Abstract
Many host defense cationic antimicrobial peptides (HDPs) perturb the staphylococcal cell membrane (CM) and alter transmembrane potential (ΔΨ) as key parts of their lethal mechanism. Thus, a sense-response system for detecting and mediating adaptive responses to such stresses could impact organism survival; the Staphylococcus aureus LytSR two-component regulatory system (TCRS) may serve as such a ΔΨ sensor. One well-known target of this system is the lrgAB operon, which, along with the related cidABC operon, has been shown to be a regulator in the control of programmed cell death and lysis. We used an isogenic set of S. aureus strains: (i) UAMS-1, (ii) its isogenic ΔlytS and ΔlrgAB mutants, and (iii) plasmid-complemented ΔlytSR and ΔlrgAB mutants. The ΔlytS strain displayed significantly increased in vitro susceptibilities to all HDPs tested (neutrophil-derived human neutrophil peptide 1 [hNP-1], platelet-derived thrombin-induced platelet microbicidal proteins [tPMPs], and the tPMP-mimetic peptide RP-1), as well as to calcium-daptomycin (DAP), a cationic antimicrobial peptide (CAP). In contrast, the ΔlrgAB strain exhibited no significant changes in susceptibilities to these cationic peptides, indicating that although lytSR positively regulates transcription of lrgAB, increased HDP/CAP susceptibilities in the ΔlytS mutant were lrgAB independent. Further, parental UAMS-1 (but not the ΔlytS mutant) became more resistant to hNP-1 and DAP following pretreatment with carbonyl cyanide m-chlorophenylhydrazone (CCCP) (a CM-depolarizing agent). Of note, lytSR-dependent survival against CAP/HDP killing was not associated with changes in either surface positive charge, expression of mprF and dlt, or CM fluidity. The ΔlytS strain (but not the ΔlrgAB mutant) displayed a significant reduction in target tissue survival in an endocarditis model during DAP treatment. Collectively, these results suggest that the lytSR TCRS plays an important role in adaptive responses of S. aureus to CM-perturbing HDPs/CAPs, likely by functioning as a sense-response system for detecting subtle changes in ΔΨ.
INTRODUCTION
The interplay between bacterial and host factors plays a crucial role in the initiation, progression, and outcome of staphylococcal infection. One of the critical elements in host defense against Staphylococcus aureus infections is the innate immune system, particularly in the context of elaboration of numerous cationic antimicrobial peptides (CAPs) (1, 2). Host defense CAPs (HDPs) are typically small amphipathic peptides (<50 amino acids) with a high net positive charge and are found in most mammalian tissues (3–5). In the context of bloodstream infections, HDPs localized within the skin, nasal mucosa, white blood cells and platelets are especially relevant in the initial successful colonization of S. aureus, as well as the subsequent hematogenous dissemination and organ invasion of this organism (3, 6–8). Therefore, for colonization of the host, the organism must resist the microbicidal action of a diverse cadre of HDPs. Since HDPs may target the staphylococcal cell membrane (CM) and perturb the CM electrical potential (Δψ) and are rapidly bactericidal (1, 5), an “early warning” sense-response system for rapidly detecting and adapting to subtle changes in Δψ would foster bacterial survival. The LytSR two-component regulatory system (TCRS) has become a leading candidate to serve these functions (9–11). Recent studies have demonstrated that LytS senses decreases in Δψ and presumably initiates phosphorylation and activation of the response regulator, LytR. Activated LytR is then hypothesized to regulate key downstream genes that are involved in HDP resistance (10, 11). For example, one well-studied target of the LytSR TCRS is the lrgAB operon, which, along with the cidABC operon, is directly involved in the control of programmed cell death and lysis (10–12). Thus, the LytSR system has been hypothesized to function as a staphylococcal “voltmeter,” rapidly and universally sensing changes in Δψ and then triggering adaptive countermeasures that enable resistance to HDP killing through regulation of key adaptive pathways. Several lines of evidence support this hypothesis: (i) lytSR rapidly responds to changes in Δψ induced by a variety of perturbations (10, 11), (ii) its activation phenotypically impacts cell death and autolysis (10, 11, 13, 14), and (iii) it regulates expression of key downstream genes involved in programmed cell death (e.g., lrgAB) (10, 11, 15).
Although the LytSR system has been examined in vitro, little is known about the role of this system with respect to (i) responsivity to changes in Δψ, role in biofilm formation, or murein hydrolase activity and autolysis, in the context of HDP resistance (10, 11, 13, 15), or (ii) the impact of this locus on in vivo virulence or antimicrobial treatment outcomes. In the present study, we utilized isogenic lytS and lrgAB mutant strains of a well-characterized clinical S. aureus strain, UAMS-1 (16), to examine the potential role of the LytSR TCRS in adaptive HDP resistance. Specifically, we examined the role of this system in the following: (i) in vitro resistance to a group of HDPs of distinct structures, net charges, and mammalian cell origins; (ii) modulation of key phenotypes frequently linked to adaptive resistance to HDPs (i.e., surface envelope charge, CM order [fluidity/rigidity], and CM fatty acid profiles); (iii) in vivo virulence during the induction and maintenance of a prototypical endovascular infection (infective endocarditis [IE]); (iv) in vivo efficacy of antimicrobial treatment (calcium-daptomycin [DAP]) that targets the CM and collapses the Δψ as part of its lethal mechanism (17, 18); and (v) role of LrgAB in modulating the impacts of LytSR in the context of in vivo outcome metrics.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. The lytS mutation in S. aureus UAMS-1 was generated by inserting an erythromycin cassette using the allelic replacement strategy described previously (11). The lytS mutation eliminates expression of both the lytS and lytR genes, indicating that these genes form a dicistronic operon (11). Complementation of the lytS mutation was achieved by cloning a DNA fragment encompassing the UAMS-1 lytSR operon into a shuttle plasmid (pBK5) (11). All S. aureus strains were grown in either tryptic soy broth (TSB) (Difco Laboratories, Detroit, MI) or Mueller-Hinton broth (MH) (Difco Laboratories, Detroit, MI) as indicated, depending on the individual experiments. Liquid cultures were grown in Erlenmeyer flasks at 37°C with shaking (250 rpm) in a volume that was no greater than 10% of the flask volume.
Table 1.
