Abstract
Health care-associated infections, including Pseudomonas aeruginosa bloodstream infection, have been linked to delays in appropriate antibiotic therapy and an increased mortality rate. The objective of this study was to evaluate intrinsic virulence, bacterial resistance, and clinical outcomes of health care-associated bloodstream infections (HCABSIs) in comparison with those of community-acquired bloodstream infections (CABSIs) caused by P. aeruginosa. We conducted a retrospective multicenter study of consecutive P. aeruginosa bacteremia patients at two university-affiliated hospitals. Demographic, clinical, and treatment data were collected. Microbiologic analyses included in vitro susceptibility profiles and type III secretory (TTS) phenotypes. Sixty CABSI and 90 HCABSI episodes were analyzed. Patients with HCABSIs had more organ dysfunction at the time of bacteremia (P = 0.05) and were more likely to have been exposed to antimicrobial therapy (P < 0.001) than those with CABSIs. Ninety-two percent of the carbapenem-resistant P. aeruginosa infections were characterized as HCABSIs. The 30-day mortality rate for CABSIs was 26% versus 36% for HCABSIs (P = 0.38). The sequential organ failure assessment score at the time of bacteremia (hazard ratio [HR], 1.2; 95% confidence interval [CI], 1.1 to 1.3) and the TTS phenotype (HR 2.1; 95% CI, 1.1 to 3.9) were found to be independent predictors of the 30-day mortality rate. No mortality rate difference was observed between CABSIs and HCABSIs caused by P. aeruginosa. Severity of illness and expression of TTS proteins were the strongest predictors of the 30-day mortality rate due to P. aeruginosa bacteremia. Future P. aeruginosa bacteremia trials designed to neutralize TTS proteins are warranted.
INTRODUCTION
Bloodstream infections (BSIs) are serious clinical events with life-threatening consequences. The total number of deaths resulting from nosocomial BSIs is difficult to estimate and varies greatly, depending on the etiology. However, the attributable mortality rate may be as high as 80% among patients in intensive care units (1). Pseudomonas aeruginosa accounts for 3 to 7% of all BSIs and 23 to 26% of Gram-negative bacteremias (2, 3). Pneumonia, pancreaticobiliary tract infection, indwelling catheters, and urinary tract infection have all been implicated as potential sources of infection (1, 4). Despite recent advances in critical-care management, mortality rates due to P. aeruginosa BSI remain high, ranging between 27 and 48% (1, 5).
Poor outcomes of P. aeruginosa BSIs have been associated with both microbial and host factors. Neutropenia, a respiratory source of bacteremia, shock, renal failure, and metastatic foci of infection are among the host factors implicated in increased mortality rates (4, 6). Bacterial attributes include a high degree of intrinsic virulence (7, 8) and widespread antibiotic resistance. P. aeruginosa is intrinsically resistant to many structurally unrelated antimicrobial agents (9) because of the low permeability of its outer membrane, the constitutive expression of various efflux pumps with wide substrate specificity, and the naturally occurring chromosomal AmpC beta-lactamase (10). In addition, the timely administration of adequate antimicrobial therapy to which the bacteria are susceptible has been identified as an important factor in improving the outcomes of P. aeruginosa BSIs (2, 4, 11–14).
With the progressive changes in the health care system whereby health care services are shifted from hospitals to outpatient facilities, a new classification scheme for BSIs has been proposed to distinguish among infections acquired (i) in the community, (ii) by outpatients having recurrent contact with the health care system, and (ii) by inpatients with hospital-acquired infections (15). This distinction was driven by observations indicating an increased risk of antimicrobial resistance and a higher mortality rate among patients with health care-acquired BSIs (HCABSIs) than among those with community-acquired BSIs (CABSIs) (16–18). As a result, a growing consensus has advocated a different empirical antibiotic regimen based on the antimicrobial susceptibility profile anticipated for each of these categories.
Despite the fact that P. aeruginosa is a predominantly nosocomial pathogen, the distinction between HCABSIs and CABSIs as it relates to P. aeruginosa in terms of its virulence traits (type III secretory [TTS] system) and its impact on clinical outcomes has not been previously examined. The objective of this study was to assess the epidemiology and outcome of P. aeruginosa BSIs by acquisition classification among all of the adults admitted to two tertiary-care hospitals. Hence, we performed a multicenter retrospective analysis of P. aeruginosa BSIs to compare the clinical and bacteriologic characteristics of community- and health care-associated P. aeruginosa bacteremias.
