Abstract
Colistin is an old antibiotic used in the treatment of Gram-negative infections. It was once suspended because of its nephrotoxic effect but has since been reintroduced due to multidrug-resistant bacterial infections. The pathogenesis of colistin-associated nephropathy has not been clarified, and there is currently no effective therapeutic or prophylactic agent available. The aim of this study was to investigate the roles of caspase-associated apoptosis and caspase 1, calpain 1, inducible nitric oxide synthase (iNOS), and endothelial nitric oxide synthase (eNOS) expression in the pathogenesis of colistin-associated nephrotoxicity and the effect of grape seed proanthocyanidin extract (GSPE) in preventing it. Twenty-four rats were divided into three groups: control, colistin, and colistin plus GSPE (colistin+GSPE). Colistin-associated nephropathy was induced by the administration of 300,000 IU/kg of body weight/day colistin intraperitoneally for 7 days. The experiment was discontinued on the seventh day. Blood was collected for measurements of blood urea nitrogen (BUN) and creatinine levels. Histopathological examination of kidney tissue and caspase 1 and 3, iNOS, eNOS, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL), and calpain 1 staining was also performed. Significant increases in BUN levels; creatinine levels; renal histopathological scores; and TUNEL, caspase 1 and 3, calpain 1, iNOS, and eNOS staining were observed for the colistin group compared to the control group. Significant decreases in BUN levels; creatinine levels; renal histopathological scores; and TUNEL, caspase 1 and 3, calpain 1, iNOS, and eNOS staining were observed in the colistin+GSPE group compared to the colistin group. Our study shows, for the first time in the literature, that caspase-mediated apoptosis, iNOS, caspase 1, and calpain 1 are involved in the pathogenesis of colistin-associated nephropathy. GSPE had a renoprotective effect, as shown by the lowered levels of these mediators.
INTRODUCTION
Polymyxin group antibiotics have been used in the treatment of Gram-negative infections for approximately half a century. Polymyxin E (colistin) and polymyxin B were the forms frequently used in clinical practice (1). Despite being effective agents, they were discontinued because of their nephro- and neurotoxic effects and with the discovery of antibiotics with fewer side effects. However, they have reentered clinical use in recent years with the growth of multidrug-resistant Gram-negative pathogens, and the previously forgotten side effects of nephro- and neurotoxicity have begun to be observed again on a frequent basis (2, 3).
The nephrotoxic effect of colistin is dose dependent, and a reversal can be observed when the drug is discontinued. Different levels have been reported in different publications, and a nephrotoxic effect as high as 40% has been observed (3). The nephrotoxicity mechanism has not been fully established, although increased cell membrane permeability; cell swelling and lysis associated with an increased influx of cations, anions, and water; and, finally, apoptosis have been implicated (4). Another nephrotoxic mechanism is rhabdomyolysis, as previously reported for a case from our clinic (5). Apoptosis is currently thought to be involved in the pathogenesis of nephropathy secondary to colistin, although it is not known whether apoptosis is caspase dependent. There are also no studies showing the role of calpain 1 (6), a calcium-dependent cysteine protease, and caspase 1 (7), which converts the proinflammatory cytokine interleukin-1β (IL-1β) into the active form, in pathogenesis.
In the same way in which the pathogenesis of colistin-associated nephropathy is not fully understood, there is also currently no effective treatment. Grape seed proanthocyanidin extract (GSPE) is a natural molecule obtained from grape seeds that has many health benefits, including antioxidant, antiapoptotic, anticarcinogenic, and anti-inflammatory properties (8). Previous studies in our clinic have shown the organ-protective effects of this molecule in several acute kidney injury models, but its protective effect against nephropathy secondary to colistin treatment has not yet been investigated (9–11).
The purpose of our study was to investigate the role of oxidative damage and apoptosis in the as-yet-unclear pathogenesis of nephropathy secondary to colistin treatment; whether apoptosis is associated with caspase; and the role of caspase 1, calpain 1, inducible nitric oxide (NO) synthase (iNOS), and endothelial nitric oxide synthase (eNOS) in pathogenesis. The secondary aim of the study was to evaluate the role of GSPE in preventing nephropathy.
MATERIALS AND METHODS
Twenty-four adult Sprague-Dawley rats, 8 to 10 weeks old and weighing 200 to 250 g, were used. The rats were kept in cages in a 12-h-light and 12-h-dark cycle at 22°C ± 2°C and were provided with food and water ad libitum. The study was approved by the Karadeniz Technical University School of Medicine Animal Ethics Committee. Experimental animals were cared for and used in accordance with National Institute of Health guidelines (24).
Chemicals.
GSPE (0.5 ml of extract solution with 66.7 mg/g of total phenolic substance and an oligomeric proanthocyanidin level of 95% containing 100 mg of GSPE) (Kale Natural Herbal Products Food, Cosmetic and Agricultural Products Ltd., Edremit, Balikesir, Turkey) and colistimethate sodium (1 million IU colistin in an injectable vial) were used.
