Abstract
In the absence of an effective vaccine against HIV-1 infection, anti-HIV-1 strategies play a major role in disease control. However, the rapid emergence of drug resistance against all currently used anti-HIV-1 molecules necessitates the development of new antiviral molecules and/or strategies against HIV-1 infection. In this study, we have identified a benzamide derivative named AH0109 that exhibits potent anti-HIV-1 activity at an 50% effective concentration of 0.7 μM in HIV-1-susceptible CD4+ C8166 T cells. Mechanistic analysis revealed that AH0109 significantly inhibits both HIV-1 reverse transcription and viral cDNA nuclear import. Furthermore, our infection experiments indicated that AH0109 is capable of disrupting the replication of HIV-1 strains that are resistant to the routinely used anti-HIV-1 drugs zidovudine, lamivudine, nevirapine, and raltegravir. Together, these findings provide evidence for a newly identified antiviral molecule that can potentially be developed as an anti-HIV-1 agent.
INTRODUCTION
AIDS is a slow degenerative disease of the immune and nervous systems resulting from human immunodeficiency virus type 1 (HIV-1) infection. Global estimates of the HIV-1 pandemic indicate that there are about 34 million people living with HIV-1 and that there have been 12 million cumulative AIDS-related deaths thus far (1). Although anti-HIV-1 chemotherapy has achieved dramatic success by suppressing viral replication to an undetectable level and has improved the quality of life and life expectancy of HIV-1-infected individuals, complete, long-term suppression of HIV-1 replication in HIV-1-infected individuals is still a major challenge due to the rapid emergence of drug resistance (2, 3). Hence, identification of new anti-HIV-1 molecules and novel targets is still an urgent priority as part of a global strategy to combat the spread of HIV-1 infection.
HIV-1 encodes three enzymatic proteins, reverse transcriptase (RT), integrase (IN), and protease (PR), which are critical for its replication. RT and IN are critical during the early steps of the viral replication cycle since they are necessary for reverse transcription and integration of the viral genome, respectively. Soon after HIV-1 enters the cell, RT catalyzes the conversion of viral genomic RNA into double-stranded cDNA (4), and IN mediates the insertion of this newly synthesized cDNA into the host genome (reviewed in reference 5). Moreover, IN also plays a crucial role in HIV-1 cDNA nuclear import and chromatin targeting (6–9). In contrast to RT and IN, HIV-1 PR is involved in virus maturation during the late stages of HIV-1 replication. Due to the essential nature of these viral enzymatic proteins for HIV-1 replication, extensive studies have focused on developing new molecules that specifically target these viral enzymatic proteins. Proteins involved in other viral replication steps, such as HIV-1 nuclear import, membrane fusion, and uncoating, are also targeted by various antiviral molecules (reviewed in reference 10). After 25 years of research, more than 25 anti-HIV-1 compounds have been licensed for clinical use against HIV-1 infection (11–15). The successful development of new anti-HIV-1 agents with novel targets would greatly complement continued efforts to control HIV-1 infection and dissemination.
Based on our preliminary screening of ∼1,500 synthesized molecules, we have identified a 4-chloro-3-{[(2,5-dimethylphenyl)amino]sulfonyl-N-(2-pyridinylmethyl) benzamide with potent anti-HIV-1 activity. This molecule was named AH0109 and has a molecular weight of 429. Subsequent infection analysis indicated that AH0109 was able to inhibit virus replication by affecting the early stages of HIV-1 infection. More detailed mechanistic studies pinpointed that AH0109 specifically impairs HIV-1 reverse transcription and cDNA nuclear import.
MATERIALS AND METHODS
Cells and viruses.
Human embryonic kidney 293T and CD4+ C8166 T cells were cultured in Dulbecco modified Eagle medium and RPMI 1640 medium, each supplemented with 10% fetal bovine serum. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy adult volunteers and cultured as described in previous study (16). The nucleoside reverse transcriptase inhibitor (NRTI) zidovudine (AZT) was purchased from Sigma, Inc. The non-nucleoside reverse transcriptase inhibitors (NNRTI) etravirine (catalog no. 11609) and integrase inhibitor raltegravir (Isentress/MK-0518, catalog no. 11680) were obtained from the NIH AIDS Research and Reference Reagent Program.
HIV-1 HxBru and pNL4.3-GFP+ viruses were generated by transfecting 293T cells with corresponding HIV-1 proviral DNAs, as described previously (17). To produce vesicular stomatitis virus glycoprotein (VSV-G)-pseudotyped viruses, a VSV-G expresser and pNL4.3ΔBgl/Luc+/Nef− proviral DNA were cotransfected into 293T cells (18). At 49 h posttransfection, the supernatants were collected and subjected to ultracentrifugation (35,000 rpm for 1.5 h at 4°C) to concentrate viral particles. AZT-, lamivudine (3TC)-, and nevirapine-resistant HIV-1 viruses (catalog numbers 2526, 2527, 2970, and 1392) were obtained through the NIH AIDS Research and Reference Reagent Program. Raltegravir-resistant HIV-1 was described previously (19). Infectivity of each virus stock was quantified by endpoint titration in C8166 T cells in a 96-well plate by measuring HIV-1-induced syncytium formation and/or anti-HIV-1 p24 immunofluorescence. For different infection experiments, cells were infected at a multiplicity of infection (MOI) of either 0.1 or 1, as determined by the 50% tissue culture infective doses per cell (TCID50/cell) (20).
Compound screening.
Approximately 1,500 synthesized small molecules were obtained from ChemBridge, Inc. (San Diego, CA), and individually dissolved in dimethyl sulfoxide at 5 mg/ml and stored at −20°C until use. To screen the anti-HIV-1 activity of these small molecules, each compound was added to a single well of 96-well plates at a final concentration of 20 μM. Meanwhile, 106 C8166 T cells were mixed with HIV-1 pNL4.3-GFP+ virus (MOI of 0.1), and this cell-virus mixture was immediately transferred to the 96-well plate containing the synthesized molecules such that each well contained a final concentration of 104 cells. At 3 days postinfection, the level of HIV-1-infected cells (as determined by green fluorescent protein [GFP] fluorescence) in each well was measured using a POLARstar Optima microplate reader (BMG Labtech, Germany) to determine whether any compound had an inhibitory effect on HIV-1 infection. AZT-treated C8166 T cells were included as a positive control.
Cytotoxicity assay.