MATERIALS AND METHODS
Setting and design.
Clinical records of patients who had documented episodes of P. aeruginosa BSI at two university-affiliated hospitals (Veterans Affairs of Western New York and Northwestern Memorial Hospital) between August 1999 and January 2010 were examined retrospectively. A subset of the data has been published previously (19, 20). The data collected included age, gender, the presence of polymicrobial bacteremia, the presumed source of bacteremia, the duration of the hospital stay, the antimicrobial therapy regimen used, the time to the administration of active antimicrobial therapy, prior exposure to antibiotics, and death during the hospital stay, including the 30-day mortality rate. The duration of the hospital stay was defined as the time from the first positive blood culture result until discharge. Patients who died during the first 30 days after the onset of bacteremia were excluded for the length-of-stay analysis. The concomitant presence of coagulase-negative staphylococci was deemed a contaminant and thus not included in the analyses. Severity of acute illness was measured by the sequential organ failure assessment (SOFA) score on the day the blood culture was obtained (21). Comorbidities were assessed by the Charlson weighted index (22). The BSI source was determined from the infection control designation, in accordance with Centers for Disease Control guidelines (23). In cases of recurrent episodes of P. aeruginosa bacteremia, only the first event was entered into the database. This study was approved by the Institutional Review Board of the VA Western New York Healthcare System. In view of the retrospective nature of this study, informed consent was not required.
Microbiology.
Blood cultures from hospitalized patients were processed with the Bactec 9240 blood culture system (Becton, Dickinson, Sparks, MD), with each set consisting of aerobic and anaerobic cultures. Identification of bacteria from positive cultures to the genus and species levels was performed by the Vitek II System (bioMérieux, Balmes-les-Grottes, France) or manual biochemical assays when necessary. Cultures that grew P. aeruginosa were grown in LB medium and stored at −70°C in 25% glycerol until analyzed. Antimicrobial susceptibility and multidrug resistance (MDR) tests of all of the isolates were performed with different antibiotics (penicillins, cephalosporins, monobactams, carbapenems, and quinolones) by either Vitek II or Etest (AB Biodisk, Solna, Sweden) to determine MICs. MICs were interpreted according to Clinical and Laboratory Standards Institute guidelines (24).
Immunoblot analysis.
Analysis of P. aeruginosa TTS protein phenotypes was performed as described previously (25). In brief, bacterial strains were grown in MINS medium (26) for approximately 18 h at 37°C with vigorous shaking. Bacterial supernatants were obtained from 5-ml cultures by centrifugation at 6,000 × g at 4°C for 20 min. Proteins in supernatants were precipitated by the addition of ammonium sulfate to a final concentration of 55% (wt/vol). Following incubation on ice for 2 h, the precipitated material was collected by centrifugation at 13,000 × g at 4°C for 20 min. The pellet was boiled in 500 μl of 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer for 5 min, and 50 μl of each sample was subjected to sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (27). Proteins were then electrotransferred to nitrocellulose (Bio-Rad) and exposed to mixtures of polyclonal antisera against ExoS, ExoT, ExoU, PopB, and PopD (PopB and PopD are proteins secreted through the membrane-associated secretion needle, where they form the pore-like translocon in the host cell plasma membrane.) (7, 28, 29). Goat anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (Jackson ImmunoResearch Laboratories, Inc.) diluted 1:3,000 was used as the secondary antibody. Proteins were visualized by incubating the membranes in 225 μM coumaric acid (Sigma Chemical Co.)–1.25 mM 3-aminophthalhydrazide (Sigma)–0.009% hydrogen peroxide (Fisher Scientific Co.)–100 mM Tris (pH 8.5) for 1 min and then exposing the membranes to autoradiography film. P. aeruginosa isolates that secreted at least one effector (toxin) or component of the translocon apparatus under these conditions were designated “secretors” (secretion positive). Isolates that did not secrete any effectors or translocon components under these culture conditions were designated nonsecretors (secretion negative).
Clonal relatedness of P. aeruginosa isolates.
The clonal relatedness of P. aeruginosa isolates was assessed by repetitive-element-based PCR as described previously (25). The isolates were considered indistinguishable (no difference in bands on visual inspection), related (a difference of one or two bands), or distinct (a difference of three or more bands) (30).
Definitions.