Study protocol.
Following a 5-day adaptation period, the 24 rats were divided into control, colistin, and colistin plus GSPE (colistin+GSPE) groups, with 8 animals each, and placed into separate cages. All rats had access to food and drink ad libitum throughout the experiment. The control group was given 1 ml/kg of body weight saline, and the colistin and colistin+GSPE groups were given 300,000 IU/kg colistin per day for 6 days intraperitoneally (i.p.) (12). Additionally, the control and colistin groups were given 1 ml saline and the colistin+GSPE group was given 1 ml GSPE by gavage daily for 6 days. On the seventh day, the rats were anesthetized with 90 mg/kg of ketamine and 10 mg/kg of xylazine, and a midline incision was performed. At the end of the experiment, the rats were exsanguinated. Kidney tissue was removed immediately before exsanguination in order for the tissue not to be affected by the ischemic process. The experiment was terminated after obtaining 2 ml intracardiac blood samples for biochemical analysis.
Biochemical analysis.
Measurements of blood urea nitrogen (BUN) and creatinine levels were performed by using a Roche autoanalyzer (Modular System, GmbH, Mannheim, Germany). Decapsulation of the kidneys was performed. Half the tissue samples were stored at −80°C until malondialdehyde (MDA), total antioxidant system (TAS), total oxidant system (TOS), and oxidative stress index (OSI) measurements were performed. At the time of analysis, rat kidney tissues were weighed and homogenized in an ice-cold 1.15% KCl solution containing 0.50 ml Triton X-100 by using an Ultra Turrax T18 basic homogenizer (IKA, Wilmington, NC, USA). The homogenate was centrifuged at 4,000 rpm for 5 min at 4°C, and the supernatant was used for the determination of MDA, TAS, and TOS levels. Tissue TAS, TOS, and OSI values were determined as described previously (13), using commercial assay kits (product no. RL0017 and RL0024, respectively; Rel Assay Diagnostics, Gaziantep, Turkey). Tissue TAS levels are given as μmol Trolox equivalents/g wet tissue, and TOS levels are given as μmol H2O2 equivalents/g wet tissue. OSI values were calculated by using the formula (TOS/TAS) × 100. Tissue MDA levels were assigned according to a method described by Mihara and Uchiyama (14). Tetramethoxypropane was used as a standard, and tissue MDA levels are given as nmol/g wet tissue.
Histopathological evaluation.
The other halves of the decapsulated kidneys were used for pathological examination. Specimens were fixed in a 10% formalin solution. For light microscopy, the specimens were dehydrated by using 70% and 100% alcohol solutions, processed in an Autotechnicon instrument, and embedded in paraffin. Sections 4 μm thick were cut by using a microtome and stained with hematoxylin-eosin (HE). Stained specimens were assessed by two pathologists in a blinded fashion by using an Olympus BX50 (Japan) light microscope. Lesions were classified under the following three previously described grades (15): grade 1, mild acute tubular damage with tubular dilation, prominent nuclei, and a few pale tubular casts; grade 2, severe acute tubular damage with necrosis of tubular epithelial cells and numerous tubular casts; and grade 3, acute cortical necrosis/infarction of tubules and glomeruli with or without papillary necrosis. The grades were given the following scores: grade 1 was given a score of 1, grade 2 was given a score of 4, and grade 3 was given a score of 10. The percentages of the kidney slices affected were assigned the following scores: 0 for <1%, 1 for 1% to <5%, 2 for 5% to <10%, 3 for 10% to <20%, 4 for 20% to <30%, 5 for 30% to <40%, and 6 for ≥40%. The overall score was calculated as the product of the percentage score and the grade score. Finally, a semiquantitative score (SQS) for renal histological changes was assigned, as follows: SQS of 0 for no significant change (overall score, <1), SQS of +1 for mild damage (overall score, 1 to <15), SQS of +2 for mild to moderate damage (overall score, 15 to <30), SQS of +3 for moderate damage (overall score, 30 to <45), SQS of +4 for moderate to severe damage (overall score, 45 to <60), and SQS of +5 for severe damage (overall score, 60) (15).
TUNEL analysis.
For the labeling of apoptotic cells, all paraffin-embedded tissue samples were cut into 4-μm sections. Terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) staining of sections was performed by using a standard TUNEL kit (Roche, Mannheim, Germany), in accordance with the manufacturer's instructions (11). Color was then developed with a kit containing 3,3′-diaminobenzidine (DAB) (Sigma, St. Louis, MO, USA). The TUNEL-stained cells appear as brown stains, and other cells' nuclei appear as blue stains. Brown-stained tubular and glomerular cells in each slide were counted under a light microscope with the Analysis 5 Research program (Olympus Soft Imaging Solutions, Germany) at a ×400 magnification. One hundred cells were counted in five microscopic fields per tissue slide. The percentage of TUNEL-positive apoptotic cells was measured, and this measurement represents the apoptotic index (AI).
