant for any of the comparisons (see Fig. S3 in the supplemental material). Although both PaMZ and RHZ regimens demonstrated bactericidal activity, treatment with PaMZ resulted in a mean lung CFU count that was 0.6 log10 lower than that observed after treatment with RHZ (P = 0.11). Each of the single-drug regimens showed bactericidal activity in the following rank order: Pa = M > Z. One month of RHZ treatment reduced the CFU count by 4.5 log10. For comparison, 1 month of treatment with PaMZ reduced the CFU count by 5.7 log10 (P < 0.001) (Fig. 2; see Fig. S3). The two-drug combinations showed equivalent activities, and the order of activity of the single-drug regimens was unchanged. After completion of 2 months of treatment with PaMZ or RHZ, all lungs were culture negative.
Fig 2.
Antitubercular activities of the indicated drugs in infected guinea pigs. Animals were infected via aerosol with ∼102 CFU of M. tuberculosis H37Rv and were either left untreated or treated with drugs daily (5 days/week) beginning 6 weeks after infection. R, rifampin at 100 mg/kg once daily; H, isoniazid at 60 mg/kg once daily; Z, pyrazinamide at 300 mg/kg once daily; Pa, PA-824 at 25 mg/kg twice daily (q8/16h); M, moxifloxacin at 90 mg/kg twice daily (q8/16h).
Sterilizing activity of RHZ and PaMZ in guinea pigs.
Groups of 10 guinea pigs were held without treatment for 3 months following completion of 1 and 2 months of RHZ or PaMZ treatment in order to assess lung culture-positive relapse rates. After 1 month of treatment, relapse rates in guinea pig lungs were 80% (8/10 animals) and 50% (5/10 animals) for those treated with RHZ (mean log10 CFU = 0.4 ± 0.2) and PaMZ (log10 CFU = 0.24 ± 0.3), respectively. The relapse rate in guinea pig lungs was 0% (0/10 animals) after 2 months of treatment with RHZ or PaMZ. One of the limitations of this study is that we assessed bacillary burdens in the lungs only, not in any extrapulmonary organs such as the spleen or liver, which is why our data do not allow us to conclude that guinea pigs were truly “cured” of TB infection.
DISCUSSION
In this study, we compared the bactericidal and sterilizing activities of the standard antitubercular regimen RHZ against chronic TB infection in guinea pigs with those of the novel regimen PaMZ and investigated the contribution of each single and two-drug component to the activity of each combination regimen. We found that PaMZ was well tolerated and that animals receiving this novel regimen had a more rapid reduction in lung CFU count and achieved a stable cure at least as rapidly as those receiving RHZ.
A key component of PaMZ is Pa, a nitroimidazole effective against drug-sensitive and drug-resistant TB (5, 6, 29–31). In addition to inhibiting ketomycolic acid and protein synthesis, Pa also kills M. tuberculosis through a novel mechanism involving generation of intracellular nitric oxide (32). Pa is a prodrug that undergoes nitroreduction to one or more active compounds (17, 20). Because it may kill M. tuberculosis by different mechanisms under aerobic and anaerobic conditions (33) and may be activated more efficiently under hypoxic conditions, it warrants evaluation in models that differ in their degree of tissue hypoxia. In an experimental mouse model of TB infection, which lacks hypoxic lesions (34, 35), Pa given as monotherapy at 100 mg/kg exhibited bactericidal activity similar to that of the human-equivalent dose of H during the intensive phase of treatment (36, 37). Subsequently, the PaMZ combination was shown to achieve a more rapid cure than that by the standard RHZ regimen in the murine model of acute TB infection (3). Given its unique activity under hypoxic conditions, Pa has also been studied in the guinea pig model of TB infection, in which animals develop hypoxic necrotic granulomas histologically resembling their human counterparts (38). After 30 days of treatment, Pa given orally at 40 mg/kg exhibited antitubercular activity equivalent to that of H at 25 mg/kg in the lungs and spleens of chronically infected guinea pigs (33). More recently, Pa given as a dry powder via aerosol showed more modest killing in the guinea pig model of TB infection (17). However, in the latter study, guinea pig plasma drug concentrations were significantly lower than corresponding human steady-state values following dosing with Pa at 200 mg (29). Regarding the activity of PA-824 in the murine model (21, 30), the fTMIC provided the best fit of the data, while the fAUC/MIC ratio was not far behind (fTMIC was 5% better over 12 to 72 h, based on the R2 value, and AUC was 1% better over 12 to 48 h). Within each dosing frequency, higher values for all three parameters (fTMIC, fAUC/MIC, and fCmax/MIC) were associated with a greater kill capacity. All regimens dosed every 144 h resulted in an increase in CFU counts or, in one case, bacteriostasis. Since the best PD parameter clearly depended on the conditions of the analysis, we matched Pa exposures in guinea pigs and humans based on AUC values for the drug. Such doses were recently shown to have early bactericidal activity in clinical trials (29). Based on our simulations, the 25-mg/kg q8/16h dosing strategy produced an fTMIC somewhat similar to that for the 200-mg dose in humans (29, 30).