Health care-associated bacteremia was defined as follows: (i) bacteremia that occurred >72 h after hospital admission, (ii) bacteremia that occurred <72 h after admission in patients who had been hospitalized for >2 days within the preceding 2 weeks before the BSI, (iii) bacteremia that occurred <72 h after hospital admission in patients who had been transferred from another hospital or a nursing home, or (iv) renal failure requiring hemodialysis in the last 30 days prior to the BSI. All other cases of bacteremia were classified as community acquired. P. aeruginosa strains were defined as carbapenem resistant (CR) if the MIC of imipenem or meropenem was ≥16 μg/ml. MDR was defined as resistance to three or more of the following classes of agents (31): antipseudomonal carbapenems, antipseudomonal beta-lactams (penicillins and cephalosporins), aminoglycosides, and fluoroquinolones.
Effective initial antimicrobial therapy was defined as therapy administered within 48 h after blood culture samples were obtained and consisting of an initial empirical regimen containing at least one antipseudomonal antibiotic (such as antipseudomonal penicillin, ceftazidime, carbapenem, or fluoroquinolone) that was later proved to be active in vitro against blood isolates of P. aeruginosa (6). A delay of effective antimicrobial therapy was defined as the administration of empirical antibiotics ineffective against the P. aeruginosa isolate prior to the availability of the results of antibiotic susceptibility testing, with a delay of >48 h after blood culture samples were obtained.
To calculate SOFA scores, we collected the necessary laboratory and clinical data from patients' medical records for the appropriate day. If multiple values were obtained on a given day, the most abnormal value was used. If a value was missing from the day of interest, the measurement from the nearest day was used in its place. If no values were available, the measurement was assumed to be normal. Points were assigned for increasing degrees of failure of six different organ systems, respiratory, coagulation, hepatic, cardiovascular, central nervous system, and renal, as previously described (21). Values were transformed into component dysfunction scores of 0 (normal) to 4 (the most abnormal) for each organ system (21), thus providing a composite SOFA score between 0 and 24.
Statistical analysis.
Data are expressed as means ± standard deviations (SDs) or medians (interquartile ranges [IQRs]). The Student t test and Mann-Whitney test were used to compare continuous variables, and the χ2 test, Fisher's exact test, or linear-by-linear association was used to compare categorical variables, as needed. Time to death was analyzed by Kaplan-Meier survival analysis and the log-rank test. To determine independent risk factors for death, a Cox regression hazard model was used to control for the effects of confounding variables. Variables with P values of <0.2 in the univariate analyses were candidates for multivariate analysis. Interactions between variables were not introduced into the models. All P values were two tailed, and P values of ≤0.05 were considered to be statistically significant. SPSS statistics for Windows (SPPS Inc., Chicago, IL), version 18.0, was used for these analyses.
RESULTS
Clinical data of P. aeruginosa isolates causing BSIs.
During the study period, a total of 150 patients with P. aeruginosa bacteremia were identified. Ninety cases (60%) met the definition of HCABSI, and 60 (40%) were classified as CABSIs. Demographics, comorbid diseases, and primary sites of infection are shown in Table 1. The median age of the population was 65.5 (range, 18 to 93) years. Men represented 67% of the entire cohort. There was no difference in age, gender, or the burden of comorbidities between the CABSI and HCABSI groups. However, HCABSI patients had more organ dysfunction at the time of bacteremia (P = 0.05) and were more likely to have been exposed to antimicrobial therapy (P < 0.001) than those with CABSIs. The admission characteristics were also different. Of the HCABSI patients, 57% had been hospitalized in an acute-care hospital prior to BSI, 33% had undergone dialysis or received intravenous chemotherapy, and 9% had resided in a nursing home or a long-term care facility.
Table 1.