Immunohistochemistry. (i) iNOS and caspase 3.
For iNOS and caspase 3, sections 4 μm thick were cut from paraffin-embedded rat kidney tissues. The sections were stained with primary antibodies for iNOS (clone RB-9242-R7, ready to use; Thermo Fisher Scientific Anatomical Pathology, Cheshire, United Kingdom) and caspase 3 (clone RB-1197-R7, ready to use; Thermo Fisher Scientific Anatomical Pathology, Cheshire, United Kingdom). Immunohistochemical reactions of iNOS and caspase 3 were carried out on a BenchMark XT automated slide stainer (Ventana Medical System, Inc., Tucson, AZ, USA). The labeling index (LI) was calculated by two pathologists blinded to the study groups by using the percentage of positively stained cells counted (manually) in 100 cells on each slide.
(ii) eNOS, caspase 1, and calpain 1.
Sections were stained with primary antibodies for caspase 1 (clone ab1872; Abcam PLC, Cambridge, United Kingdom), calpain 1 (clone ab108400; Abcam PLC, Cambridge, United Kingdom.), and eNOS (clone RB-9279; Thermo Fisher Scientific Anatomical Pathology, Cheshire, United Kingdom). The LI was calculated by two pathologists blinded to the study groups by using the percentage of positively stained cells counted (manually) in 100 cells on each slide.
Statistical analysis.
Statistical analysis of data was performed by using SPSS 13.0., with results expressed as means ± standard deviations. Groups were examined for normal distribution. Kruskal-Wallis variance analysis and the Mann-Whitney U test were then used for comparison of BUN levels, creatinine levels, renal pathological findings, AI, caspase 1, caspase 3, calpain 1, iNOS, and eNOS. P values of <0.05 were considered to be statistically significant.
RESULTS
Effect of GSPE on kidney functions.
There were significant increases in BUN (25.78 ± 7.90 mg/dl) and creatinine (0.56 ± 0.15 mg/dl) values in the colistin group compared to the control group. P values were <0.05 for both. The increase in BUN and creatinine levels in the colistin group decreased significantly in the colistin+GSPE group (BUN, 15.48 ± 2.84 mg/dl; creatinine, 0.40 ± 0.04 mg/dl). P values were <0.05 for both (Table 1).
Table 1.
Comparison of the groups' BUN, creatinine, and oxidative parametersa
| Parameter | Mean value ± SD for group |
P value |
||||
|---|---|---|---|---|---|---|
| G1 (control) | G2 (colistin) | G3 (colistin+GSPE) | G1 vs G2 | G1 vs G3 | G2 vs G3 | |
| BUN level (mg/dl) | 14.40 ± 1.80 | 25.78 ± 7.90 | 15.48 ± 2.84 | <0.05 | NS | <0.05 |
| Creatinine level (mg/dl) | 0.36 ± 0.02 | 0.56 ± 0.15 | 0.40 ± 0.04 | <0.05 | NS | <0.05 |
| TOS level (μmol H2O2 equivalents/g wet tissue) | 0.31 ± 0.45 | 0.41 ± 0.10 | 0.36 ± 0.42 | <0.05 | <0.05 | NS |
| TAS level (μmol Trolox equivalents/g wet tissue) | 24.23 ± 4.57 | 21.21 ± 3.41 | 23.32 ± 3.39 | NS | NS | NS |
| OSI | 1.30 ± 0.19 | 1.98 ± 0.43 | 1.56 ± 0.16 | <0.05 | <0.05 | <0.05 |
| MDA level (nmol/g wet tissue) | 78.48 ± 15.11 | 95.16 ± 13.82 | 70.84 ± 13.15 | <0.05 | NS | <0.05 |
Data are given as means ± standard deviations, and a P value of <0.05 was regarded as significant. Abbreviations: GSPE, grape seed proanthocyanidin extract; G1 group 1; G2, group 2; G3, group 3; BUN, blood urea nitrogen; TOS, total oxidant system; TAS, total antioxidant system; OSI, oxidative stress index [calculated as (TOS/TAS) × 100]; MDA, malondialdehyde; NS, not significant.
Effect of GSPE on histopathological injury and apoptosis.
Significant histopathological injury (score of 1.85 ± 0.37) developed in the colistin group compared to the control group (P < 0.001). However, this injury decreased significantly for the colistin+GSPE group (0.28 ± 0.48) (P < 0.001). The decrease for the colistin+GSPE group left no significant difference between it and the control group (Table 2 and Fig. 1). The AI calculated from TUNEL staining in order to show apoptosis was again significantly higher for the colistin group (32.58 ± 4.38) than for the control group (P < 0.001). This increase in the AI was decreased significantly in the colistin+GSPE group (17.67 ± 2.29) (P < 0.001) (Table 2 and Fig. 1).