Strains used in this study
| Strain | Description | Reference or source | MIC (μg/ml)a |
|
|---|---|---|---|---|
| DAP | GENT | |||
| UAMS-1 | Clinical osteomyelitis isolate | 16 | 0.5 | 0.75 |
| KB999 | ΔlytS in UAMS-1 | 11 | 0.125 | 0.5 |
| KB999(pBK5) | Complementation of lytSR in KB999 | 11 | 0.38 | 0.75 |
| KB1045 | ΔlrgAB in UAMS-1 | 13 | 0.5 | 0.75 |
| KB1045(pDR45) | Complementation of lrgAB in KB1045 | 13 | 0.5 | 0.75 |
DAP, daptomycin; GENT, gentamicin.
CAPs/HDPs.
DAP was purchased from Cubist Pharmaceuticals (Lexington, MA), and gentamicin (GENT) was purchased from Fisher Scientific (Fair Lawn, NJ); both agents were reconstituted according to the manufacturer's recommendations. All DAP assays were done in the presence of 50 μg/ml calcium as recommended by the manufacturer. Human neutrophil peptide 1 (hNP-1), a prototypical α-defensin, was purchased from Peptide International (Louisville, KY). RP-1 (a synthetic 18-amino-acid congener modeled in part upon α-helical microbicidal domains of the platelet factor 4 family of kinocidins) was prepared and authenticated as detailed before (19, 20). Of note, the antistaphylococcal mechanisms of RP-1 recapitulate that of the native thrombin-induced platelet microbicidal protein 1 (tPMP-1) (19). The tPMP preparations were obtained as previously described from fresh rabbit platelets and contain a mixture of tPMPs, predominantly tPMP-1 (21–23). The bioactivity of these preparations (μg/ml equivalents) was determined by the standard Bacillus subtilis killing assay as detailed before (21, 22).
CAP/HDP susceptibility assays.
Standard MIC testing in nutrient broth may underestimate HDP activities (22, 24). Accordingly, in vitro bactericidal assays were carried out with hNP-1, RP-1, and tPMPs as described previously using a 2-h microdilution method in Eagle's minimal essential medium (24, 25). These assays were performed with a well-described tPMP preparation (bioactivity of 1 or 2 μg/ml), hNP-1 (20 and 40 μg/ml), and RP-1 (0.5 and 1 μg/ml) using an initial inoculum of 5 × 103 CFU S. aureus cells (24, 25). These HDP concentrations were selected based on extensive pilot studies showing their inability to completely eradicate the starting inocula of the parental UAMS strain over the 2-h exposure period. Data were calculated and expressed as the relative percentage of surviving CFU (± standard deviation [SD]) of HDP-exposed versus HDP-unexposed cells, with the survival of each parental strain set at 100%. A minimum of three independent runs were performed for each CAP.
In addition to the HDP assays described above, DAP and GENT MICs were determined by standard Etest as recommended by the manufacturer (AB bioMérieux).
Role of LytSR and LrgAB in HDP susceptibilities following CM potential (Δψ) perturbations.
In parallel to the above-described HDP susceptibility assays, we assessed the same HDP profiles following Δψ collapse and the roles of LytSR and LrgAB in circumventing this process. It has been demonstrated previously that LytSR senses changes in Δψ caused by agents that disrupt this parameter, such as carbonyl cyanide m-chlorophenylhydrazone (CCCP) and gramicidin (10, 11). Bacterial cultures were grown to mid-exponential phase (2.75 h) and then treated with 10 μM CCCP to collapse Δψ; this is a standard CCCP concentration used in prior investigations that is known to reliably perturb the Δψ of S. aureus (10, 11). The cultures were then incubated for an additional 15 min and harvested by centrifugation, following which HDP susceptibility assays were performed as described above.
To confirm that the perturbed Δψ was responsible for the changes in CAP susceptibilities, a parallel experiment was carried out in which the CCCP-treated UAMS-1 culture was washed and resuspended in glucose-containing medium (1%, wt/vol) and incubated for an additional 15 min and hNP-1 susceptibility assays performed as described above.
CM fatty acid composition.
One important adaptive mechanism that S. aureus utilizes to resist CAP/HDP attack is to alter its CM order (i.e., fluidity/rigidity properties) (21, 24, 26–28). Modulation of CM fatty acid content is one key method to accomplish such adaptations (26, 27, 29). Fatty acids of total CM lipids, extracted from S. aureus, were analyzed using gas-liquid chromatography after conversion to their methyl ester form (courtesy of Microbial ID Inc., Newark, DE) (25, 28). All assays were performed a minimum of two times on separate days.
CM fluidity.
As noted above, CM biophysical characteristics can influence interaction of S. aureus with CAPs/HDPs (21, 24, 26–28). To determine the relative CM order characteristics (i.e., fluidity/rigidity) among the study strains, CM fluidity assays were performed by fluorescence polarization spectroscopy using the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene (DPH) as described before (27, 29). Data were quantified by determination of the polarization index (PI) (mean ± SD) (24, 25). The lower the PI value, the more fluid is the CM. These assays were performed at least five times for each strain on separate days.
Surface charge assays.
The relative positivity of the S. aureus envelope can have a profound and independent impact on CAP/HDP susceptibilities, presumably related to a surface charge-repulsive milieu (30–32). To quantify the relative positive cell surface charge in our strains, cytochrome c binding assays were performed as described before (33, 34). The binding of cytochrome c (pI = 10; Sigma) was measured with a spectrophotometric assay (optical density at 530 nm [OD530]) which quantifies the amount of the polycation remaining within reaction mixture supernatants following exposure to the study strains for 30 min; larger amounts of residual cytochrome c in the supernates correlate with a more relatively positive surface charge (25, 28, 33, 35). A minimum of three independent runs were performed.
RNA isolation and quantitative real-time PCR (qRT-PCR).
As discussed above, activation of the lytSR system is associated with upregulation of lrgAB expression (10, 11). In addition, it is well known that activation of certain TCRS sensors in S. aureus by specific HDPs (e.g., GraSR [33, 36, 37]) can augment the expression of at least two downstream genes involved in maintenance of surface envelope positive charge (dltA and mprF) (5, 36–39). To quantify expression of these genes, total RNA was isolated from the S. aureus cell pellets using the RNeasy kit (Qiagen, Valencia, CA) and the FastPrep FP120 instrument (BIO 101, Vista, CA), according to the manufacturer's recommended protocols.