The WST-1 cell proliferation assay (Roche) was used to determine the cytotoxicity of AH0109, as described earlier (16). Briefly, C8166 T cells were cultured at 4 × 103 cells/well in a 96-well format and maintained at 37°C in the presence of various concentrations of AH0109. At day 4, 10 μl of WST-1 reagent was added to each well and incubated at 37°C for 4 h. After continuous shaking for 1 min, the absorbance at an 490-nm wavelength was recorded using a POLARstar Optima microplate reader. The concentration of AH0109 that resulted in a 50% decrease in cell proliferation was defined as the cell culture inhibition concentration (CCID50) of the compound.
Viral infection.
To determine the 50% effective concentration (EC50) of AH0109 against HIV-1, 106 C8166 T cells were infected with HIV-1 pNL4.3-GFP+ virus (MOI of 0.1) in the presence of various concentrations of AH0109. At 3 days postinfection, HIV-1 replication was monitored by measuring HIVp24 antigen in the supernatant by anti-p24 enzyme-linked immunosorbent assay (ELISA). The EC50 was defined as the compound concentration that caused a 50% reduction in HIV-1 p24 level compared to the control that lacked AH0109.
VSV-G-pseudotyped pNL4.3-Luc+ virus (MOI of 0.1) was also used to infect 106 C8166 T cells in the presence of various concentrations of AH0109. At 48 h postinfection, 106 cells from each sample were collected and lysed in 50 μl of luciferase (Luc) lysis buffer (Promega), from which 10 μl of the cell lysate was subjected to the Luc assay using a POLARstar Optima microplate reader. Luc activity was expressed as relative luciferase units. Meanwhile, 106 C8166 T cells were infected with HxBru virus (MOI of 0.1) in the absence or presence of 4.6 μM AH0109. After 48 h of infection, HIV-1-induced syncytium formation was viewed by microscopy.
To determine the ability of AH0109 to inhibit the replication of various strains of drug-resistant HIV-1, 0.5 × 106 C8166 T cells or human PBMCs were infected with HIV-1 wild type or mutants resistant to AZT, 3TC, nevirapine, or raltegravir (MOI of 0.1) in the presence of various concentrations of AH0109 or etravirine. At 3 days postinfection, HIV-1 replication was monitored by measuring p24 antigen in supernatants. The EC90 was defined as the compound concentration that inhibited HIV-1 p24 level production by 90%.
Quantitative real-time PCR analysis.
The effect of AH0109 on HIV-1 cDNA synthesis and nuclear import was evaluated by infecting CD4+ T cells with DNase treated virus, followed by quantitative real-time PCR analysis, as described previously (21). Briefly, 106 C8166 T cells were infected with DNase treated HxBru strain virus (MOI of 1) in the presence or absence of AH0109. Heat-inactivated virus (pretreated at 80°C for 30 min) treated with DNase was included as a plasmid DNA carryover contamination control. At 20 h postinfection, infected cells were collected and DNA was isolated using a QIAamp DNA blood minikit (Qiagen). The levels of total HIV-1 DNA, 2-long terminal repeat (2-LTR) circles, and integrated proviral DNA were quantified by using a Mx3000P real-time PCR system (Stratagene, CA). The quantitative PCR (Q-PCR) for total HIV-1 DNA was carried out using primers targeting the 5′LTR-Gag region of the HIV-1 genomic sequence: TD-Gag Fr (5′-TCTCGACGCAGGACTCG-3′), TD-Gag Rv (5′-TACTGACGCTCTCGCACC-3′), and the TD-Gag probe (5′-6FAM-CTCTCTCCTTCTAGCCTC-MGBNFQ-3′). 2-LTR circle DNA was quantified using the following primers: MH535 (5′-AACTAGGGAACCCACTGCTTAAG-3′), MH536 (5′-TCCACAGATCAAGGATATCTTGTC-3′), and the 2-LTR probe (5′-6FAM-ACACTACTTGAAGCACTCAAGGCAAGCTTT-6TAMRA-3′), targeting LTR-LTR junctions. Integrated proviral DNA was quantified by a modified nested Alu-PCR (8). Total DNA, 2-LTR circle DNA, and integrated DNA were expressed as copy numbers per cell with the DNA template normalized by β-globin gene amplification using the primers β-glob-Fr (5′-CAACTTCATCACGTTCACC-3′) and β-glob-Rv (5′-GAAGAGCCAAGGACAGGTAC-3′).
Virion-associated reverse transcriptase assay.
Virion-associated RT activity of HIV-1 stocks was assayed using a colorimetric reverse transcriptase assay (Roche Applied Science, Indianapolis, IN) (22, 23). The viruses were pelleted by centrifugation at 35,000 rpm for 1 h at 4°C. Reverse transcriptase concentrations of viruses were determined by using several dilutions of recombinant HIV-1 RT as a standard curve. Viruses containing 100 pg of reverse transcriptase (equal to an MOI of 1) were resuspended and lysed by adding 20 μl of lysis buffer per reaction tube. Reactions were performed by adding 20 μl of AH0109 or RT inhibitor etravirine diluted in lysis buffer, 20 μl of reaction mixture into each reaction tube containing lysed viruses. The reaction mixture contained primer-template hybrid poly(A)-oligo(dT)15 (750 mA260nm/ml), digoxigenin, and biotin-labeled nucleotides (10 μM). Reactions were incubated at 37°C for 15 h, followed by a sandwich ELISA. Briefly, the samples were transferred into a streptavidin-coated microplates for 1 h of incubation at 37°C, followed by adding an anti-digoxigenin antibody conjugated to peroxidase (anti-DIG-POD) to the plate and allowed to bind to the digoxigenin-labeled nucleotides. Finally, the peroxidase substrate ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid)] was added, and the absorbances of samples were measured by using a microplate (ELISA) reader at 405 nm. The levels of RT activity in the presence of AH0109 or etravirine were calculated as a percentage of that of the control.
In vitro integration assay.
The effect of AH0109 on HIV-1 integrase activity was determined by using an in vitro HIV-1 integration assay kit (XpressBio Life Science Products), according to the manufacturer's instructions. Briefly, 100 μl of HIV-1 integrase protein (200 nM) was added onto streptavidin-coated 96-well plate coated with a double-stranded HIV-1 LTR U5 donor substrate (DS) oligonucleotide containing an end-labeled biotin. Various concentrations of AH0109 or raltegravir were then added to the reaction, followed by the addition of different double-stranded target substrate (TS) oligonucleotides containing 3′-end modifications. HIV-1 integrase cleaves the terminal two bases from the exposed 3′ end of the HIV-1 LTR and catalyzes a strand-transfer reaction to integrate the DS into the TS. The reaction products were colorimetrically detected using a horseradish peroxidase-labeled antibody directed against the TS 3′-end modification. HIV-1 integrase activity in the presence of AH0109 or raltegravir was calculated as a percentage of the control.