Previous studies have shown that each drug contributes substantially to the activity of PaMZ in a murine model of acute TB (3). The addition of the third drug to any regimen comprising the other two drugs led to a >100-fold increase in bacillary killing after 1 month and 2 months of treatment. Similarly, in the current study, we found that the addition of each drug significantly increased the bactericidal activity of the companion two-drug regimens after 2 weeks and 1 month of treatment in the guinea pig model of chronic TB. These data are consistent with human EBA studies, in which PaMZ was more effective than PaZ (8), which likewise was more effective than Pa alone (3, 31). Historically, mouse models have represented the activity of existing TB drugs well (3). However, pathological differences between M. tuberculosis-infected mice and humans have raised concerns about the “predictiveness” of mouse models with respect to novel drugs or drug regimens, which require more human-like pathology for optimal activity. Human TB is characterized histologically by the presence of necrotic granulomas, which are absent in BALB/c mice (2, 39). Like rabbits and nonhuman primates, guinea pigs infected with M. tuberculosis develop necrotic granulomas characterized by tissue hypoxia (9–14, 25, 38), which is not a feature of mouse granulomas (9–14, 25, 34, 35). Evidence in humans (40) and guinea pigs suggests that persistent bacilli reside in the extracellular compartment of such necrotic granulomas (41). In particular, the hypoxia-dependent sterilizing activity of Pa against M. tuberculosis persisters may be represented better in the guinea pig model than in the standard mouse model. Although upon first consideration the guinea pig model of TB appears overly “optimistic,” in so far as guinea pigs are cured with the standard anti-TB regimen within 2 months, this model has proven useful in discriminating between purely tuberculocidal drugs and those with more potent sterilizing activity (9–13, 42). Thus, the bactericidal drugs isoniazid and streptomycin showed early potent activity in acutely infected guinea pigs that was dramatically reduced against persistent bacilli (13, 14). The uniquely sterilizing drug Z was found to have dose-dependent activity against chronic TB infection in guinea pigs and to exhibit synergy with R, as in humans (12). Furthermore, substitution of rifapentine for R in the standard antitubercular regimen did not shorten the time to cure in the guinea pig model of chronic TB (10), matching results from human studies (43) but unlike those derived from the mouse model (44). Interestingly, the PaMZ combination appeared less effective than RHZ in preliminary studies in C3HeB/FeJ mice, which develop human-like necrotic lung granulomas characterized by tissue hypoxia (45), raising concern that the relative sterilizing activity of a particular regimen may be dependent on species-specific pathology. However, the selection of Pa-resistant mutants in the latter study may have confounded results. A direct comparison of the sterilizing activities of PaMZ in BALB/c and C3HeB/FeJ mice is ongoing. The experimental design of our study did not allow us to find monotherapy regimens that lead to emergence of drug resistance, as occurs when M (46) or Z (47) is given as monotherapy in humans. It is important that the lung bacillary burden in guinea pigs at the start of treatment in our study was 106 CFU, whereas in most cases of human cavitary TB, the number of bacilli exceeds 109 (48). We reported earlier that H-resistant mutants may have reduced virulence in this model (14).
Our results indicate that the potent sterilizing activity of PaMZ is sufficient to cure guinea pigs at least as quickly as RHZ does, thus marking for the first time a regimen without R and H that has prevented relapse at least as effectively as the first-line regimen in this model. An oral regimen that cures TB in 6 months or less may open the door to improved treatments for MDR-TB, which currently consist of complex regimens of 18 to 24 months that include the use of injectable agents. Because Pa, which does not induce hepatic metabolism, was able to effectively substitute for R, a potent metabolic inducer of many drugs, including antiretroviral agents and the newly approved TB drug bedaquiline, Pa-containing regimens may provide attractive alternatives to R-containing combinations in evaluations of new drug candidates.