For qRT-PCR analyses, two micrograms of DNase-treated RNA was reverse transcribed using the SuperScript III first-strand synthesis kit (Invitrogen) according to the manufacturer's protocols. Quantification of cDNA levels was performed following the instructions of the Power SYBR Green Master Mix kit (Applied Biosystems) on an ABI Prism 7000 sequence detection system (Applied Biosystems). The lrgA, mprF, dltA, and gyrB genes were detected using the respective specific primers as described before (37, 40). Expression levels of lrgA, mprF, and dltA were quantified in relation to that of gyrB.
Experimental rabbit IE model.
Animals were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care criteria. The Animal Research Committee (IACUC) of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center approved these animal studies. A well-characterized catheter-induced rabbit model of aortic IE was used as described previously (41–43) to assess the role of lytSR gene expression in relative virulence and in vivo efficacy outcomes with DAP therapy. Briefly, female New Zealand White rabbits (2.0- to 2.5-kg body weight; Harlan Laboratories, Indianapolis, IN) underwent indwelling transcarotid-transaortic valve catheterization with a polyethylene catheter. Twenty-four hours after catheter placement, animals were infected intravenously (i.v.) with ∼1 × 106 CFU/animal, which is a 95% infective dose (ID95) for this strain as established in pilot studies.
For the relative virulence and treatment studies, four strains were compared: the parental UAMS-1, its lytS and lrgAB knockout mutants, and the lytS-complemented strain. For the virulence assessments, the four infecting strains were compared for bacterial burdens achieved in three target tissues (see below). For treatment studies, at 24 h postinfection, animals were randomized to receive either (i) no therapy (control) or (ii) DAP at 12 mg/kg, i.v., once daily for 3 days. This DAP dose regimen (12 mg/kg) mimics human-like pharmacokinetics of the standard clinical dose recommended for IE (6 mg/kg once daily) (30, 44). Control animals and antibiotic-treated animals were sacrificed by a rapid i.v. injection of sodium pentobarbital (200 mg/kg; Abbott Laboratories) at either 24 h postinfection (untreated controls) or 24 h after the last antibiotic dose, respectively. At the time of sacrifice, cardiac vegetations, kidneys, and spleens were sterilely removed and quantitatively cultured as described before (45).
For verification of plasmid (pBK5) maintenance within the ΔlytS complemented strain during in vivo studies, all three tissue samples from animals infected with this construct were quantitatively cultured in the presence and absence of the plasmid antibiotic selection marker (chloramphenicol; 10 μg/ml). These studies confirmed stability of the plasmid during animal passage (data not shown).
The mean log10 CFU/g of tissue (± standard deviation [SD]) was calculated for each target tissue in each group for statistical comparisons. The lower limit of microbiologic detection in the target tissues is ≤1 log10 CFU/g of tissue.
Statistical analysis.
To statistically compare S. aureus tissue bacterial densities among the various groups, we used the Kruskal-Wallis analysis of variance (ANOVA) test with the Tukey post hoc correction for multiple comparisons. Significance was determined at a P value of <0.05.
RESULTS
CAP/HDP susceptibility profiles.
To determine the roles of lytSR and lrgAB in HDP resistance, we assessed in vitro susceptibility profiles of ΔlytS and ΔlrgAB strains against three prototypical antistaphylococcal cationic peptides, i.e., hNP-1 (a human neutrophil HDP), tPMPs (of platelet origin) (25, 38), and RP-1 (a synthetic congener of the platelet factor 4 family of kinocidins) (20, 21). As shown in Table 2, deletion of lytS in the UAMS-1 parental strain resulted in significantly increased susceptibilities to all three peptide exposures compared with that of the parental strain UAMS-1 (P < 0.05). Complementation of the ΔlytS strain with a lytSR-expressing plasmid restored parental-level susceptibilities to hNP-1, tPMPs, and RP-1 in most instances. Furthermore, the ΔlytS strain exhibited a 4-fold reduction in susceptibility to the cationic peptide DAP compared to those of the wild-type and complemented strains. In contrast to the ΔlytS strain, the ΔlrgAB strain showed no significant differences in susceptibilities to the three HDPs, as well as parental-level MICs to DAP. There were no substantive differences in susceptibility to the nonpeptide cationic agent GENT in terms of MICs among the strain set.
Table 2.
In vitro CAP susceptibilities of the strains
| Straina | % Survival (mean ± SD)b after 2 h of exposure to: |
|||||
|---|---|---|---|---|---|---|
| tPMP-1 |
hNP-1 |
RP-1 |
||||
| 1 μg/ml | 2 μg/ml | 20 μg/ml | 40 μg/ml | 0.5 μg/ml | 1 μg/ml | |
| UAMS-1 | 59 ± 19 | 33 ± 11 | 71 ± 19 | 43 ± 11 | 32 ± 7 | 21 ± 3 |
| ΔlytS mutant | 30 ± 18* | 15 ± 4* | 58 ± 24 | 29 ± 4* | 17 ± 12* | 4 ± 3* |
| ΔlytS Comp | 56 ± 16 | 43 ± 17 | 70 ± 16 | 53 ± 19 | 27 ± 9 | 11 ± 5* |
| ΔlrgAB mutant | 60 ± 18 | 40 ± 12 | 82 ± 13 | 48 ± 19 | 26 ± 11 | 22 ± 9 |
| ΔlrgAB Comp | 64 ± 21 | 48 ± 16 | 72 ± 22 | 59 ± 13 | 31 ± 12 | 24 ± 6 |
Comp, complemented strain.
*, P < 0.05 versus the parental UAMS-1.
Net cell surface charge.
The relative net surface charge positivity did not significantly differ among the five study strains (data not shown).
Expression of mprF and dlt genes.
As demonstrated in Fig. 1A and B, paralleling the surface charge analysis data, expression of mprF and dltA did not show any significant differences among the strain set. As anticipated (10, 11), the ΔlytSR strain exhibited ∼12-fold-lower lrgA expression than its parental strain during exponential growth, confirming the normally positive modulation of the lrgAB operon by lytSR; the complemented ΔlytSR strain exhibited ∼7-fold-higher lrgA expression than the parental UAMS-1. As expected, the ΔlrgAB strain showed no detectable level of lrgA expression, while the ΔlrgAB complemented variant overexpressed lrgA from the complementation plasmid (Fig. 1C).
Fig 1.