RESULTS
Characterization of anti-HIV-1 activity of AH0109.
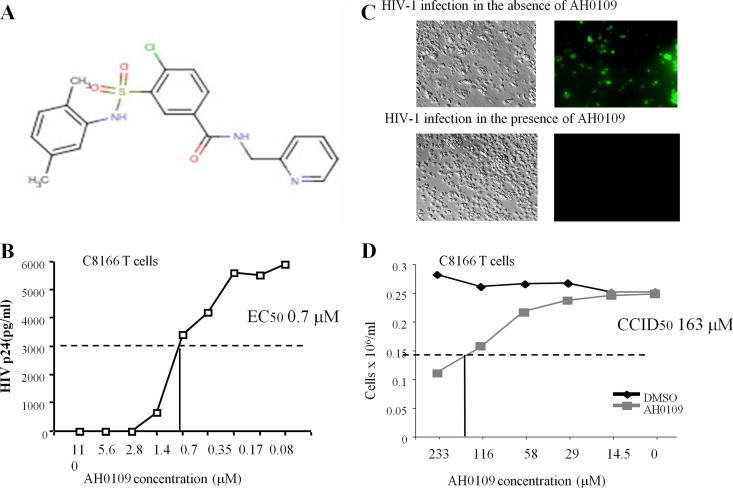
Initially, a large scale screening of ∼1,500 undefined synthesized compounds was performed to select molecules that have anti-HIV-1 activity. Briefly, C8166 T cells were infected with pNL4.3-GFP+ virus in the presence of different compounds in 96-well plates and the extent of HIV-1 infection (as determined by GFP fluorescence) was measured using a POLARstar Optima microplate reader. This screening identified a compound, named AH0109, capable of significantly inhibiting HIV-1 replication. This compound is a benzamide derivative and its chemical structure is 4-chloro-3-{[(2,5-dimethylphenyl)amino]sulfonyl}-N-(2-pyridinylmethyl) benzamide (Fig. 1A). To further evaluate the efficiency of AH0109 against HIV-1 infection, we determined its 50% effective dose (EC50) in C8166 T cells. To this end, C8166 T cells were infected with HIV-1 pNL4.3-GFP+ virus in the presence of various concentrations of AH0109, as shown in Fig. 1B. At 3 days postinfection, the HIV-1 replication levels were determined by measuring the HIV-1 p24 antigen released into the supernatant. The EC50 of AH0109 was determined to be 0.7 μM. A 100% inhibition of HIV-1 infection was achieved when AH0109 was used at a concentration of 2.8 μM (Fig. 1B and C).
Fig 1.
Characterization of anti-HIV-1 activity and cytotoxicity of AH0109. (A) Chemical structure of AH0109. (B) Determination of the 50% effective concentration (EC50) of AH0109 against HIV-1 pNL4.3-GFP+ virus infection (MOI of 0.1) in 0.5 × 106 C8166 T cells. (C) At 3 days postinfection with pNL4.3-GFP+ virus, the infected C8166 T cells (GFP-positive) in the presence or absence of AH0109 were visualized by fluorescence microscopy. (D) Determination of 50% cell culture inhibitory dose (CCID50) of AH0109 in C8166 T cells by using WST-1 cell proliferation and a cytotoxicity assay kit (Roche). The results in panels B and D are representative of three independent experiments, respectively.
Meanwhile, we evaluated the cytotoxicity of AH0109 in C8166 T cells. C8166 T cells were treated with different concentrations of AH0109 as indicated in Fig. 1D. After 4 days of treatment, cell proliferation was detected using the WST-1 assay. The results indicate that the 50% cell culture inhibitory dose (CCID50) of AH0109 is ∼163 μM (Fig. 1D); the calculated therapeutic index (CCID50/EC50) of AH0109 is 233.
AH0109 inhibits HIV-1 replication by targeting the postentry step.
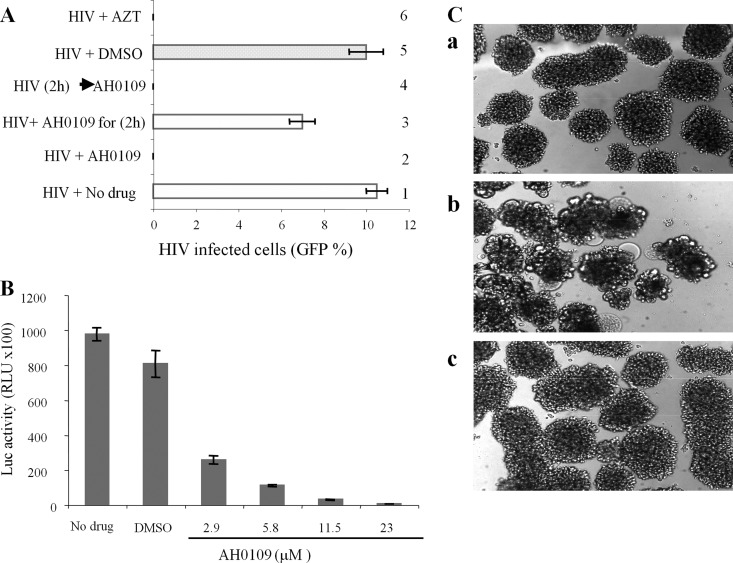
To gain insight into the mechanism of action of AH0109 on HIV-1 replication, we first evaluated steps in the HIV-1 replication cycle that could be blocked by AH0109. C8166 T cells were infected with HIV-1 pNL4.3-GFP+ virus in the presence (Fig. 2A, bars 2 and 3) or absence (Fig. 2A, bars 1, 4, and 5) of AH0109. After 2 h of infection, the residual virus was washed away, and the cells were then cultured in medium either with AH0109 (bars 2 and 4) or without AH0109 (bars 1, 3, and 5). Meanwhile, HIV-1-infected C8166 T cells were cultured either in the presence of the reverse transcriptase inhibitor AZT (Fig. 2A, bar 6) as a positive control. After 48 h, HIV-1 replication was analyzed by examining HIV-1-infected cells expressing-GFP fluorescence. The results showed that the presence of AH0109 for only 2 h at the beginning of the infection did not block HIV-1 replication (Fig. 2A, bar 3), suggesting that this compound does not affect HIV-1 entry. In contrast, the addition of AH0109 after 2 h of infection still significantly inhibited HIV-1 infection (Fig. 2A, bar 4), indicating that AH0109, blocks HIV-1 replication at a postentry step.