Characteristics of the study population
| Characteristic | CABSI (n = 60) | HCABSI (n = 90) | P value |
|---|---|---|---|
| Mean age (yr) ± SD | 65.4 ± 15.9 | 62.1 ± 19.1 | 0.32 |
| No. (%) of males | 39 (65) | 62 (69) | 0.75 |
| Median Charlson index (IQR) | 4 (2–7) | 4 (2–7) | 0.98 |
| No. (%) with polymicrobial infections | 8 (13) | 17 (19) | 0.50 |
| Median SOFA score (IQR) | 5 (3–8) | 7 (4–10) | 0.05 |
| No. (%) with prior antibiotic treatment | 24 (40) | 68 (76) | <0.001 |
| Median length of stay (days) before bacteremia (IQR) | 0 (0–1) | 9 (0–26) | <0.001 |
| No. (%) who also had: | |||
| Diabetes mellitus | 13 (22) | 23 (26) | 0.73 |
| COPDa | 7 (12) | 11 (12) | 0.88 |
| Hepatobiliary tract disease | 8 (13) | 14 (16) | 0.89 |
| Hematologic malignancies | 4 (7) | 15 (17) | 0.12 |
| Solid tumors | 7 (12) | 17 (19) | 0.34 |
| No. (%) whose infection source was: | |||
| Respiratory tract infection | 12 (20) | 26 (29) | 0.30 |
| Urinary tract infection | 16 (27) | 20 (22) | 0.67 |
| Abdominal infection | 5 (8) | 9 (10) | 0.95 |
| Soft tissue infection | 7 (12) | 5 (6) | 0.29 |
| Central venous catheter | 1 (2) | 12 (13) | 0.03 |
| Unknown | 19 (32) | 18 (20) | 0.15 |
COPD, chronic obstructive pulmonary disease.
Among the sites identified, lower respiratory tract infections (25%) and urinary tract infections (24%) were the most common P. aeruginosa BSI sources overall. Respiratory tract infection (29%) was the most frequent HCABSI source, whereas urinary tract infection (27%) was the most common CABSI source. An indwelling catheter was the only infection source more common among HCABSI patients than among CABSI patients (P = 0.03).
Seventeen percent of the patients had polymicrobial BSIs with secondary pathogens other than coagulase-negative staphylococci (Table 2). Among the concomitant organisms isolated were Enterococcus species (n = 7), Klebsiella pneumoniae (n = 2), Escherichia coli (n = 4), Proteus mirabilis (n = 3), methicillin-resistant Staphylococcus aureus (n = 2), Streptococcus species (n = 1), Enterobacter cloacae (n = 2), and Bacteroides species (n = 1).
Table 2.
Concomitant bacteria isolated during P. aeruginosa-related BSI
| Bacterium | CABSIa | HCABSIb |
|---|---|---|
| Staphylococcus aureus | 0 | 2 |
| Streptococcus species | 0 | 1 |
| Enterococcus species | 2 | 5 |
| Enterobacter species | 0 | 2 |
| Klebsiella pneumonia | 1 | 1 |
| Escherichia coli | 1 | 3 |
| Proteus species | 1 | 2 |
| Bacteriodes | 0 | 1 |
| Other | 0 | 3 |
There were 60 CABSI patients.
There were 90 HCABSI patients.
Antimicrobial susceptibility testing.
The antimicrobial susceptibility of the bacteremic isolates of P. aeruginosa is shown in Table 3. CABSIs and HCABSIs showed the highest frequency of resistance to aztreonam (35 and 40%), ciprofloxacin (20 and 41%), and gentamicin (18 and 25%, respectively). However, health care-acquired isolates had a higher overall frequency of resistance to multiple antibiotics. In particular, resistance to ciprofloxacin and ceftazidime was significantly higher in health care-associated isolates than in community-acquired isolates (P = 0.02 and P = 0.01, respectively).
Table 3.
Antimicrobial susceptibility patterns of P. aeruginosa isolates
| Antimicrobial agent | No. (%) of susceptible isolates |
P value | |
|---|---|---|---|
| CABSI | HCABSI | ||
| Gentamicin | 49 (82) | 67 (75) | 0.41 |
| Tobramycin | 56 (93) | 74 (82) | 0.09 |
| Amikacin | 56 (93) | 83 (96) | 0.95 |
| Piperacillin-tazobactam | 57 (95) | 78 (87) | 0.17 |
| Ceftazidime | 57 (95) | 73 (81) | 0.03 |
| Imipenem | 55 (92) | 82 (91) | 0.86 |
| Meropenem | 60 (100) | 85 (94) | 0.16 |
| Ciprofloxacin | 48 (80) | 53 (59) | 0.01 |
| Aztreonam | 39 (65) | 54 (60) | 0.66 |
There were 60 CABSI patients.
There were 90 HCABSI patients.
MDR occurred in 11 cases (7%). All 11 patients had been exposed to antimicrobial therapy in the previous 90 days. Although HCABSIs accounted for 9 (82%) of these cases, the difference between CABSIs and HCABSIs in the prevalence of MDR P. aeruginosa did not attain statistical significance (P = 0.19).
Microbiological data of P. aeruginosa isolates causing BSIs.