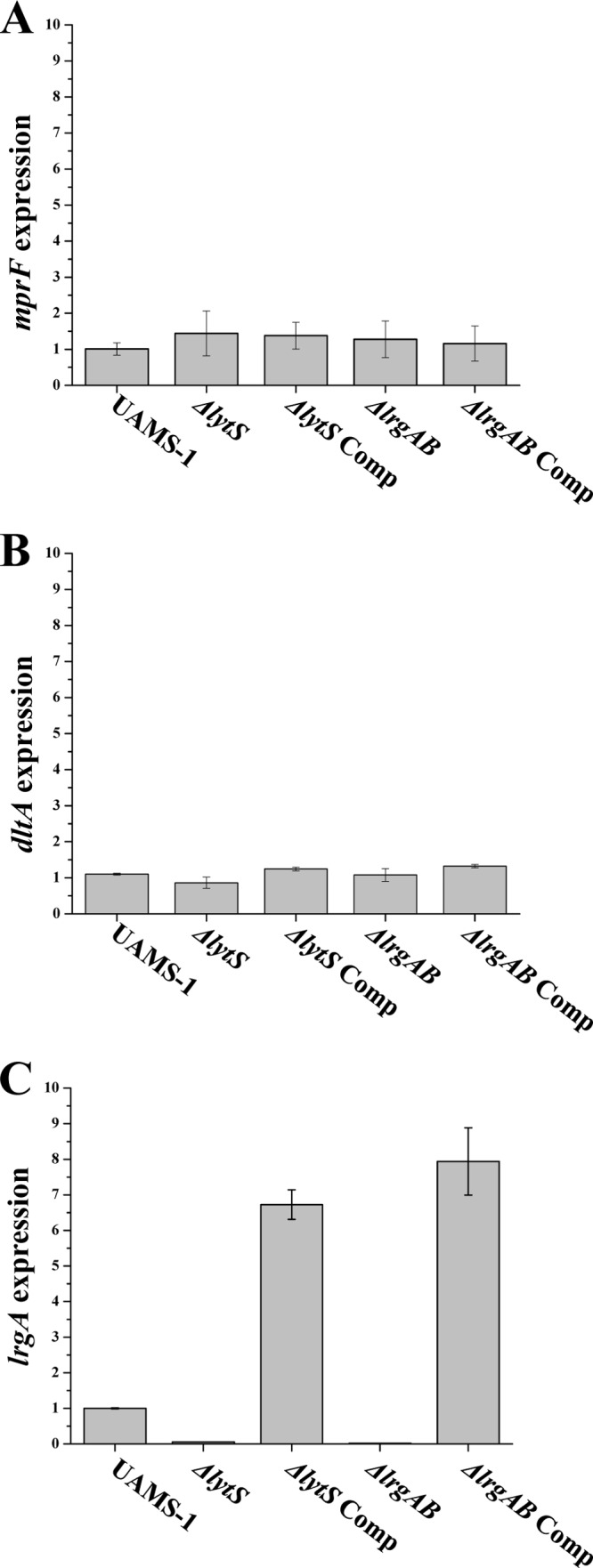
Effect of lytSR and lrgAB on expression of the mprF and dlt genes. RNA samples were isolated from mid-exponential-growth-phase cultures of the strain set, and were subjected to qRT-PCR to detect and quantify transcription of mprF (A), dltA (B), and lrgA (C). The mid-exponential time point has been determined based on our pilot studies showing that expression of mprF and dlt is maximal at this time point. Comp, complemented strain.
CM fatty acid composition.
Fatty acid analyses revealed no significant differences among the five strains in terms of unsaturation indices, acyl chain length profiles, ratio of branched-chain to straight-chain fatty acids, or iso- versus anteiso-branched-chain fatty acid contents (data not shown).
CM fluidity.
Paralleling the data showing equivalency in fatty acid composition, CM fluidity/rigidity analyses revealed no significant differences among the parental UAMS-1, ΔlytS, and ΔlrgAB strains (with polarization index [PI] values ranging from 0.3674 to 0.3823 [data not shown]).
LytSR-mediated adaptations to CAPs.
As shown in Fig. 2A and B, pretreating the parental UAMS-1 strain with CCCP resulted in significant increases in survivability in the presence of hNP-1 and DAP (∼2.5- to 3-fold) compared to that for cells without prior treatment with CCCP (P < 0.01). For hNP-1, the use of glucose to rescue the CCCP impact resulted in maintenance of parental-level susceptibility (data not shown). In contrast, the ΔlytS strain failed to show increased protection against hNP-1- and DAP-mediated killing after CCCP pretreatment. Importantly, the ΔlytS complemented strain also exhibited protection against hNP-1 and DAP killing following CCCP pretreatment (P < 0.05). Although the parental UAMS-1 strain displayed a moderate increase in survivability to RP-1-induced killing following CCCP treatment, this failed to reach statistical significance (data not shown).
Fig 2.
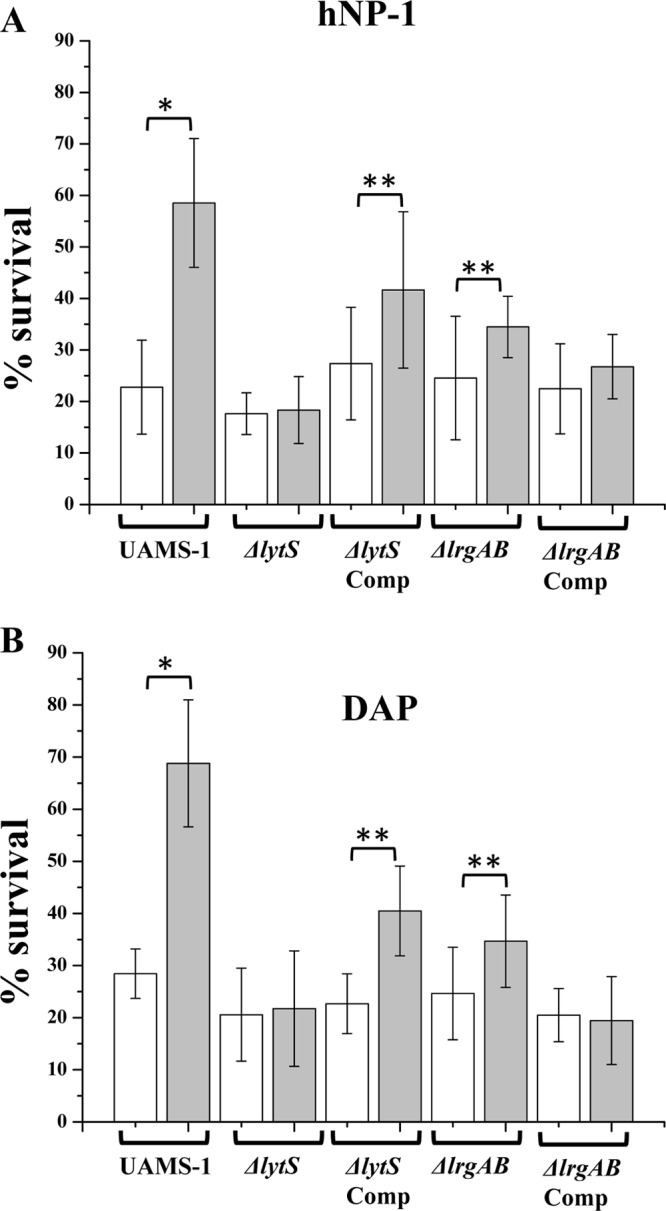
In vitro HDP/CAP susceptibility assays following CCCP exposure. The strains tested were the S. aureus UAMS-1 parent, the ΔlytS mutant, the ΔlytS mutant containing the lytSR complementation plasmid (ΔlytS Comp), the ΔlrgAB mutant, and the ΔlrgAB mutant containing the lrgAB complementation plasmid (ΔlrgAB Comp). Cells were grown to mid-exponential phase (2.75 h) and then treated with CCCP (10 μM) for 15 min. The CCCP-treated cells were then assessed for hNP-1 (A) and DAP (B) susceptibility profiles compared to CCCP-unexposed cells. White bars, CCCP-free cultures; gray bars, CCCP-treated cultures. *, P < 0.01; **, P < 0.05.