Fig 2.
AH0109 inhibits HIV-1 replication by targeting the postentry step. (A) 106 CD4+ C8166 T cells were infected with HIV-1 pNL4.3-GFP+ virus (MOI of 0.1) in the presence of AH0109 (at a final concentration of 4.6 μM). After 2 h of infection, the cells were either continued culture in medium containing AH0109 for 48 h (bar 2) or without AH0109 (bar 3). In parallel, C8166 T cells were first infected by virus for 2 h and then treated with AH0109 (bar 4). Meanwhile, HIV-1-infected C8166 T cells were cultured in the presence of the RT inhibitor AZT (bar 6) or without the compound (bars 1 and 5), as positive and negative controls. After 48 h, GFP-positive cells were counted by fluorescence microscopy and calculated as the percentage of HIV-1-infected cells in culture. (B) 106 C8166 T cells were infected with a VSV-G-pseudotyped pNL4.3-Luc+ virus (MOI of 0.1) in the presence or absence of AH0109. At 48 h postinfection, the cell-associated luciferase activity was measured, as described in Materials and Methods. The results in panels A and B are mean values ± the standard deviations from three independent experiments. (C) AH0109 inhibited HIV-1-induced cytopathic effect. A total of 106 CD4+ C8166 cells were mock infected (a) or infected with HIV-1 HxBru strain (MOI of 0.1) in the absence (b) or presence (c) of 4.6 μM AH0109. After 48 h, HIV-1-induced syncytium formation was investigated by light microscopy, and images were captured. The results are representative of two independent experiments.
To further confirm that AH0109 inhibits HIV-1 replication through a postentry step, we also used a VSV-G-pseudotyped pNL4.3-Luc+ HIV-1 to infect C8166 T cells in the presence of AH0109, because VSV-G-pseudotyped HIV-1 entry is independent of the CD4 receptor and the CXCR4 coreceptor (24). As expected, AH0109 also inhibited VSV-G-pseudotyped HIV-1 replication in a dose-dependent manner (Fig. 2B).
We also tested the effect of AH0109 on infection of HxBru virus, another HIV-1 laboratory strain (25). The results clearly indicated that AH0109 completely inhibited the infection of HxBru virus in C8166 T cells and that no p24 antigens were detected in AH0109-treated HIV-infected cells (data not shown). Also, the results indicate that HIV-1 induced cytopathic effect (syncytium formation) was blocked by AH0109 (Fig. 2C). All of these observations indicate that AH0109 targets a postentry step during HIV-1 infection and blocks viral replication and cytopathic effect.
HIV-1 reverse transcription is affected by AH0109.
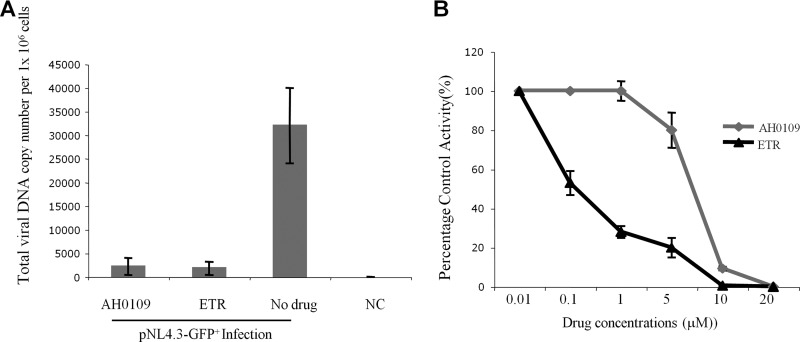
Since AH0109 targets a postentry step during HIV-1 infection, we next sought to determine whether HIV-1 reverse transcription might be affected by the presence of the compound. Briefly, C8166 T cells were infected with HIV-1 pNL4.3-GFP+ virus in the presence of either AH0109 or a known reverse transcriptase inhibitor, etravirine (26), which was used as a positive control. At 20 h postinfection, the cells were harvested, and the total HIV-1 DNA level was measured by real-time Q-PCR. The results showed that the level of HIV-1 cDNA was drastically reduced in infected cells treated with AH0109 or etravirine (Fig. 3A), suggesting that HIV-1 reverse transcription is inhibited by AH0109 (Fig. 3A).
Fig 3.
AH0109 disrupts HIV-1 DNA synthesis by targeting reverse transcriptase. (A) 106 CD4+ C8166 T cells were infected with HIV-1 pNL4.3-GFP+ virus (MOI of 1) in the presence or absence of AH0109 (4.6 μM). An NNRTI, etravirine (ETR; 4.6 μM), was also used as a control. After 20 h of infection, the cells were collected, and DNA was isolated by using a QIAamp DNA blood minikit (Qiagen). The total HIV-1 DNA levels were measured by real-time Q-PCR analysis. (B) Effect of AH0109 on virion-associated reverse transcriptase activity. A colorimetric enzyme immunoassay that quantifies retroviral reverse transcriptase activity based on incorporation of digoxigenin- and biotin-labeled dUTP into DNA was used. Etravirine was included as a positive control. The percent control activity (%) indicates a ratio of reverse transcriptase activity in the presence of AH0109 or etravirine versus the absence of any compound. These data are means of results from three independent experiments and are expressed with the standard deviations.
To further confirm the observation described above, we utilized a reverse transcription assay to test the effect of AH0109 on virion-associated reverse transcriptase activity. Figure 3B shows the relative virion-associated RT activity in the presence of different concentrations of AH0109 or etravirine. HIV-1 reverse transcription was inhibited in a dose-dependent manner in the presence of AH0109 (Fig. 3B), indicating that AH0109-mediated disruption of HIV-1 DNA synthesis is attributable to its inhibition of viral reverse transcriptase. However, etravirine (IC50 of 0.1 μM) was more effective than AH0109 (IC50 of 7.5 μM) (Fig. 3B).
AH0109 disrupts HIV-1 nuclear import.