Table 2.
Comparison of the groups' histopathological injury and apoptotic indicesa
| Parameter | Mean value ± SD for group |
P value |
||||
|---|---|---|---|---|---|---|
| G1 (control) | G2 (colistin) | G3 (colistin + GSPE) | G1 vs G2 | G1 vs G3 | G2 vs G3 | |
| HE score | 0.00 ± 0.00 | 1.85 ± 0.37 | 0.28 ± 0.48 | <0.001 | NS | <0.001 |
| AI | 8.89 ± 2.66 | 32.58 ± 4.38 | 17.67 ± 2.29 | <0.001 | <0.001 | <0.001 |
Data are given as means ± standard deviations, and a P value of <0.05 was regarded as significant. Abbreviations: GSPE, grape seed proanthocyanidin extract; G1, group 1; G2, group 2; G3, group 3; HE, hematoxylin-eosin; AI, apoptotic index.
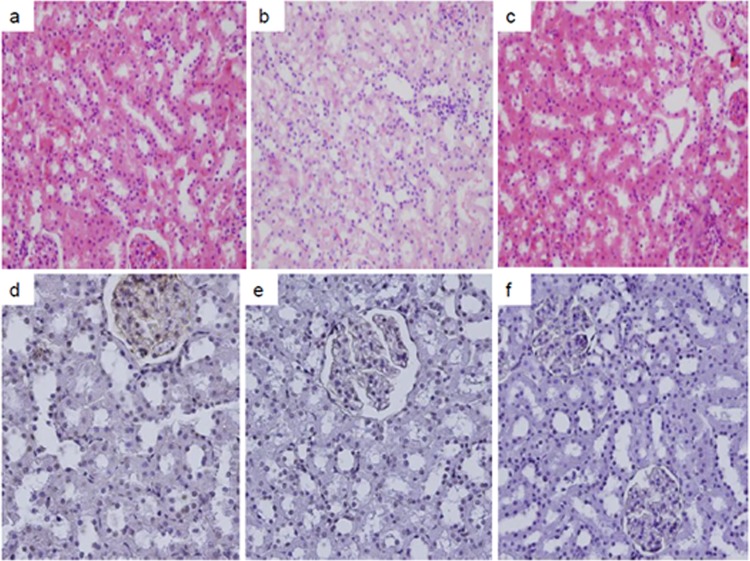
Fig 1.
Hematoxylin-eosin and TUNEL staining images. (a to c) HE-stained images of the control (a), colistin (b), and colistin+GSPE groups. (d to e) TUNEL images of the control (d), colistin (e), and colistin+GSPE groups. Tubular desquamation, vacuolization, and necrosis were present in the colistin group, while these injury findings decreased significantly for the colistin+GSPE group.
Effect of GSPE on immunohistochemical staining.
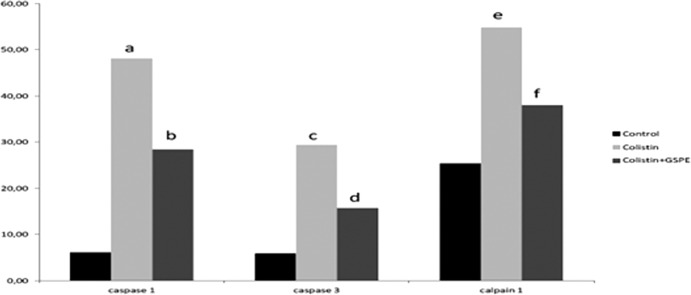
There was a significant increase in caspase 1 (48.14 ± 13.13), caspase 3 (29.42 ± 6.16), calpain 1 (54.85 ± 10.66), iNOS (22.28 ± 7.49), and eNOS (26.85 ± 3.93) staining in the colistin group compared to the control group (P values of <0.001, <0.001, <0.001, <0.05, and < 0.05, respectively). Significant decreases in these increased values were determined for the colistin+GSPE group (caspase 1, 28.42 ± 12.60; caspase 3, 15.71 ± 4.64; calpain 1, 38.00 ± 6.60; iNOS, 10.85 ± 2.34; eNOS, 11.14 ± 3.38) (P values of <0.05, <0.05, <0.05, <0.001, and < 0.05, respectively) (Fig. 2 to 6).
Fig 2.
Comparison of the groups' caspase 1, caspase 3, and calpain 1 labeling indices. A P value of <0.05 was regarded as significant. (a) P value of <0.001 for the caspase 1 labeling index for the control group versus the colistin group; (b) P value of < 0.05 for the caspase 1 labeling index for the colistin+GSPE group versus the colistin group; (c) P value of <0.001 for the caspase 3 labeling index for the control group versus the colistin group; (d) P value of <0.05 for the caspase 3 labeling index for the colistin+GSPE group versus the colistin group; (e) P value of <0.001 for the calpain 1 labeling index for the control group versus the colistin group; (f) P value of <0.05 for the calpain 1 labeling index for the colistin+GSPE group versus the colistin group.