It has also been reported that Δψ-inducible lrgAB transcription in S. aureus is dependent on the LytSR TCRS (10, 11, 31, 46). However, as shown in Fig. 2A and B, the lrgAB mutant strain also exhibited significantly increased protection against both hNP-1 and DAP killing following preexposure to CCCP. This suggested that lytSR-mediated protection against the antistaphylococcal effects of HDPs and CAPs following Δψ perturbation are lrgAB independent.
Our pilot studies confirmed that the CCCP pretreatment (10 μM, 15 min) alone did not affect cell viability (data not shown).
In vivo virulence and DAP treatment outcomes.
As observed in previous studies (6, 39, 47, 48), S. aureus cell densities in cardiac vegetations were significantly higher than those in kidneys and spleens for each strain at 24 h postinfection (P < 0.01) (Table 3). In the comparative virulence assessments, except for the ΔlytSR complemented and ΔlrgAB strains, which showed rather moderate decreases in cell densities in kidneys (<1 log10-fold; P < 0.05), no significant differences in bacterial densities were observed among the strain set in the three target tissues. However, during DAP treatment of animals infected with the UAMS-1 parental strain, there were significant reductions in bacterial counts in all three target tissues (with reductions ranging from ∼3 to 4 log10 CFU/g) (Table 3). DAP treatment of animals infected with the ΔlytS mutant yielded an even more extensive and significant bacterial clearance from target tissues (with reductions ranging from ∼4 to 6 log10 CFU/g). DAP treatment of animals infected with the ΔlytS complementation strain resulted in return of near-parental bacterial clearance profiles. Consistent with our in vitro results described above, DAP treatment of animals infected with the lrgAB mutant resulted in only a modest reduction in target tissue counts.
Table 3.
In vivo virulence and DAP treatment outcomes
| Group (no. of animals)a |
S. aureus density (log10 CFU/g tissue ± SD)b in: |
||
|---|---|---|---|
| Vegetation | Kidney | Spleen | |
| Without treatment | |||
| UAMS-1 (7) | 7.30 ± 1.01 | 5.59 ± 0.65 | 5.60 ± 0.41 |
| ΔlytS mutant (7) | 7.65 ± 0.52 | 5.21 ± 0.88 | 5.35 ± 0.39 |
| ΔlytS Comp (7) | 7.12 ± 0.87 | 4.63 ± 0.86§ | 4.93 ± 0.70 |
| ΔlrgAB mutant (7) | 6.89 ± 0.93 | 4.52 ± 0.80§ | 4.97 ± 0.82 |
| DAP treatment (12 mg/kg, i.v., once daily, 3 days) | |||
| UAMS-1 (6) | 3.31 ± 0.59† | 2.67 ± 1.09† | 2.10 ± 0.65† |
| ΔlytS mutant (7) | 1.74 ± 0.69†* | 0.97 ± 0.71†* | 0.96 ± 0.58†* |
| ΔlytS Comp (7) | 2.56 ± 0.52† | 1.53 ± 0.65†¥ | 1.63 ± 0.75† |
| ΔlrgAB mutant (7) | 4.48 ± 1.85† | 4.33 ± 1.01¥ | 4.50 ± 1.27¥ |
Comp, complemented strain.
†, P < 0.05 versus same strain without DAP treatment; §, P < 0.05 versus UAMS-1 without DAP treatment; *, P < 0.001 versus UAMS-1 with DAP treatment; ¥, P < 0.05 versus UAMS-1 with DAP treatment.
DISCUSSION
Host defense antimicrobial peptides (HDPs) are crucial components of the innate immune system, and homologues are evolutionarily conserved in virtually all groups of organisms (3, 21, 49–51). Despite HDP-specific differences in amino acid sequences, net charge, and secondary/tertiary structures, these peptides may exert their lethal mechanisms predominantly after interacting with the target bacterial cytoplasmic membrane (CM) (1, 4, 49). This initial HDP interaction with the target CM can involve direct binding of the peptide to the CM via an electrostatic mechanism, followed by integration into the CM with or without formal oligomerization. In some contexts, when this process occurs, perturbation of membrane potential (Δψ) leading to cell death may ensue (1, 3, 17, 32, 52, 53). It should be emphasized that certain HDPs (e.g., hNP-1) both depolarize and permeabilize the CM of S. aureus, while others (e.g., the PMP family) appear to permeabilize, without initially depolarizing, and some may hyperpolarize the CM of S. aureus (53). In the latter circumstance, ultimate depolarization of the CM may occur as a secondary phenomenon following CM permeabilization (53).
The persistence and progression of endovascular S. aureus infections, such as infective endocarditis (IE), clearly require the organism to resist the bactericidal action of (i) CM-targeting HDPs, especially those likely to be encountered within the bloodstream or at sites of damaged endothelium (i.e., those of polymorphonuclear leukocyte [PMN] or platelet origins [22, 23, 32]), and (ii) clinically utilized CAPs (e.g., calcium-daptomycin).