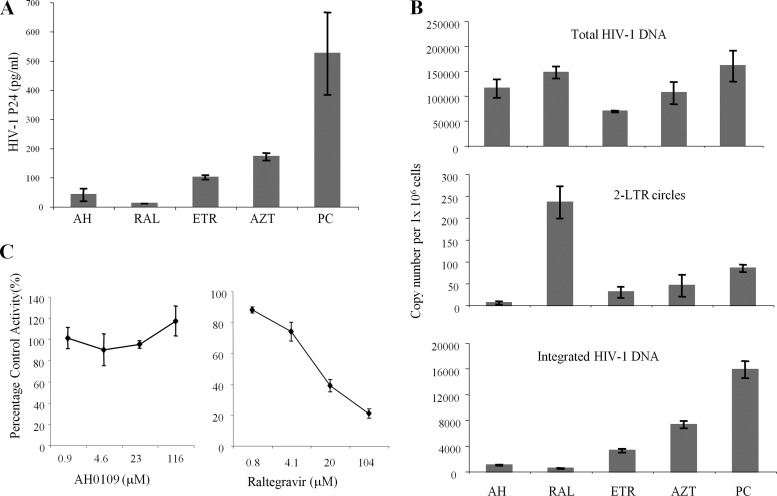
Next, we sought to determine whether AH0109 also affected other early step(s) of HIV-1 replication. To answer this question, C8166 T cells were infected with HIV-1 HxBru for 8 h prior to the addition of AH0109 to allow viral reverse transcription to be processed. Then, AH0109 (at final concentration of 4.6 μM) was added into the cell culture medium and was maintained for 48 h (Fig. 4A). Meanwhile, an NRTI (AZT), NNRTI (etravirine), or integrase inhibitor (raltegravir) was used as a control. At 48 h postinfection, the HIV-1 replication was monitored by measuring HIV-1 p24 levels in the supernatant (Fig. 4A). As expected, adding integrase inhibitor raltegravir 8 h after infection almost completely blocked virus replication, whereas the addition of HIV-1 RT inhibitors (AZT or etravirine) resulted in 3- to 5-fold-lower HIV-1 p24 production. Significantly, after AH0109 was added at 8 h postinfection, the HIV-1 p24 level was even lower than that of samples treated with AZT or etravirine, suggesting that this compound also inhibited other step(s) downstream of reverse transcription.
Fig 4.
Effect of AH0109 on HIV-1 nuclear import and replication. (A) 106 C8166 T cells were infected with HIV-1 HxBru strain (MOI of 0.1) for 8 h, followed by the addition of AH0109 (AH), raltegravir (RAL), or etravirine (ETR) at a concentration of 4.6 μM or added AZT at 1 μM. At 48 h postinfection, the level of HIV-1 p24 in the supernatant was measured. (B) 2 × 106 C8166 T cells were infected with HIV-1 HxBru strain (MOI of 1) for 8 h, and AH0109, RAL, ETR, or AZT was then added to the cell culture. After 20 h of infection, the levels of total HIV-1 DNA (upper panel), 2-LTR circles (middle panel) and integrated DNA (lower panel) were measured by real-time PCR. (C) Effect of AH0109 on HIV-1 integrase activity in vitro using an in vitro HIV-1 integration assay kit (XpressBio Life Science Products) according to the manufacturer's instructions. These data are means of results from three independent experiments and are expressed with the standard deviations.
To explore the possibility that AH0109 inhibits additional steps in the viral replication cycle, viral DNA synthesis, nuclear import (2-LTR circle DNA), and integration were further analyzed by real-time Q-PCR. C8166 T cells were first infected with HIV-1 HxBru for 8 h, followed by the addition of AH0109, and collected at 20 h postinfection. The results revealed that, although reverse transcription already has been processing after 8 h of infection, the addition of the RT inhibitor etravirine still significantly reduced the total HIV-1 DNA levels to 43% of the control. However, similar to AZT, AH0109 only resulted in an about 30% decrease in viral DNA synthesis (Fig. 4B, upper panel). The reduction of HIV-1 total DNA induced by etravirine or AZT led to a corresponding decrease in viral 2-LTR circular DNA and integrated DNA, whereas raltegravir almost completely blocked viral DNA integration and resulted in the accumulation of viral 2-LTR circular DNA in the nuclei of infected cells (Fig. 4B, middle and lower panels). Interestingly, despite the fact that AH0109 only resulted in a slight decrease in viral DNA synthesis, both the viral 2-LTR circular DNA levels and the levels of integrated DNA in AH0109-treated cells were ∼6-fold lower than in the control group (Fig. 4B, middle and lower panels), indicating that AH0109 not only affected reverse transcription but also inhibited HIV-1 cDNA nuclear translocation.
Similar decreased ratios of viral 2-LTR circular DNA and integrated DNA levels were noted in AH0109-treated HIV-1-infected T cells suggested that HIV-1 integration was not significantly affected by the compound. To further confirm this observation, we used an in vitro HIV-1 integration assay to test whether AH0109 could affect the catalytic activity of IN. Consistently, the results did not show any negative effect of AH0109 on integrase-mediated integration reaction in vitro (Fig. 4C), whereas raltegravir, a known specific integrase inhibitor (27), significantly reduced this activity in a dose-dependent manner. Thus, we conclude that AH0109 is able to inhibit both HIV-1 reverse transcription and the nuclear import of the HIV-1 preintegration complex (PIC).
Effect of AH0109 on NRTI and NNRTI-resistant virus infection.