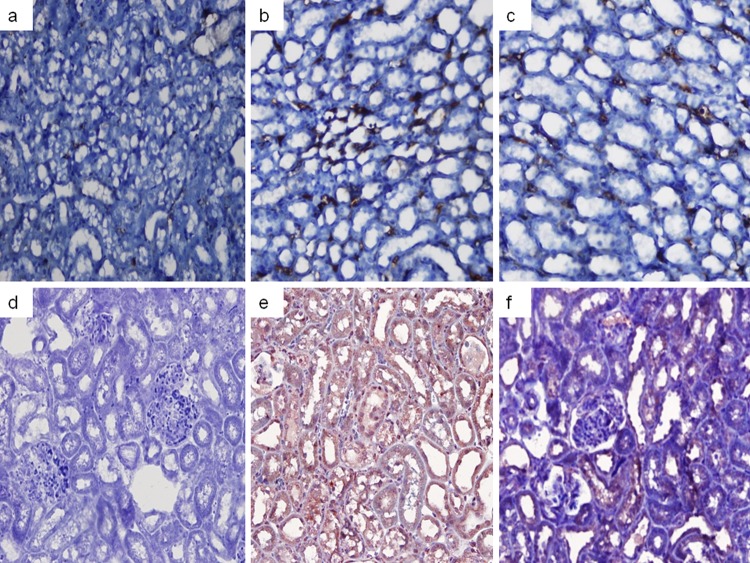
Fig 6.
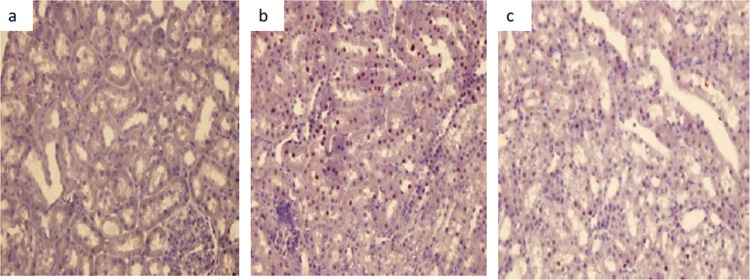
Calpain 1 staining images. (a) Control group image; (b) colistin group image; (c) colistin+GSPE group image.
Fig 3.
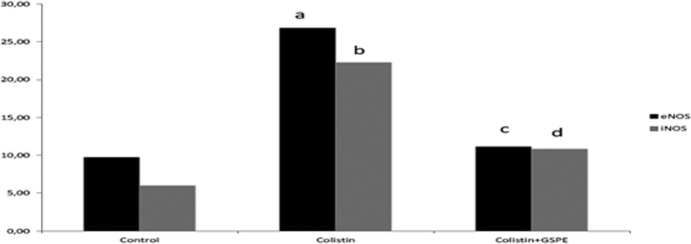
Comparison of the groups' eNOS and iNOS labeling indices. A P value of <0.05 was regarded as significant. (a) P value of <0.05 for the eNOS labeling index for the control group versus the colistin group; (b) P value of <0.05 for the iNOS labeling index for the control group versus the colistin group; (c) P value of <0.001 for the eNOS labeling index for the colistin+GSPE group versus the colistin group; (d) P value of <0.05 for the iNOS labeling index for the colistin+GSPE group versus the colistin group.
Fig 4.
Caspase 3 and caspase 1 staining images. (a to c) Caspase 3 staining images for the control (a), colistin (b), and colistin+GSPE (c) groups. (d to f) Caspase 1 staining images for the control (d), colistin (e), and colistin+GSPE (f) groups.
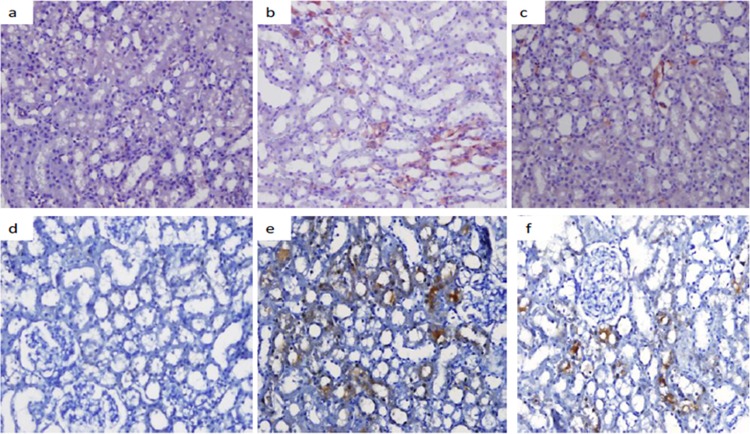
Fig 5.
iNOS and eNOS staining images. (a to c) iNOS staining images for the control (a), colistin (b), and colistin+GSPE (c) groups. (d to f) eNOS staining images for the control (d), colistin (e), and colistin+GSPE (f) groups.