Two-component regulatory systems (TCRSs) are widely utilized by bacteria to sense and respond to a broad range of external stimuli. Recently, it has been shown that the S. aureus GraRS TCRS (also called Aps, for antibiotic peptide sensor) is involved in promoting resistance to distinct HDPs and other CAPs by upregulating downstream genes such as mprF and dltABCD to modify the net positive surface charge (36, 37). The observation that expression of such GraRS-mediated effector genes is induced only by specific CAPs implies that the S. aureus GraRS system senses and responds to CAPs selectively (36, 37). Since most HDPs and clinical CAPs target the staphylococcal CM and ultimately perturb Δψ (3, 7, 52), additional sense-response systems for more rapidly and promiscuously detecting and adapting to subtle changes in Δψ would likely enhance the survival of the organism.
The S. aureus LytSR TCRS is a compelling candidate for such a sense-response module, potentially serving as a Δψ sensor by detecting changes in Δψ caused universally by HDPs and other CAPs. It has been demonstrated previously that the S. aureus LytSR TCRS regulates murein hydrolase activity and autolysis (11, 31). One well-known downstream target of this system is lrgAB, which, along with the cidABC operon, has been shown to be involved in regulation of programmed cell death and autolysis (8, 14, 15). More recently, it has also been shown that the LytSR TCRS plays a crucial role in the control of biofilm development, presumably by controlling lrgAB expression, autolysis, and the regulation of extracellular DNA (eDNA) release (11, 13). In addition to these roles, we examined whether the LytSR system in S. aureus can rapidly respond to a range of HDPs relevant to bloodstream infections by sensing changes in Δψ and then triggering adaptive countermeasures through the induction of lrgAB expression. In the present study, we used a well-characterized S. aureus strain, UAMS-1, and its isogenic ΔlytS and ΔlrgAB strains, along with their complemented variants. This parental strain was selected because (i) it is a well-characterized clinical isolate (from a patient with osteomyelitis) (8), (ii) it is easily manipulated using standard genetic techniques, and (iii) it has demonstrated virulence in a variety of animal infection models (54, 55).
The results of this study yielded several interesting findings that demonstrate the role of LytSR in sensing and responding to HDPs. First, the ΔlytS mutant displayed significantly increased in vitro susceptibilities to the tested HDPs (hNP-1, tPMPs, and RP-1), which are known to be important in host defense against staphylococcal endovascular infections (19, 21). In addition, this mutant was more susceptible to DAP, whose in vivo calcium-complexed form renders it a de facto CAP (25, 52). In contrast, no significant changes in susceptibilities to these CAPs were found for the ΔlrgAB strain, suggesting that even though the LytSR system can directly modulate lrgAB expression, the increase in HDP susceptibilities in ΔlytS was largely an lrgAB-independent phenomenon.
Second, since the bactericidal mechanisms of action of the study peptides, as well as DAP, involve disruption of Δψ associated with the CM (either primarily or secondarily), we examined the role of the lytSR-lrgAB pathway in CAP/HDP-induced survival adaptations. Previously, Sharma-Kuinkel et al. and Patton et al. demonstrated that exposure of S. aureus cells to CCCP, an agent that dissipates the CM Δψ, enhanced expression of lrgAB in a lytSR-dependent manner (10, 11). In our studies, the parental UAMS-1 strain exhibited enhanced survival against hNP-1 and DAP following pretreatment with CCCP, while the ΔlytS variant showed no such differences in survival. Of note, when the parental UAMS-1 strain was exposed to glucose-containing medium to reestablish CM Δψ after the CCCP treatment, susceptibility to hNP-1 was restored to the level for CCCP-nontreated cells. The ΔlrgAB mutant strain also showed enhanced survival to killing by hNP-1 and DAP when cells were preexposed to CCCP, although this impact was rather modest. Taken together, these data indicate that the S. aureus lytSR TCRS responds to changes in Δψ and triggers adaptive responses that enable resistance to CAP-induced killing through the regulation of gene expression other than, or in combination with, lrgAB. Thus, although changes in CM Δψ can activate the lrgAB pathway via lytSR, the consequences of this activation may be largely dedicated to the tasks of programmed cell death, autolysis, and biofilm formation and remodeling, as detailed above. These observations support the notion that the lytSR-mediated protection against HDP and CAP antistaphylococcal effects following altered CM Δψ necessitates the contribution of downstream pathways other than lrgAB.
Third, recently published studies by our group and others have suggested that there are two prominent CAP/HDP-adaptive phenotypic mechanisms in S. aureus: (i) increased positive surface charge (25, 38, 39, 41) and (ii) adaptive “recalibration” of CM biophysical order (fluidity/rigidity) (26–28). However, in the current study, we found that lytSR-dependent survival against CAP/HDP killing was not associated with changes in surface charge or with altered expression of mprF and dltA (two well-characterized genes involved in maintenance of staphylococcal surface charge) (38, 39, 41, 56). In addition, CM fluidity analyses failed to reveal any differences in the strains used in this study. Thus, the current data suggest that lytSR-dependent CAP/HDP resistance is not mediated by several previously described mechanisms of CAP/HDP resistance. Previous studies have assessed the impact of lytSR on global gene expression and revealed a large number of lytSR-regulated genes (∼460 genes) (11). In contrast, our ongoing investigations are more focused on defining changes in global gene expression profiles in comparing our ΔlytS mutant and its parental UAMS-1 strain under Δψ-depolarizing conditions. Such information should facilitate the identification of genes or regulatory pathways that may be involved in the CAP/HDP protective phenomenon mediated by LytSR.
Fourth, our in vitro data above led us to evaluate the role of the LytSR TCRS in endovascular infections, using the IE model (6, 41, 43). In the current study, both the ΔlytS and ΔlrgAB strains were found to be equivalent to the parental strain in their capabilities to induce experimental IE, proliferate within the vegetative lesion, and then disseminate hematogenously to distant target organs. In contrast, organism survivability and dissemination profiles were maximally reduced in animals infected with the ΔlytS mutant during treatment with DAP. As both hNP-1 and tPMPs play a key role in bacterial clearance from infected valves and other target tissues in IE (1, 8, 13, 49), the combined effect of increased susceptibilities to such HDPs, as well as to DAP, may explain the highly significant reduction in bacterial burden in these target tissues of ΔlytS mutant-infected animals. Consistent with our in vitro CAP/HDP susceptibility data, the profile of DAP-mediated target tissue bacterial clearances in animals infected with the ΔlrgAB strain was quite modest; these in vivo findings additionally underscore the concept that the lytSR-mediated adaptive response against CAPs/HDP is predominantly an lrgAB-independent process.