While the PaMZ regimen is expected to be just as effective against the majority of MDR-TB isolates as it is against fully drug-susceptible isolates, Z and M are unlikely to be active against extensively drug-resistant TB (XDR-TB). Moreover, both Z resistance and fluoroquinolone resistance are on the rise in MDR-TB patients (49–52), indicating that use of PaMZ in this population must be accompanied by careful evaluation of drug susceptibility. The findings of this study are timely, as the dosing mirrors that in a clinical trial investigating the same regimen given during the intensive phase of therapy, the results of which are expected soon.
Supplementary Material
ACKNOWLEDGMENTS
We have no transparency declarations to declare.
This work was supported by the National Institutes of Health (grant AI083125 to P.C.K.) and the FDA (grant U18FD004004 to K.E.M., E.L.N., and P.C.K.).
Footnotes
Published ahead of print 3 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00761-13.
REFERENCES
- 1. Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. 2010. Global tuberculosis drug development pipeline: the need and the reality. Lancet 375:2100–2109 [DOI] [PubMed] [Google Scholar]
- 2. Dutta NK, Karakousis PC. 2012. Tuberculosis chemotherapy: present situation, possible solutions, and progress towards a TB-free world. Indian J. Med. Microbiol. 30:261–263 [DOI] [PubMed] [Google Scholar]
- 3. Nuermberger E, Tyagi S, Tasneen R, Williams KN, Almeida D, Rosenthal I, Grosset JH. 2008. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 52:1522–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nuermberger EL, Yoshimatsu T, Tyagi S, Williams K, Rosenthal I, O'Brien RJ, Vernon AA, Chaisson RE, Bishai WR, Grosset JH. 2004. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am. J. Respir. Crit. Care Med. 170:1131–1134 [DOI] [PubMed] [Google Scholar]
- 5. Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. 2011. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob. Agents Chemother. 55:5485–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL. 2012. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 56:3114–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tasneen R, Tyagi S, Williams K, Grosset J, Nuermberger E. 2008. Enhanced bactericidal activity of rifampin and/or pyrazinamide when combined with PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 52:3664–3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, van Niekerk C, Everitt D, Winter H, Becker P, Mendel CM, Spigelman MK. 2012. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 380:986–993 [DOI] [PubMed] [Google Scholar]
- 9. Ahmad Z, Nuermberger EL, Tasneen R, Pinn ML, Williams KN, Peloquin CA, Grosset JH, Karakousis PC. 2010. Comparison of the ‘Denver regimen’ against acute tuberculosis in the mouse and guinea pig. J. Antimicrob. Chemother. 65:729–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dutta NK, Illei PB, Peloquin CA, Pinn ML, Mdluli KE, Nuermberger EL, Grosset JH, Karakousis PC. 2012. Rifapentine is not more active than rifampin against chronic tuberculosis in guinea pigs. Antimicrob. Agents Chemother. 56:3726–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmad Z, Fraig MM, Pinn ML, Tyagi S, Nuermberger EL, Grosset JH, Karakousis PC. 2011. Effectiveness of tuberculosis chemotherapy correlates with resistance to Mycobacterium tuberculosis infection in animal models. J. Antimicrob. Chemother. 66:1560–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmad Z, Fraig MM, Bisson GP, Nuermberger EL, Grosset JH, Karakousis PC. 2011. Dose-dependent activity of pyrazinamide in animal models of intracellular and extracellular tuberculosis infections. Antimicrob. Agents Chemother. 55:1527–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahmad Z, Pinn ML, Nuermberger EL, Peloquin CA, Grosset JH, Karakousis PC. 2010. The potent bactericidal activity of streptomycin in the guinea pig model of tuberculosis ceases due to the presence of persisters. J. Antimicrob. Chemother. 65:2172–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmad Z, Klinkenberg LG, Pinn ML, Fraig MM, Peloquin CA, Bishai WR, Nuermberger EL, Grosset JH, Karakousis PC. 2009. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J. Infect. Dis. 200:1136–1143 [DOI] [PubMed] [Google Scholar]
- 15. Sanchez F, Lopez Colomes JL, Villarino E, Grosset J. 2011. New drugs for tuberculosis treatment. Enferm. Infecc. Microbiol. Clin. 29(Suppl 1):47–56 [DOI] [PubMed] [Google Scholar]