Effect of GSPE on oxidative damage.
Significant increases in the TOS (0.41 ± 0.10), OSI (1.98 ± 0.43), and MDA (95.16 ± 13.82) values were determined for the colistin group compared to the control group (P values of <0.05, <0.05, and < 0.05, respectively). Significant decreases in the OSI (1.56 ± 0.16) and MDA (70.84 ± 13.15) values were determined for the colistin+GSPE group (P < 0.05 for both) (Table 1).
DISCUSSION
In this study, we showed that colistin caused nephrotoxicity and that this toxicity was prevented by concomitant use of GSPE. Our study results also indicate that oxidative damage, caspase-dependent apoptosis, caspase 1, calpain 1, iNOS, and eNOS were involved in the pathogenesis of colistin-induced nephropathy.
Colistin is a cyclic cationic polypeptide antibiotic from the polymyxin group. It was effectively used to treat Gram-negative infections approximately 70 years ago but was discontinued due to its toxic effects, which were as high as 40%, being replaced by less nephrotoxic carbapenem group antibiotics (1, 2). However, with the spread in subsequent years of multidrug-resistant Gram-negative infections and with no new antibiotics being produced, this old antibiotic was revisited (1, 2). While its nephrotoxic effect still has not been fully clarified, the mechanisms postulated are lysis associated with an increase in cell membrane ion passage, oxidative damage, an increase in iNOS levels, and renal injury secondary to rhabdomyolysis (3, 4, 5, 12). The increases in TOS, OSI, and MDA values in our study support the presence of oxidative damage. The significant increase in TUNEL staining in the colistin group in our study supports the presence of apoptotic injury.
Nitric oxide (NO) is a highly unstable, free radical gas synthesized from l-arginine mediated by NO synthase (16). There are three forms of NO synthase: cytokine-inducible NO synthase (iNOS), endothelial NO synthase (eNOS), and neuronal NO synthase (nNOS). Increased NO production after iNOS induction can lead to direct DNA damage, mitochondrial membrane damage, or apoptosis. Although NO is a vasodilator molecule, excess production may lead to dose-dependent apoptotic or necrotic injury (17). Few studies have investigated the place of NO in the pathogenesis of colistin-associated nephropathy. Ozyilmaz et al. determined an increase in the iNOS expression level in tubular epithelial cells developing colistin-associated damage. That study did not analyze apoptotic injury but reported that colistin nephropathy may be associated with oxidative damage and increased iNOS expression levels (12). Similarly, we determined oxidative damage and an increase in the iNOS expression level. Increased iNOS expression was parallel to apoptotic injury. We therefore thought that the increased iNOS expression level may have led to apoptotic as well as necrotic injury.
Apoptosis is a physiological process involved in cell death. Under some pathological conditions, however, it may lead to undesired, negative consequences by accelerating or decelerating. Apoptosis may occur dependently or independently of caspase (18). Caspases are members of the cysteine protease family. Caspase 3 is activated as a result of activation of the intrinsic and extrinsic apoptotic pathways and leads to DNA breakdown, one of the characteristic cellular changes of apoptosis (19). There have been very few studies of the role of apoptosis in the development of colistin-associated nephropathy (4) and none showing whether apoptosis is caspase dependent. Since the AI obtained with TUNEL staining in the colistin group was significantly higher than that in the control group, we think that apoptosis is involved in the pathogenesis of colistin-related nephropathy. Additionally, since the caspase 3 staining percentage of the colistin group was higher than that of the control group, we also think that apoptosis may be caspase dependent.
There have been studies showing the involvement of caspase 1, known as inflammatory caspase, in sepsis-dependent acute kidney injury models and in neurological injury in particular (7). It has been suggested in recent years that caspase 1 may also be involved in the development of apoptotic injury in cisplatin-induced renal damage (19). The role of caspase 1 in colistin-associated nephropathy, the pathogenesis of which is as yet unclear, is unknown. We determined an increase in caspase 1 staining in the colistin group in our study. We therefore think that caspase 1 is involved in the pathogenesis of nephropathy. Since the increase in caspase 1 staining is parallel to the increase in caspase 3 staining, we also think that caspase 1 may be associated with apoptotic injury.
Calpain 1 is a calcium-associated cysteine protease involved in apoptotic and necrotic cell damage (6, 20). There have been few studies showing the role of calpain in the development of AKI and none showing its role in the development of colistin-associated nephropathy. We determined a significant increase in staining at the calpain 1 level in our colistin group. The increase in calpain 1 staining in the colistin group was parallel to the increases in caspase 3 and AI values obtained from TUNEL staining. At the same time, as it was parallel with the increase in the semiquantitative score, our evaluation with hematoxylin-eosin staining suggested that it is also associated with necrotic injury. We therefore think that calpain 1 may affect the development of colistin-related necrotic and apoptotic renal injury.