Of the 150 isolates, 119 had a unique fingerprint. Twenty-seven isolates were considered related strains, and two pairs of isolates were identical. During the study period, 13 (9%) P. aeruginosa isolates were identified as CR. The clinical characteristics of the infected patients are listed in Table 4. None of the demographics or bacteriological factors was significantly associated with CR P. aeruginosa. There was also no difference in the severity of organ dysfunction, the burden of comorbidities, or the source of infection between carbapenem-sensitive and CR P. aeruginosa-infected patients.
Table 4.
Clinical characteristics of CR P. aeruginosa isolates
| Patient characteristic | Carbapenem-sensitive isolatesa | CR isolatesb | P value |
|---|---|---|---|
| Mean age (yr) ± SD | 63.9 ± 17.9 | 62.5 ± 18.0 | 0.77 |
| No. (%) of males | 92 (67) | 7 (54) | 0.51 |
| Median Charlson index (IQR) | 4.0 (2.0–7.0) | 4.0 (2.75–5.25) | 0.65 |
| No. (%) with polymicrobial infections | 22 (16) | 3 (23) | 0.79 |
| Median SOFA score (IQR) | 6.0 (4.0–9.0) | 8.0 (4.5–9.5) | 0.41 |
| Median HCABSI (IQR) | 78 (57) | 12 (92) | 0.03 |
| No. (%) with prior antibiotic therapy | 79 (58) | 13 (100) | 0.007 |
| No. (%) with TTS phenotype | 91 (66) | 8 (62) | 0.96 |
| No. (%) who died within 30 days | 44 (32) | 7 (54) | 0.20 |
| No. (%) whose source of infection was: | |||
| Respiratory tract infection | 35 (26) | 3 (23) | 0.89 |
| Urinary tract infection | 36 (26) | 0 | 0.08 |
| Abdominal infection | 12 (9) | 2 (15) | 0.78 |
| Soft tissue infection | 9 (7) | 3 (23) | 0.11 |
| Central venous catheter | 11 (8) | 2 (15) | 0.70 |
| Unknown | 34 (25) | 3 (23) | 0.84 |
There were 137 carbapenem-sensitive isolates.
There were 13 CR isolates.
Ninety-two percent of the CR P. aeruginosa infections were characterized as HCABSIs. Prior exposure to antibiotics was reported more frequently in cases with CR P. aeruginosa than in cases with carbapenem-sensitive P. aeruginosa (100% versus 58%, respectively; P = 0.007). Based on in vitro susceptibility, the CR P. aeruginosa strains were highly resistant to other antimicrobial agents (ciprofloxacin in 77% of the isolates, gentamicin in 69%, and piperacillin-tazobactam in 46%).
Ninety-nine P. aeruginosa isolates (66%) were identified as TTS secretors, of which 61 were classified as HCABSIs and 38 were classified as CABSIs (P = 0.71). ExoU, ExoT, and ExoS were produced by 25, 63, and 37% of the isolates, respectively. The prevalence of ExoU, ExoT, and ExoS in CABSIs was 28, 63, and 32% versus 22, 64 and 41% in HCABSIs (P = 0.51, P = 0.92, and P = 0.32, respectively). One isolate tested positive for PopB and PopD, but there was no secretory exotoxin detected.
Clinical outcomes.
Empirical antimicrobial therapy was adequate in 48 (80%) CABSI and 78 (87%) HCABSI patients (P = 0.39). However, inappropriate antimicrobial therapy was more often observed in patients with CR P. aeruginosa bacteremia than in those with carbapenem-sensitive P. aeruginosa bacteremia (46% versus 12%; P = 0.005). The length of hospital stay of HCABSI patients (median, 10 [IQR, 4 to 28] days) was significantly longer than that of CABSI patients (median, 8 [IQR, 3 to 16] days), P = 0.05. The overall crude in-hospital mortality rate was 37%. The 30-day mortality rate of CABSI patients was 26% versus 36% for HCABSI patients (P = 0.38) (Fig. 1). Twenty-two (39%) of 56 patients died within 48 h of bacteremia onset. The mortality rate was greatest in individuals with a pneumonic origin of bacteremia and least in individuals bacteremic from a urinary source (odds ratio, 5.4; 95% confidence interval [CI], 2.4 to 11.8). There was no difference in the 30-day mortality rate between mono- and polymicrobial BSIs (28% versus 35%, respectively, P = 0.64). According to the univariate analysis, the SOFA score was the only variable independently associated with the mortality rate (Table 5). In a Cox regression analysis, the SOFA score at the time of bacteremia (hazard ratio [HR], 1.18; 95% CI, 1.10 to 1.26) and the TTS phenotype (HR, 2.24; 95% CI, 1.26 to 4.67) were found to be independent predictors of the 30-day mortality rate of P. aeruginosa bacteremia patients after adjustment for other confounding variables (Table 6).