Our investigations have several limitations. For example, the in vitro HDP susceptibility testing was performed in austere artificial media, in the absence of host factors (e.g., serum proteins). Moreover, both PMNs and platelets contain a large cadre of HDPs which were not individually tested in this study (e.g., LL-37 [57]). Additionally, nonbloodstream HDPs (e.g., of cutaneous or mucosal origin) were not explored; these may be very important in modulating the initial colonization of S. aureus to skin or nasal mucosa, a frequent prerequisite stage prior to bloodstream invasion (49, 58, 59). Furthermore, it is likely that S. aureus cells within the bloodstream are simultaneously exposed to multiple HDPs. Finally, the concentrations of HDPs utilized in our in vitro assays are unquestionably lower than what organisms would encounter in vivo (58, 60). Nonetheless, the current data provide important insights into the potential for the LytSR system with respect to sense-and-response adaptations for S. aureus survival in the face of HDP or CAP exposure in vitro and in vivo.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (AI-39108 to A.S.B., AI-39001 to M.R.Y., AI-097657 to Y.Q.X., and AI-83211 to K.W.B.), an American Heart Association grant (12BGIA11780035 to S.-J.Y.), and the Institutional Junior Faculty Seed Grant Program (20526-01 to S.-J.Y.) from the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
We thank Kuan-Tsen Chen and Steven N. Ellison for excellent technical assistance with CAP/HDP susceptibility testing.
Footnotes
Published ahead of print 3 June 2013
REFERENCES
- 1. Peschel A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179–186 [DOI] [PubMed] [Google Scholar]
- 2. Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175:3907–3919 [DOI] [PubMed] [Google Scholar]
- 3. Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. 2005. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122:461–472 [DOI] [PubMed] [Google Scholar]
- 4. Brogden KA. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238–250 [DOI] [PubMed] [Google Scholar]
- 5. Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen WF, Jung G, Tarkowski A, van Kessel KPM, van Strijp JAG. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xiong YQ, Hady WA, Bayer AS, Chen L, Kreiswirth BN, Yang S-J. 2012. Telavancin in therapy of experimental aortic valve endocarditis in rabbits due to daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 56:5528–5533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koo S-P, Bayer AS, Yeaman MR. 2001. Diversity in antistaphylococcal mechanisms among membrane-targeting antimicrobial peptides. Infect. Immun. 69:4916–4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rice KC, Firek BA, Nelson JB, Yang Patton S-JTG, Bayles KW. 2003. The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. J. Bacteriol. 185:2635–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li M, Lai Y, Villaruz AE, Cha DJ, Sturdevant DE, Otto M. 2007. Gram-positive three-component antimicrobial peptide-sensing system. Proc. Natl. Acad. Sci. U. S. A. 104:9469–9474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patton TG, Bayles KW, Yang S-J. 2006. The role of proton motive force in expression of the Staphylococcus aureus cid and lrg operons. Mol. Microbiol. 59:1395–1404 [DOI] [PubMed] [Google Scholar]
- 11. Sharma-Kuinkel BK, Mann EE, Ahn JS, Kuechenmeister LJ, Dunman PM, Bayles KW. 2009. The Staphylococcus aureus LytSR two-component regulatory system affects biofilm formation. J. Bacteriol. 191:4767–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sadykov MR, Bayles KW. 2012. The control of death and lysis in staphylococcal biofilms: a coordination of physiological signals. Curr. Opin. Microbiol. 15:211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW. 2009. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 4:e5822. 10.1371/journal.pone.0005822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rice KC, Bayles KW. 2003. Death's toolbox: examining the molecular components of bacterial programmed cell death. Mol. Microbiol. 50:729. [DOI] [PubMed] [Google Scholar]
- 15. Groicher KH, Firek BA, Fujimoto DF, Bayles KW. 2000. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J. Bacteriol. 182:1794–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 63:3373–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silverman JA, Perlmutter NG, Shapiro HM. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steenbergen JN, Alder J, Thorne GM, Tally FP. 2005. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 55:283–288 [DOI] [PubMed] [Google Scholar]
- 19. Xiong YQ, Bayer AS, Elazegui L, Yeaman MR. 2006. A synthetic congener modeled on a microbicidal domain of thrombin-induced platelet microbicidal protein-1 recapitulates staphylocidal mechanisms of the native molecule. Antimicrob. Agents Chemother. 50:3786–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeaman MR, Gank KD, Bayer AS, Brass EP. 2002. Synthetic peptides that exert antimicrobial activities in whole blood and blood-derived matrices. Antimicrob. Agents Chemother. 46:3883–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeaman MR, Bayer AS, Koo SP, Foss W, Sullam PM. 1998. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J. Clin. Invest. 101:178–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yeaman MR, Puentes SM, Norman DC, Bayer AS. 1992. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect. Immun. 60:1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeaman MR, Sullam PM, Dazin PF, Bayer AS. 1994. Platelet microbicidal protein alone and in combination with antibiotics reduces Staphylococcus aureus adherence to platelets in vitro. Infect. Immun. 62:3416–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xiong YQ, Mukhopadhyay K, Yeaman MR, Adler-Moore J, Bayer AS. 2005. Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3114–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones T, Yeaman MR, Sakoulas G, Yang SJ, Proctor RA, Sahl HG, Schrenzel J, Xiong YQ, Bayer AS. 2008. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 52:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mishra NN, Liu GY, Yeaman MR, Nast CC, Proctor RA, McKinnell J, Bayer AS. 2011. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 55:526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mishra NN, Yang SJ, Sawa A, Rubio A, Nast CC, Yeaman MR, Bayer AS. 2009. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus (MRSA). Antimicrob. Agents Chemother. 53:2312–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukhopadhyay K, Whitmire W, Xiong YQ, Molden J, Jones T, Peschel A, Staubitz P, Adler-Moore J, McNamara PJ, Proctor RA, Yeaman MR, Bayer AS. 2007. In vitro susceptibility of Staphylococcus aureus to thrombin-induced platelet microbicidal protein-1 (tPMP-1) is influenced by cell membrane phospholipid composition and asymmetry. Microbiology 153:1187–1197 [DOI] [PubMed] [Google Scholar]