Another aim of our study was to evaluate the effectiveness of GSPE in preventing colistin nephropathy, which we frequently encounter in clinical practice, and to determine the mechanism of such effectiveness if it exists. GSPE is a molecule obtained from the seed of the black grape that possesses natural antioxidant, antiapoptotic, anticarcinogenic, vasodilator, and anti-inflammatory properties (8). Ray et al. showed that administration of up to 5,000 mg/kg of GSPE by gavage caused no organ toxicity in rats (21). In addition, administration of 100 mg/kg by gavage for 7 to 10 days has been shown to establish an organ-protective effect (8). We have shown, in several studies in our clinic, that the use of 100 mg/kg in various models of AKI established effective renal protection by the antioxidant and antiapoptotic mechanism (9–11). However, its effectiveness in preventing colistin-associated nephropathy has not been investigated to date. This study shows, for the first time in the literature, that GSPE significantly reduces colistin-associated nephropathy. Our data suggest that its renoprotective effect functions by reducing the effects of oxidative damage, apoptosis, and iNOS. The finding that levels of TOS and OSI, oxidative damage markers, and the AI, an apoptosis marker, were significantly lower in the colistin+GSPE group than in the colistin group in this study suggests that GSPE establishes a renoprotective effect by reducing oxidative and apoptotic damage. Cui et al. achieved significant increases in iNOS levels at the NO level with the administration of GSPE to rats over a 5-week period and showed that this had a pronounced antihypertensive effect (22). Additionally, increased iNOS expression is known to be associated with apoptotic damage (17). To the best of our knowledge, however, there are no studies showing the effect of GSPE on iNOS expression in renal injury. This study showed that GSPE may have exhibited its nephroprotective effect by also reducing iNOS expression levels.
Studies have shown how GSPE exhibits its antiapoptotic effect in hepatic injury (23), although it is unclear how it achieves this in renal injury. We think that GSPE exhibited its antiapoptotic effect in our study since it reduced caspase 3 levels by reducing caspase-mediated apoptosis. In addition, its reduction of calpain 1 and caspase 3 levels may also have assisted the antinecrotic effect.
In conclusion, colistin, an old antibiotic, has reentered clinical use, and nephropathy, its much-feared complication, has been encountered once again. This study shows that increases in oxidative damage, caspase-mediated apoptosis, and caspase 1, calpain 1, and iNOS levels may be involved in the pathogenesis of colistin-associated nephropathy. We have also shown that GSPE significantly reduced nephropathy with the oral administration of 100 mg/kg from the first day of colistin use and that it produced this effect by reducing oxidative damage, caspase-mediated apoptosis, and caspase 1 and calpain 1 levels. In light of our data, GSPE is clearly an important potential agent in the prevention of colistin-related nephropathy. However, new research on this subject, and especially clinical studies, will determine the place of this potential agent in the treatment of colistin nephropathy.
ACKNOWLEDGMENTS
We report no conflict of interest.
We thank Kale Natural Herbal Products Food Cosmetics and Agriculture Ltd., which provided the grape seed extract used in the study.
This study was supported by the Scientific Researches Fund of Karadeniz Technical University.
Footnotes
Published ahead of print 29 April 2013
REFERENCES
- 1. Yahav D, Farbman L, Leibovici L, Paul M. 2012. Colistin: new lessons on an old antibiotic. Clin. Microbiol. Infect. 18:18–29 [DOI] [PubMed] [Google Scholar]
- 2. Falagas ME, Kasiakou SK. 2006. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit. Care 10:R27. 10.1186/cc3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pogue JM, Lee J, Marchaim D, Yee V, Zhao JJ, Chopra T, Lephart P, Kaye KS. 2011. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin. Infect. Dis. 53:879–884 [DOI] [PubMed] [Google Scholar]
- 4. Yousef JM, Chen G, Hill PA, Nation RL, Li J. 2012. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J. Antimicrob. Chemother. 67:452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Özkan G, Ulusoy S, Gazioğlu S, Cansız M, Kaynar K, Arı D. 2012. Rhabdomyolysis and severe muscle weakness secondary to colistin therapy. Ren. Fail. 34:926–929 [DOI] [PubMed] [Google Scholar]