Fig 1.
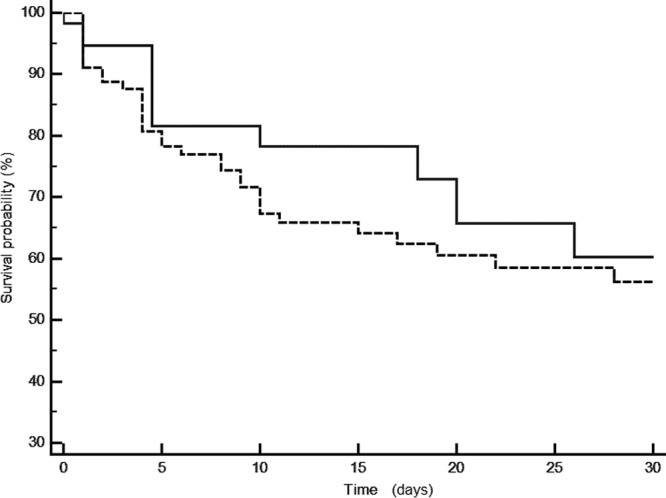
Kaplan-Meier curve comparing the 30-day survival rates of patients with community-acquired bacteremia (continuous line) and those with health care-associated bacteremia (dotted line) caused by P. aeruginosa (P = 0.46 by log-rank test).
Table 5.
Univariate predictors of the P. aeruginosa BSI patient 30-day mortality rate
| Predictor | Survivorsa | Nonsurvivorsb | P value |
|---|---|---|---|
| Mean age (yr) ± SD | 62.6 ± 16.7 | 65.2 ± 19.9 | 0.42 |
| No. (%) of males | 64 (65) | 35 (69) | 0.76 |
| Median Charlson index (IQR) | 4.0 (2.0–7.0) | 4.0 (2.0–7.0) | 0.52 |
| No. (%) with polymicrobial infections | 18 (18) | 7 (14) | 0.64 |
| Median SOFA score (IQR) | 5.0 (3.0–8.0) | 9.0 (5.0–12.0) | <0.001 |
| No. (%) with HCABSI | 55 (56) | 35 (69) | 0.17 |
| No. (%) with prior antibiotic therapy | 59 (60) | 33 (65) | 0.67 |
| No. (%) with TTS phenotype | 59 (60) | 39 (76) | 0.06 |
| No. (%) with carbapenem resistance | 6 (6) | 7 (14) | 0.20 |
| No. (%) with adequate empirical therapy | 84 (85) | 42 (82) | 0.87 |
There were 99 survivors.
There were 51 nonsurvivors.
Table 6.
Cox regression analysis of risk factors for death within 30 days
| Covariate | HR | P value | 95% CI of HR |
|---|---|---|---|
| SOFA | 1.18 | <0.0001 | 1.10 to 1.26 |
| TTS | 2.42 | 0.0085 | 1.26 to 4.67 |
| HCABSI | 1.24 | 0.68 | 0.84 to 1.62 |
| CR | 1.53 | 0.30 | 0.68 to 3.42 |
DISCUSSION
The findings of this study suggest that the incidence of health care-associated P. aeruginosa bacteremia is greater than that of community-acquired P. aeruginosa. CR P. aeruginosa was isolated predominantly from HCABSI patients. Despite improved antimicrobial stewardship over the last decade, the mortality rate of P. aeruginosa bacteremia patients has remained constant, suggesting intrinsic virulence as a potential target for future adjuvant therapy.
Our annual incidence rate of P. aeruginosa bacteremia of 1.2 cases/1,000 admissions was comparable to that of other hospital-based studies, which reported a range of 0.39 to 4.7 cases/1,000 admissions (4, 32–34). Unlike previous investigations, where the clinical characteristics of HCABSI patients are usually distinct from those of CABSI patients (17, 35, 36), we found no significant demographic or clinical parameters that would distinguish these two entities in cases of P. aeruginosa bacteremia apart from the higher severity of illness and the higher exposure to prior antibiotics in the HCABSI group. The lack of difference in age, gender, and underlying comorbid diseases between the two groups reflects the opportunistic trait of P. aeruginosa infection. Contrary to others' findings, we failed to note that those with HCABSIs were more likely than those with CABSIs to receive inappropriate empirical therapy. One reason is that all of the patients were treated according to established guidelines at each corresponding institution. These results are encouraging, since it may represent an increase in awareness that is due to the growing body of research and policy importance in the area of BSIs.