- 16. Srivastava S, Gumbo T. 2011. In vitro and in vivo modeling of tuberculosis drugs and its impact on optimization of doses and regimens. Curr. Pharm. Des. 17:2881–2888 [DOI] [PubMed] [Google Scholar]
- 17. Garcia-Contreras L, Sung JC, Muttil P, Padilla D, Telko M, Verberkmoes JL, Elbert KJ, Hickey AJ, Edwards DA. 2010. Dry powder PA-824 aerosols for treatment of tuberculosis in guinea pigs. Antimicrob. Agents Chemother. 54:1436–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karakousis PC, Williams EP, Bishai WR. 2008. Altered expression of isoniazid-regulated genes in drug-treated dormant Mycobacterium tuberculosis. J. Antimicrob. Chemother. 61:323–331 [DOI] [PubMed] [Google Scholar]
- 19. Dutta NK, Alsultan A, Peloquin CA, Karakousis PC. 2013. Preliminary pharmacokinetic study of repeated doses of rifampin and rifapentine in guinea pigs. Antimicrob. Agents Chemother. 57:1535–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sung JC, Garcia-Contreras L, Verberkmoes JL, Peloquin CA, Elbert KJ, Hickey AJ, Edwards DA. 2009. Dry powder nitroimidazopyran antibiotic PA-824 aerosol for inhalation. Antimicrob. Agents Chemother. 53:1338–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nuermberger E, Rosenthal I, Tyagi S, Williams KN, Almeida D, Peloquin CA, Bishai WR, Grosset JH. 2006. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 50:2621–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson JL, Hadad DJ, Boom WH, Daley CL, Peloquin CA, Eisenach KD, Jankus DD, Debanne SM, Charlebois ED, Maciel E, Palaci M, Dietze R. 2006. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 10:605–612 [PubMed] [Google Scholar]
- 23. Peloquin CA, Hadad DJ, Molino LP, Palaci M, Boom WH, Dietze R, Johnson JL. 2008. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 52:852–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenthal IM, Williams K, Tyagi S, Vernon AA, Peloquin CA, Bishai WR, Grosset JH, Nuermberger EL. 2005. Weekly moxifloxacin and rifapentine is more active than the Denver regimen in murine tuberculosis. Am. J. Respir. Crit. Care Med. 172:1457–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klinkenberg LG, Sutherland LA, Bishai WR, Karakousis PC. 2008. Metronidazole lacks activity against Mycobacterium tuberculosis in an in vivo hypoxic granuloma model of latency. J. Infect. Dis. 198:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grosset J, Truffot-Pernot C, Lacroix C, Ji B. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob. Agents Chemother. 36:548–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dhillon J, Dickinson JM, Sole K, Mitchison DA. 1996. Preventive chemotherapy of tuberculosis in Cornell model mice with combinations of rifampin, isoniazid, and pyrazinamide. Antimicrob. Agents Chemother. 40:552–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. 2009. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob. Agents Chemother. 53:3720–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diacon AH, Dawson R, du Bois J, Narunsky K, Venter A, Donald PR, van Niekerk C, Erondu N, Ginsberg AM, Becker P, Spigelman MK. 2012. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob. Agents Chemother. 56:3027–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahmad Z, Peloquin CA, Singh RP, Derendorf H, Tyagi S, Ginsberg A, Grosset JH, Nuermberger EL. 2011. PA-824 exhibits time-dependent activity in a murine model of tuberculosis. Antimicrob. Agents Chemother. 55:239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, Venter A, Donald PR, van Niekerk C, Whitney K, Rouse DJ, Laurenzi MW, Ginsberg AM, Spigelman MK. 2010. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob. Agents Chemother. 54:3402–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, Dowd CS, Lee IY, Kim P, Zhang L, Kang S, Keller TH, Jiricek J, Barry CE., 3rd 2008. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 322:1392–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, Anderson SW, Towell JA, Yuan Y, McMurray DN, Kreiswirth BN, Barry CE, Baker WR. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962–966 [DOI] [PubMed] [Google Scholar]
- 34. Aly S, Wagner K, Keller C, Malm S, Malzan A, Brandau S, Bange FC, Ehlers S. 2006. Oxygen status of lung granulomas in Mycobacterium tuberculosis-infected mice. J. Pathol. 210:298–305 [DOI] [PubMed] [Google Scholar]