- 6. Liu X, Harriman JF, Schnellmann RG. 2002. Cytoprotective properties of novel nonpeptide calpain inhibitors in renal cells. J. Pharmacol. Exp. Ther. 302:88–94 [DOI] [PubMed] [Google Scholar]
- 7. Denes A, Lopez-Castejon G, Brough D. 2012. Caspase-1: is IL-1 just the tip of the ICEberg? Cell Death Dis. 3:e338. 10.1038/cddis.2012.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bagchi D, Bagchi M, Stohs S, Ray SD, Sen CK, Preuss HG. 2002. Cellular protection with proanthocyanidins derived from grape seeds. Ann. N. Y. Acad. Sci. 957:260–270 [DOI] [PubMed] [Google Scholar]
- 9. Ozkan G, Ulusoy S, Orem A, Ersoz S, Alkanat M, Yucesan FB, Kaynar K, Al S. 2012. Protective effect of the grape seed proanthocyanidin extract in a rat model of contrast-induced nephropathy. Kidney Blood Press. Res. 35:445–453 [DOI] [PubMed] [Google Scholar]
- 10. Ulusoy S, Ozkan G, Ersoz S, Orem A, Alkanat M, Yucesan FB, Kaynar K, Al S. 2012. The effect of grape seed proanthocyanidin extract in preventing amikacin-induced nephropathy. Ren. Fail. 34:227–234 [DOI] [PubMed] [Google Scholar]
- 11. Ulusoy S, Ozkan G, Yucesan FB, Ersöz S, Orem A, Alkanat M, Yuluğ E, Kaynar K, Al S. 2012. Anti-apoptotic and anti-oxidant effects of grape seed proanthocyanidin extract in preventing cyclosporine A-induced nephropathy. Nephrology (Carlton) 17:372–379 [DOI] [PubMed] [Google Scholar]
- 12. Ozyilmaz E, Ebinc FA, Derici U, Gulbahar O, Goktas G, Elmas C, Oguzulgen IK, Sindel S. 2011. Could nephrotoxicity due to colistin be ameliorated with the use of N-acetylcysteine? Intensive Care Med. 37:141–146 [DOI] [PubMed] [Google Scholar]
- 13. Harma M, Harma M, Erel O. 2003. Increased oxidative stress in patients with hydatidiform mole. Swiss Med. Wkly. 133:563–566 [DOI] [PubMed] [Google Scholar]
- 14. Mihara M, Uchiyama M. 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86:271–278 [DOI] [PubMed] [Google Scholar]
- 15. Yousef JM, Chen G, Hill PA, Nation RL, Li J. 2011. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob. Agents Chemother. 55:4044–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amore A, Emancipator SN, Cirina P, Conti G, Ricotti E, Bagheri N, Coppo R. 2000. Nitric oxide mediates cyclosporine-induced apoptosis in cultured renal cells. Kidney Int. 57:1549–1559 [DOI] [PubMed] [Google Scholar]
- 17. Tiwari MM, Messer KJ, Mayeux PR. 2006. Inducible nitric oxide synthase and apoptosis in murine proximal tubule epithelial cells. Toxicol. Sci. 91:493–500 [DOI] [PubMed] [Google Scholar]
- 18. Servais H, Ortiz A, Devuyst O, Denamur S, Tulkens PM, Mingeot-Leclercq MP. 2008. Renal cell apoptosis induced by nephrotoxic drugs: cellular and molecular mechanisms and potential approaches to modulation. Apoptosis 13:11–32 [DOI] [PubMed] [Google Scholar]
- 19. Faubel S, Ljubanovic D, Reznikov L, Somerset H, Dinarello CA, Edelstein CL. 2004. Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int. 66:2202–2213 [DOI] [PubMed] [Google Scholar]
- 20. Lee WK, Abouhamed M, Thévenod F. 2006. Caspase-dependent and -independent pathways for cadmium-induced apoptosis in cultured kidney proximal tubule cells. Am. J. Physiol. Renal Physiol. 291:F823–F832. 10.1152/ajprenal.00276.2005 [DOI] [PubMed] [Google Scholar]
- 21. Ray S, Bagchi D, Lim PM, Bagchi M, Gross SM, Kothari SC, Preuss HG, Stohs SJ. 2001. Acute and long-term safety evaluation of a novel IH636 grape seed proanthocyanidin extract. Res. Commun. Mol. Pathol. Pharmacol. 109:165–197 [PubMed] [Google Scholar]
- 22. Cui X, Liu X, Feng H, Zhao S, Gao H. 2012. Grape seed proanthocyanidin extracts enhance endothelial nitric oxide synthase expression through 5′-AMP activated protein kinase/Surtuin 1-Krüpple like factor 2 pathway and modulate blood pressure in ouabain induced hypertensive rats. Biol. Pharm. Bull. 35:2192–2197 [DOI] [PubMed] [Google Scholar]
- 23. Ray SD, Kumar MA, Bagchi DA. 1999. novel proanthocyanidin IH636 grape seed extract increases in vivo Bcl-XL expression and prevents acetaminophen-induced programmed and unprogrammed cell death in mouse liver. Arch. Biochem. Biophys. 369:42–58 [DOI] [PubMed] [Google Scholar]
- 24. National Institutes of Health 1996. Guide for the care and use of laboratory animals. National Academy of Science Press, Washington, DC [Google Scholar]