Infections with multidrug-resistant P. aeruginosa have been recognized as a growing problem in clinical settings. The carbapenems represent a realistic option for initial empirical therapy in many serious nosocomial bacteremias because of their broad spectrum of activity and limited toxicity. However, carbapenem resistance is being observed more frequently among P. aeruginosa isolates. During a 2008-2009 surveillance study of P. aeruginosa bacteremia in 10 public hospitals in Spain, 23 and 21.5% of the P. aeruginosa isolates obtained were resistant to imipenem and meropenem, respectively (37). Our rates of CR P. aeruginosa were lower and comparable to those of epidemiologic studies performed in the United States (38). It is plausible that annual variations in geographic regions, differences in antibiotic consumption, and the type of participating centers may contribute to these differences. Nevertheless, the rising trend of CR P. aeruginosa calls for early detection, strict infection control policies, and judicious prescription of antibiotics.
In line with other studies, we have found that prior antibiotic use was a common denominator among patients with CR P. aeruginosa bacteremia. In the absence of a control group, we cannot claim that previous antibiotic exposure is a risk factor for CR P. aeruginosa; however, prior fluoroquinolone (39, 40), aminoglycoside (41), and polymyxin (42) use has been implicated in the emergence of CR P. aeruginosa. The fact that more than 90% of the antibiotic-resistant strains were isolated from health care-associated bacteremic events suggests that empirical therapy in patients with prior exposure to antimicrobial therapy should avoid a monotherapy approach to the management of HCABSIs until antibiotic susceptibility is available.
The overall mortality rate among our patients was 37%. This relatively high death rate is troubling, given that most of the patients received prompt and active antimicrobial therapy. Nevertheless, our mortality rate was similar to those reported in the literature for P. aeruginosa BSIs. Kang et al. (2) and Chamot et al. (43) each reported a comparable 30-day mortality rate of 39%. A large number of deaths in our study occurred during the first few days after the diagnostic blood culture despite the use of appropriate antimicrobial therapy. This observation has been attributed to the microbiologic determinants of P. aeruginosa per se, irrespective of the initial empirical therapy (44, 45). Among the many virulence factors expressed by P. aeruginosa strains, we found that the TTS phenotype was independently associated with the 30-day mortality rate. The type III secretion system of P. aeruginosa has been previously associated with the mortality rate of patients with nosocomial pneumonia and P. aeruginosa BSI (7, 20, 46). Considering the development of targeted therapies, the knowledge of the TTS phenotype of P. aeruginosa isolates may be useful for determining the most effective therapeutic regimen for patients with these infections.
Surprisingly, we found no relationship between the adequacy of initial empirical antimicrobial therapy and death. This finding was true in our bivariate comparisons even without controlling for the higher baseline morbidity of patients who did not receive active therapy. Similar findings were reported recently by other investigators (17, 19). Several studies have highlighted specific populations with bacteremia that may be vulnerable to a delay in antimicrobial therapy (2, 6). Kang et al. (2) noted that the mean duration of the delay in starting effective antimicrobial therapy was 3.5 days. In the present study, 84% had active therapy initiated by the second calendar day. Since active therapy by 52 h is an important predictor of the 30-day mortality rate in bacteremia (6), most of the patients in our study received optimal therapy. The lack of a significant association between inadequate empirical antibiotic therapy and outcomes may also be related to the source of bacteremia. In fact, a third of BSI episodes were of urinary or vascular catheter origin, in which nonantimicrobial interventions such as catheter removal or alleviation of urinary obstruction are of critical importance in treatment, hence delegating antimicrobial therapy to a secondary role in BSI management.
This study had several limitations. First, it was a retrospective study, and thus, some of the medical records in charts may not have been complete, even though we tried to enroll patients who had more concrete information. Further, any hidden bias that was not adjusted for in our cohort may lead to underestimation or overestimation of the true relationship between antimicrobial resistance and death, even though we performed multivariate regression analysis to control for other confounding factors. Second, this study was performed in two tertiary-care hospitals, where the conditions, patient populations, and outcomes may differ from those in non-tertiary-care centers. Third, although unlikely, it is plausible that at least some of the BSIs were misclassified as CABSIs. This might have reduced the observed between-group differences.