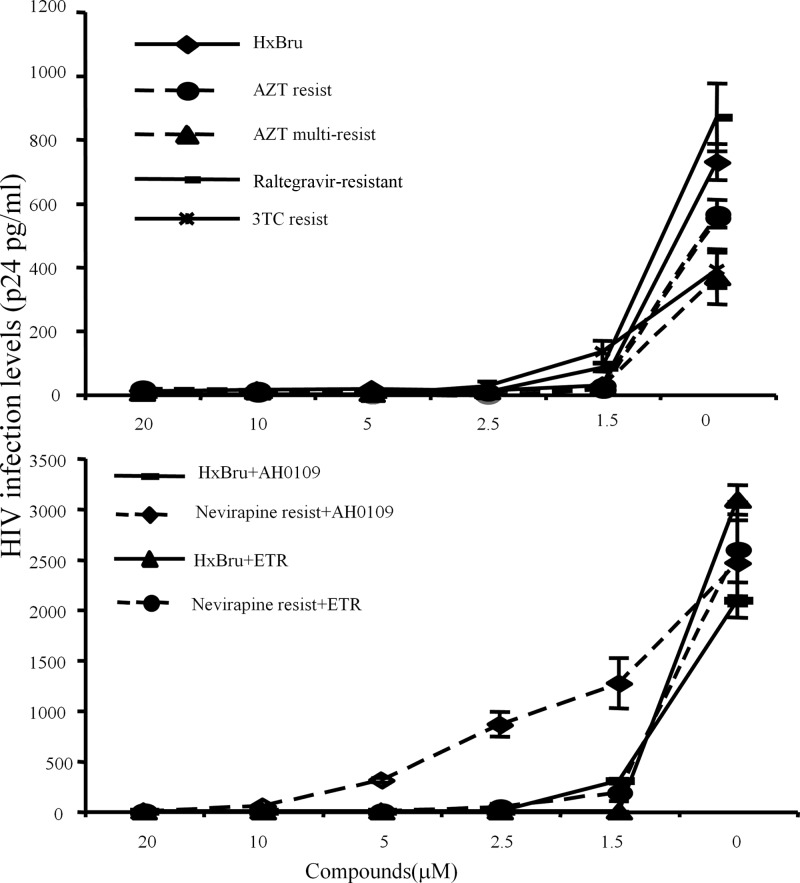
Since AH0109 exhibits a potent anti-HIV-1 activity, we next evaluated its antiviral activity against various HIV-1 drug-resistant strains. First, C8166 T cells were infected with HIV-1 that was resistant to nucleoside reverse transcriptase inhibitors (NRTIs), including AZT, 3TC, or the integrase inhibitor raltegravir, in the presence of different concentrations of AH0109 (as indicated in Fig. 5, upper panel). After 4 days of infection, the replication of each resistant variant was monitored by the measurement of HIV-1 p24 protein levels in supernatants. We found that AH0109 displayed an excellent inhibitory activity against both wild-type and NRTI- and integrase inhibitor-resistant HIV-1 viruses (Table 1, EC90 from 0.8 to 1.4 μM).
Fig 5.
HIV-1 viruses resistant to AZT, 3TC, nevirapine, or raltegravir are sensitive to AH0109. (A) 0.5 × 106 C8166 T cells were infected with wild-type HIV-1 or AZT-, 3TC-, or raltegravir-resistant HIV-1 variants (MOI of 0.1) in the presence of different concentration of AH0109, as indicated. (B) 0.5 × 106 C8166 T cells were infected with wild-type HIV-1 or nevirapine-resistant HIV-1 variants (MOI of 0.1) in the presence of different concentration of AH0109 or ETR, as indicated. After 3 days of infection, viral replication levels were determined by measuring the levels of HIV-1 p24 antigen in supernatant. These data are means of results from three independent experiments and are expressed with the standard deviations.
Table 1.
Antiviral activity of AH0109 against resistant viruses
| Virus | Mean EC90 ± SD (μM)a |
|
|---|---|---|
| C8166 T cells | PBMCs | |
| HxBru | 1.4 ± 0.01 | 1.25 ± 0.03 |
| AZT resistant | 1.0 ± 0.02 | NT |
| AZT multiresistant | 0.8 ± 0.05 | NT |
| 3TC resistant | 1.0 ± 0.03 | NT |
| Reltagravir resistant | 1.25 ± 0.10 | NT |
| Nevirapine resistant | 5 ± 0.18 | 2.5 ± 0.2 |
EC90, the concentration of AH0109 that inhibited p24 production in the antiviral assays by 90%. Data are expressed as mean values from three independent experiments. NT, not tested.
We next tried to determine the activity of AH0109 against an NNRTI-resistant virus in CD4+ C8166 T cells and human PBMCs. This virus is resistant to nevirapine and related NNRTIs because of a single RT nucleotide mutation (Y181C) (28). Meanwhile, we also included etravirine as control since it has the potential activity against HIV-1 strains resistant to newer NNRTIs. As expected, etravirine displayed a very strong antiviral activity against wild-type HIV-1 with an EC90 < 0.078 μM (data not shown). The nevirapine-resistant virus still was sensitive to etravirine but its EC90 was increased to 0.625 μM. Similar to etravirine, AH0109 remained active against nevirapine-resistant virus, with a 2- to 4-fold increased EC90 in PBMCs and CD4+ T cells (Fig. 5, lower panel, and Table 1). Therefore, AZT-, 3TC-, and raltegravir-resistant viruses are highly sensitive to AH0109, whereas the sensitivity of nevirapine-resistant virus to AH0109 was reduced 2- to 4-fold.
DISCUSSION
This study has identified a benzamide derivative (AH0109) that possesses potent anti-HIV-1 activity with an EC50 as low as 0.7 μM, which is well below the 50% cellular toxicity concentration of 163 μM. Mechanistic analysis revealed that AH0109 significantly impairs viral cDNA nuclear translocation, in addition to its effect on HIV-1 reverse transcription. Furthermore, AH0109 was also capable of effectively inhibiting the replication of AZT-, 3TC-, nevirapine-, and raltegravir-resistant HIV-1 strains.
One of the main focuses of past studies that targeted the inhibition of HIV-1 replication was reverse transcription, catalyzed by viral reverse transcriptase (RT) (13, 29). Our initial data indicated that AH0109 acts at early stages of HIV-1 replication (Fig. 2A); we therefore examined HIV-1 reverse transcription in the presence of AH0109 using Q-PCR, and the results showed that total viral DNA synthesis is greatly reduced in the presence of AH0109. Furthermore, an in vitro reverse transcription assay revealed AH0109 was able to specifically inhibit the catalytic activity of recombinant reverse transcriptase (Fig. 3B). These observations indicate that AH0109 is an HIV-1 reverse transcriptase inhibitor.