- 35. Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, Chan J. 2006. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell. Microbiol. 8:218–232 [DOI] [PubMed] [Google Scholar]
- 36. Lenaerts AJ, Gruppo V, Marietta KS, Johnson CM, Driscoll DK, Tompkins NM, Rose JD, Reynolds RC, Orme IM. 2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49:2294–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tyagi S, Nuermberger E, Yoshimatsu T, Williams K, Rosenthal I, Lounis N, Bishai W, Grosset J. 2005. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 49:2289–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., 3rd 2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76:2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roy CJ, Sivasubramani SK, Dutta NK, Mehra S, Golden NA, Killeen S, Talton JD, Hammoud BE, Didier PJ, Kaushal D. 2012. Aerosolized gentamicin reduces the burden of tuberculosis in a murine model. Antimicrob. Agents Chemother. 56:883–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vandiviere HM, Loring WE, Melvin I, Willis S. 1956. The treated pulmonary lesion and its tubercle bacillus. II. The death and resurrection. Am. J. Med. Sci. 232:30–37 [DOI] [PubMed] [Google Scholar]
- 41. Lenaerts AJ, Hoff D, Aly S, Ehlers S, Andries K, Cantarero L, Orme IM, Basaraba RJ. 2007. Location of persisting mycobacteria in a guinea pig model of tuberculosis revealed by r207910. Antimicrob. Agents Chemother. 51:3338–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dutta NK, Pinn ML, Zhao M, Rudek MA, Karakousis PC. 2013. Thioridazine lacks bactericidal activity in an animal model of extracellular tuberculosis. J. Antimicrob. Chemother. 68:1327–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dorman SE, Goldberg S, Stout JE, Muzanyi G, Johnson JL, Weiner M, Bozeman L, Heilig CM, Feng PJ, Moro R, Narita M, Nahid P, Ray S, Bates E, Haile B, Nuermberger EL, Vernon A, Schluger NW. 2012. Substitution of rifapentine for rifampin during intensive phase treatment of pulmonary tuberculosis: study 29 of the Tuberculosis Trials Consortium. J. Infect. Dis. 206:1030–1040 [DOI] [PubMed] [Google Scholar]
- 44. Rosenthal IM, Tasneen R, Peloquin CA, Zhang M, Almeida D, Mdluli KE, Karakousis PC, Grosset JH, Nuermberger EL. 2012. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob. Agents Chemother. 56:4331–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harper J, Skerry C, Davis SL, Tasneen R, Weir M, Kramnik I, Bishai WR, Pomper MG, Nuermberger EL, Jain SK. 2012. Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions. J. Infect. Dis. 205:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. El Sahly HM, Teeter LD, Jost KC, Jr, Dunbar D, Lew J, Graviss EA. 2011. Incidence of moxifloxacin resistance in clinical Mycobacterium tuberculosis isolates in Houston, Texas. J. Clin. Microbiol. 49:2942–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yeager RL, Munroe WG, Dessau FI. 1952. Pyrazinamide (aldinamide) in the treatment of pulmonary tuberculosis. Am. Rev. Tuberc. 65:523–546 [PubMed] [Google Scholar]
- 48. Canetti G. 1965. Present aspects of bacterial resistance in tuberculosis. Am. Rev. Respir. Dis. 92:687–703 [DOI] [PubMed] [Google Scholar]
- 49. Grimaldo ER, Tupasi TE, Rivera AB, Quelapio MI, Cardano RC, Derilo JO, Belen VA. 2001. Increased resistance to ciprofloxacin and ofloxacin in multidrug-resistant Mycobacterium tuberculosis isolates from patients seen at a tertiary hospital in the Philippines. Int. J. Tuberc. Lung Dis. 5:546–550 [PubMed] [Google Scholar]
- 50. Agrawal D, Udwadia ZF, Rodriguez C, Mehta A. 2009. Increasing incidence of fluoroquinolone-resistant Mycobacterium tuberculosis in Mumbai, India. Int. J. Tuberc. Lung Dis. 13:79–83 [PubMed] [Google Scholar]
- 51. Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, Pistorius C, Krause R, Bogoshi M, Churchyard G, Venter A, Allen J, Palomino JC, De Marez T, van Heeswijk RP, Lounis N, Meyvisch P, Verbeeck J, Parys W, de Beule K, Andries K, McNeeley DF. 2009. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 360:2397–2405 [DOI] [PubMed] [Google Scholar]
- 52. Mphahlele M, Syre H, Valvatne H, Stavrum R, Mannsaker T, Muthivhi T, Weyer K, Fourie PB, Grewal HM. 2008. Pyrazinamide resistance among South African multidrug-resistant Mycobacterium tuberculosis isolates. J. Clin. Microbiol. 46:3459–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.