- 29. Bayer AS, Prasad R, Chandra J, Koul A, Smriti M, Varma A, Skurray RA, Firth N, Brown MH, Koo SP, Yeaman MR. 2000. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein is associated with alterations in cytoplasmic membrane fluidity. Infect. Immun. 68:3548–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chambers HF, Basuino L, Diep BA, Steenbergen J, Zhang S, Tattevin P, Alder J. 2009. Relationship between susceptibility to daptomycin in vitro and activity in vivo in a rabbit model of aortic valve endocarditis. Antimicrob. Agents Chemother. 53:1463–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brunskill EW, Bayles KW. 1996. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J. Bacteriol. 178:611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yeaman MR, Yount NY. 2003. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 55:27–55 [DOI] [PubMed] [Google Scholar]
- 33. Meehl M, Herbert S, Gotz F, Cheung A. 2007. Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 51:2679–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405–8410 [DOI] [PubMed] [Google Scholar]
- 35. Yang S-J, Nast CC, Mishra NN, Yeaman MR, Fey PD, Bayer AS. 2010. Cell wall thickening is not a universal accompaniment of the daptomycin nonsusceptibility phenotype in Staphylococcus aureus: evidence for multiple resistance mechanisms. Antimicrob. Agents Chemother. 54:3079–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li M, Cha DJ, Lai Y, Villaruz AE, Sturdevant DE, Otto M. 2007. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 66:1136–1147 [DOI] [PubMed] [Google Scholar]
- 37. Yang S-J, Bayer AS, Mishra NN, Meehl M, Ledala N, Yeaman MR, Xiong YQ, Cheung AL. 2012. The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides. Infect. Immun. 80:74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang SJ, Kreiswirth BN, Sakoulas G, Yeaman MR, Xiong YQ, Sawa A, Bayer AS. 2009. Enhanced expression of dltABCD is associated with development of daptomycin nonsusceptibility in a clinical endocarditis isolate of Staphylococcus aureus. J. Infect. Dis. 200:1916–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang SJ, Xiong YQ, Dunman PM, Schrenzel J, Francois P, Peschel A, Bayer AS. 2009. Regulation of mprF in daptomycin-nonsusceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2636–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bertsche U, Weidenmaier C, Kuehner D, Yang SJ, Baur S, Wanner S, Francois P, Schrenzel J, Yeaman MR, Bayer AS. 2011. Correlation of daptomycin resistance in a clinical Staphylococcus aureus strain with increased cell wall teichoic acid production and D-alanylation. Antimicrob. Agents Chemother. 55:3922–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weidenmaier C, Peschel A, Kempf VAJ, Lucindo N, Yeaman MR, Bayer AS. 2005. DltABCD- and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect. Immun. 73:8033–8038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weidenmaier C, Peschel A, Xiong YQ, Kristian SA, Dietz K, Yeaman MR, Bayer AS. 2005. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J. Infect. Dis. 191:1771–1777 [DOI] [PubMed] [Google Scholar]
- 43. Xiong Y-Q, Kupferwasser LI, Zack PM, Bayer AS. 1999. Comparative efficacies of liposomal amikacin (MiKasome) plus oxacillin versus conventional amikacin plus oxacillin in experimental endocarditis induced by Staphylococcus aureus: microbiological and echocardiographic analyses. Antimicrob. Agents Chemother. 43:1737–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rose WE, Rybak MJ, Kaatz GW. 2007. Evaluation of daptomycin treatment of Staphylococcus aureus bacterial endocarditis: an in vitro and in vivo simulation using historical and current dosing strategies. J. Antimicrob. Chemother. 60:334–340 [DOI] [PubMed] [Google Scholar]
- 45. Xiong YQ, Fowler JVG, Yeaman MR, Perdreau-Remington F, Kreiswirth BN, Bayer AS. 2009. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J. Infect. Dis. 199:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rice KC, Nelson JB, Patton TG, Yang SJ, Bayles KW. 2005. Acetic acid induces expression of the Staphylococcus aureus cidABC and lrgAB murein hydrolase regulator operons. J. Bacteriol. 187:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xiong Y-Q, Bayer AS, Yeaman MR, van Wamel W, Manna AC, Cheung AL. 2004. Impacts of sarA and agr in Staphylococcus aureus strain Newman on fibronectin-binding protein A gene expression and fibronectin adherence capacity in vitro and in experimental infective endocarditis. Infect. Immun. 72:1832–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang S-J, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob. Agents Chemother. 54:3161–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hancock REW, Diamond G. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402–410 [DOI] [PubMed] [Google Scholar]
- 50. Pelegrini P, Farias L, Saude A, Costa F, Bloch C, Silva L, Oliveira A, Gomes C, Sales M, Franco O. 2009. A novel antimicrobial peptide from Crotalaria pallida seeds with activity against human and phytopathogens. Curr. Microbiol. 59:400–404 [DOI] [PubMed] [Google Scholar]
- 51. Welling MM, Hiemstra PS, van den Barselaar MT, Paulusma-Annema A, Nibbering PH, Pauwels EKJ, Calame W. 1998. Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leukocyte accumulation. J. Clin. Invest. 102:1583–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alborn WE, Jr, Allen NE, Preston DA. 1991. Daptomycin disrupts membrane potential in growing Staphylococcus aureus. Antimicrob. Agents Chemother. 35:2282–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koo SP, Yeaman MR, Nast CC, Bayer AS. 1997. The cytoplasmic membrane is a primary target for the staphylocidal action of thrombin-induced platelet microbicidal protein. Infect. Immun. 65:4795–4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beenken KE, Spencer H, Griffin LM, Smeltzer MS. 2012. Impact of extracellular nuclease production on the biofilm phenotype of Staphylococcus aureus under in vitro and in vivo conditions. Infect. Immun. 80:1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 104:8113–8118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Staubitz P, Neumann H, Schneider T, Wiedemann I, Peschel A. 2004. MprF-mediated biosynthesis of lysylphosphatidylglycerol, an important determinant in staphylococcal defensin resistance. FEMS Microbiol. Lett. 231:67. [DOI] [PubMed] [Google Scholar]
- 57. Sørensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. 1997. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90:2796–2803 [PubMed] [Google Scholar]
- 58. Ganz T. 1987. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect. Immun. 55:568–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11–16 [DOI] [PubMed] [Google Scholar]
- 60. Ganz T, Selsted ME, Lehrer RI. 1990. Defensins. Eur. J. Haematol. 44:1–8 [DOI] [PubMed] [Google Scholar]