The attractive profile of AH0109 is that it possesses a novel mode of anti-HIV action independent from HIV RT inhibition. When AH0109 was added into cell cultures after 8 h of HIV-1 infection, it only slightly inhibited total viral DNA synthesis. However, both 2-LTR circular and integrated viral DNA were greatly reduced (∼6-fold) (Fig. 4B). This suggests that AH0109 is also able to affect HIV-1 cDNA nuclear import. The mechanism underlining the action of AH0109 on HIV-1 nuclear import is not clear. It is well known that HIV-1 cDNA nuclear import is a complex process and involves several viral and/or cellular factors (reviewed in reference 30). It is possible that AH0109 targets viral components or their interactions with different cellular cofactors required for HIV-1 nuclear import. Alternatively, AH0109 may simultaneously affect both reverse transcription and viral nuclear import. A previous study reported that an anti-HIV-1 compound, CNI-H0294, binds to reverse transcriptase, and this interaction is required for CNI-H0294 to subsequently target the nuclear import signal of the HIV-1 MA protein and inhibit virus nuclear translocation (31). The latter study also suggested that modifications in a CNI-H0294 side chain would allow this compound to inhibit both RT activity and viral PIC nuclear translocation. Therefore, further investigation is going on in order to test whether AH0109 may first bind to PIC-associated RT and then target other viral and/or cellular cofactors of PIC that are required for HIV-1 nuclear import.
The use of antiretroviral therapy in developed countries is associated with an increased level of resistance to anti-HIV-1 chemotherapy. In Europe (32) and in the United States (33), an estimated 9 to 15% of antiretroviral agent-naive infected subjects harbor viruses with at least one drug resistance mutation. Thus, before a new anti-HIV-1 agent can be considered sufficient and effective, it is necessary to evaluate its effectiveness against different drug-resistant strains of HIV-1. We demonstrated here that AH0109 is capable of inhibiting the replication of AZT-, 3TC-, raltegravir-, and nevirapine-resistant HIV-1 viruses (Fig. 5 and Table 1). We also noticed that the sensitivity of nevirapine-resistant viruses to AH0109 was slightly reduced. A similar inhibition pattern was observed in the presence of etravirine. The Y181C mutation, which is carried by nevirapine-resistant virus, is one of the two most common NNTRI resistance mutations (the other is K103N), but single mutations generally do not render virus strongly resistant to etravirine (34). Interestingly, this virus had a similar level of susceptibility to AH0109, but the underlying mechanism remains to be defined. Since AH0109 acts at both the viral reverse transcription and nuclear import steps, we could not exclude the possibility that even though the RT mutation Y181C reduces the sensitivity of virus to AH0109 during DNA synthesis, it might not affect the effect of AH0109 on HIV-1 nuclear import. Further optimization of the AH0109-associated anti-HIV-1 nuclear import function would improve its activity against NNRTI-resistant viruses. In addition, the ability of HIV-1 to rapidly develop resistance to AH0109 must also be addressed in future studies.
In conclusion, we examined here a newly identified benzamide derivative, AH0109, that displayed high potency against wild-type HIV-1 and a panel of singly and multiply mutant strains isolated from HIV-1 patients treated with NRTI, NNRTI, and integrase inhibitor. Our results also demonstrate that AH0109 can be distinguished from other members of the NNTRI class due to its significantly negative effect on HIV-1 nuclear import. Thus, this compound may be clinically useful in combination with other anti-HIV agents in order to be more effective against various drug-resistant HIV-1 strains and reduce the emergency of drug resistance.
ACKNOWLEDGMENTS
We thank B. Larder, S. Kemp, J. Mellors, and R. Schinazi for the AZT- and 3TC-resistant viruses, and we thank Tibotec Pharmaceuticals, Inc., and Merck & Company, Inc., for the etravirine (catalog no. 11609) and raltegravir (Isentress/MK-0518) (catalog no. 11680), which were obtained through the AIDS Research Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health. We also greatly appreciate the editorial review by Trina Racine.
L.C. received a grant from the Natural Science Foundation of Hunan Province, China (10JJ2012), which contributed to this work. Z.A. received a postdoctoral fellowship from the CIHR International Infectious Disease and Global Health Training Program (IID&GHTP). K.D.J. received scholarships from the Manitoba Health Research Council/Manitoba Institute of Child Health and the IID&GHTP program. This study was financially supported by grants from the Canadian Foundation for AIDS Research (CANFAR grant 023-013) and the Leaders Opportunity Fund Award from the Canadian Foundation of Innovation to X.Y.
Footnotes
Published ahead of print 13 May 2013
REFERENCES
- 1. Demberg T, Robert-Guroff M. 2012. Controlling the HIV/AIDS epidemic: current status and global challenges. Front. Immunol. 3:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richman DD. 1993. HIV drug resistance. Annu. Rev. Pharmacol. Toxicol. 33:149. [DOI] [PubMed] [Google Scholar]
- 3. von Wyl V, Yerly S, Boni J, Shah C, Cellerai C, Klimkait T, Battegay M, Bernasconi E, Cavassini M, Furrer H, Hirschel B, Vernazza PL, Ledergerber B, Gunthard HF. 2011. Incidence of HIV-1 drug resistance among antiretroviral treatment-naive individuals starting modern therapy combinations. Clin. Infect. Dis. 54:131–140 [DOI] [PubMed] [Google Scholar]
- 4. Prasad VR, Goff SP. 1990. Structure-function studies of HIV reverse transcriptase. Ann. N. Y. Acad. Sci. 616:11–21 [DOI] [PubMed] [Google Scholar]
- 5. Chiu TK, Davies DR. 2004. Structure and function of HIV-1 integrase. Curr. Top. Med. Chem. 4:965–977 [DOI] [PubMed] [Google Scholar]
- 6. Ao Z, Fowke KR, Cohen EA, Yao X. 2005. Contribution of the C-terminal tri-lysine regions of human immunodeficiency virus type 1 integrase for efficient reverse transcription and viral DNA nuclear import. Retrovirology 2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gallay P, Hope T, Chin D, Trono D. 1997. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl. Acad. Sci. U. S. A. 94:9825–9830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ao Z, Danappa Jayappa K, Wang B, Zheng Y, Kung S, Rassart E, Depping R, Kohler M, Cohen EA, Yao X. 2010. Importin α3 interacts with HIV-1 integrase and contributes to HIV-1 nuclear import and replication. J. Virol. 84:8650–8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maertens G, Cherepanov P, Pluymers W, Busschots K, De Clercq E, Debyser Z, Engelborghs Y. 2003. LEDGF/p75 is essential for nuclear and chromosomal targeting of HIV-1 integrase in human cells. J. Biol. Chem. 278:33528–33539 [DOI] [PubMed] [Google Scholar]
- 10. Adamson CS, Freed EO. 2010. Novel approaches to inhibiting HIV-1 replication. Antivir. Res. 85:119–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Temesgen Z, Warnke D, Kasten MJ. 2006. Current status of antiretroviral therapy. Expert Opin. Pharmacother. 7:1541–1554 [DOI] [PubMed] [Google Scholar]
- 12. Clercq ED. 2009. Anti-HIV drugs:25 compounds approved within 25 years after the discovery of HIV. Int. J. Antimicrob. Agents 33:307–320 [DOI] [PubMed] [Google Scholar]
- 13. Le Grice SF. 2012. Human immunodeficiency virus reverse transcriptase: 25 years of research, drug discovery, and promise. J. Biol. Chem. 287:40850–40857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hazuda DJ. 2012. HIV integrase as a target for antiretroviral therapy. Curr. Opin. HIV AIDS 7:383–389 [DOI] [PubMed] [Google Scholar]
- 15. Didigu CA, Doms RW. 2012. Novel approaches to inhibit HIV entry. Viruses 4:309–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ao Z, Wang X, Bello AJ, Danappa Jayappa K, Yu Z, Fowke K, He X, Chen X, Li J, Kobinger GP, Yao X. 2011. Characterization of anti-HIV activity mediated by R88-Apobec3G mutant fusion proteins in CD4+ T cells, PBMCs, and macrophages. Hum. Gene Ther. 22:1225–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ao Z, Yu Z, Wang L, Zheng Y, Yao X. 2008. Vpr14-88-Apobec3G fusion protein is efficiently incorporated into Vif-positive HIV-1 particles and inhibits viral infection. PLoS One 3:e1995. 10.1371/journal.pone.0001995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng Y, Ao Z, Wang B, Jayappa KD, Yao X. 2011. Host protein Ku70 binds and protects HIV-1 integrase from proteasomal degradation and is required for HIV replication. J. Biol. Chem. 286:17722–17735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bar-Magen T, Sloan R, Donahue DA, Kuhl BD, Zabeida A, Xu H, Oliveira M, Hazuda DJ, Wainberg MA. 2010. Identification of novel mutations responsible for resistance to MK-2048, a second-generation HIV-1 integrase inhibitor. J. Virol. 84:9210–9216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soudeyns H, Yao XI, Gao Q, Belleau B, Kraus JL, Nguyen-Ba N, Spira B, Wainberg MA. 1991. Anti-human immunodeficiency virus type 1 activity and in vitro toxicity of 2′-deoxy-3′-thiacytidine (BCH-189), a novel heterocyclic nucleoside analog. Antimicrob. Agents Chemother. 35:1386–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jayappa KD, Ao Z, Yang M, Wang J, Yao X. 2011. Identification of critical motifs in HIV-1 integrase required for importin α3 interaction and its involvement in viral cDNA nuclear import. J. Mol. Biol. 410:847–862 [DOI] [PubMed] [Google Scholar]
- 22. Han H, He W, Wang W, Gao B. 2011. Inhibitory effect of aqueous dandelion extract on HIV-1 replication and reverse transcriptase activity. BMC Complement Altern. Med. 11:112. 10.1186/1472-6882-11-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu Z, Kuritzkes DR. 2011. Interaction of reverse transcriptase (RT) mutations conferring resistance to lamivudine and etravirine: effects on fitness and RT activity of human immunodeficiency virus type 1. J. Virol. 85:11309–11314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. U. S. A. 90:8033–8037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yao X-J, Subbramanian RA, Rougeau N, Boisvert F, Bergeron D, Cohen EA. 1995. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J. Virol. 69:7032–7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schiller DS, Youssef-Bessler M. 2009. Etravirine: a second-generation nonnucleoside reverse transcriptase inhibitor (NNRTI) active against NNRTI-resistant strains of HIV. Clin. Ther. 31:692–704 [DOI] [PubMed] [Google Scholar]
- 27. Grinsztejn B, Nguyen BY, Katlama C, Gatell JM, Lazzarin A, Vittecoq D, Gonzalez CJ, Chen J, Harvey CM, Isaacs RD. 2007. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet 369:1261–1269 [DOI] [PubMed] [Google Scholar]
- 28. Richman D, Shih CK, Lowy I, Rose J, Prodanovich P, Goff S, Griffin J. 1991. Human immunodeficiency virus type 1 mutants resistant to nonnucleoside inhibitors of reverse transcriptase arise in tissue culture. Proc. Natl. Acad. Sci. U. S. A. 88:11241–11245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maga G, Radi M, Gerard MA, Botta M, Ennifar E. 2010. HIV-1 RT inhibitors with a novel mechanism of action: NNRTIs that compete with the nucleotide substrate. Viruses 2:880–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jayappa KD, Ao Z, Yao X. 2012. The HIV-1 passage from cytoplasm to nucleus: the process involving a complex exchange between the components of HIV-1 and cellular machinery to access nucleus and successful integration. Int. J. Biochem. Mol. Biol. 3:70–85 [PMC free article] [PubMed] [Google Scholar]
- 31. Popov S, Dubrovsky L, Lee MA, Pennathur S, Haffar O, LAYa Tonge P, Ulrich P, Rexach M, Blobel G, Cerami A, Bukrinsky M. 1996. Critical role of reverse transcriptase in the inhibitory mechanism of CNI-H0294 on HIV-1 nuclear translocation. Proc. Natl. Acad. Sci. U. S. A. 93:11859–11864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vercauteren J, Wensing AM, van de Vijver DA, Albert J, Balotta C, Hamouda O, Kucherer C, Struck D, Schmit JC, Asjo B, Bruckova M, Camacho RJ, Clotet B, Coughlan S, Grossman Z, Horban A, Korn K, Kostrikis L, Nielsen C, Paraskevis D, Poljak M, Puchhammer-Stockl E, Riva C, Ruiz L, Salminen M, Schuurman R, Sonnerborg A, Stanekova D, Stanojevic M, Vandamme AM, Boucher CA. 2009. Transmission of drug-resistant HIV-1 is stabilizing in Europe. J. Infect. Dis. 200:1503–1508 [DOI] [PubMed] [Google Scholar]
- 33. Wheeler WH, Ziebell RA, Zabina H, Pieniazek D, Prejean J, Bodnar UR, Mahle KC, Heneine W, Johnson JA, Hall HI. 2010. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.-2006. AIDS 24:1203–1212 [DOI] [PubMed] [Google Scholar]
- 34. Johnson VA, Brun-Vezinet F, Clotet B, Gunthard HF, Kuritzkes DR, Pillay D, Schapiro JM, Richman DD. 2008. Update of the drug resistance mutations in HIV-1: Spring 2008. Top. HIV Med. 16:62–68 [DOI] [PubMed] [Google Scholar]