In conclusion, our findings do not support the distinction between P. aeruginosa HCABSIs and CABSIs. The outcome of P. aeruginosa bacteremia is influenced by the severity of illness and the expression of virulence traits. Randomized trials using adjuvant therapy targeting the TTS phenotype of P. aeruginosa bacteremia are warranted.
ACKNOWLEDGMENT
The study was partially supported by NIH R01AI053674 (A.R.H.).
Footnotes
Published ahead of print 3 June 2013
REFERENCES
- 1. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317 [DOI] [PubMed] [Google Scholar]
- 2. Kang CI, Kim SH, Kim HB, Park SW, Choe YJ, Oh MD, Kim EC, Choe KW. 2003. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin. Infect. Dis. 37:745–751 [DOI] [PubMed] [Google Scholar]
- 3. Gaynes R, Edwards JR, National Nosocomial Infections Surveillance System 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848–854 [DOI] [PubMed] [Google Scholar]
- 4. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. 2005. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob. Agents Chemother. 49:1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. 2005. Risk factors for antimicrobial resistance and influence of resistance on mortality in patients with bloodstream infection caused by Pseudomonas aeruginosa. Microb. Drug Resist. 11:68–74 [DOI] [PubMed] [Google Scholar]
- 6. Lodise TP, Jr, Patel N, Kwa A, Graves J, Furuno JP, Graffunder E, Lomaestro B, McGregor JC. 2007. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob. Agents Chemother. 51:3510–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hauser AR, Cobb E, Bodi M, Mariscal D, Vallés J, Engel JN, Rello J. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 30:521–528 [DOI] [PubMed] [Google Scholar]
- 8. Sadikot RT, Blackwell TS, Christman JW, Prince AS. 2005. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 171:1209–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mesaros N, Nordmann P, Plesiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, Van Laethem Y, Jacobs F, Lebecque P, Malfroot A, Tulkens PM, Van Bambeke F. 2007. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin. Microbiol. Infect. 13:560–578 [DOI] [PubMed] [Google Scholar]
- 10. Breidenstein EB, de la Fuente-Nunez C, Hancock RE. 2011. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 19:419–426 [DOI] [PubMed] [Google Scholar]
- 11. Bisbe J, Gatell JM, Puig J, Mallolas J, Martinez JA, Jimenez de Anta MT, Soriano E. 1988. Pseudomonas aeruginosa bacteremia: univariate and multivariate analyses of factors influencing the prognosis in 133 episodes. Rev. Infect. Dis. 10:629–635 [DOI] [PubMed] [Google Scholar]
- 12. Chatzinikolaou I, Abi-Said D, Bodey GP, Rolston KV, Tarrand JJ, Samonis G. 2000. Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: retrospective analysis of 245 episodes. Arch. Intern. Med. 160:501–509 [DOI] [PubMed] [Google Scholar]
- 13. Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. 1989. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am. J. Med. 87:540–546 [DOI] [PubMed] [Google Scholar]
- 14. Mallolas J, Gatell JM, Miro JM, Marco F, Soriano E. 1990. Epidemiologic characteristics and factors influencing the outcome of Pseudomonas aeruginosa bacteremia. Rev. Infect. Dis. 12:718–719 [DOI] [PubMed] [Google Scholar]
- 15. Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. 2002. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 137:791–797 [DOI] [PubMed] [Google Scholar]
- 16. Vallés J, Calbo E, Anoro E, Fontanals D, Xercavins M, Espejo E, Serrate G, Freixas N, Morera MA, Font B, Bella F, Segura F, Garau J. 2008. Bloodstream infections in adults: importance of healthcare-associated infections. J. Infect. 56:27–34 [DOI] [PubMed] [Google Scholar]
- 17. Kollef MH, Zilberberg MD, Shorr AF, Vo L, Schein J, Micek ST, Kim M. 2011. Epidemiology, microbiology and outcomes of healthcare-associated and community-acquired bacteremia: a multicenter cohort study. J. Infect. 62:130–135 [DOI] [PubMed] [Google Scholar]
- 18. Son JS, Song JH, Ko KS, Yeom JS, Ki HK, Kim SW, Chang HH, Ryu SY, Kim YS, Jung SI, Shin SY, Oh HB, Lee YS, Chung DR, Lee NY, Peck KR. 2010. Bloodstream infections and clinical significance of healthcare-associated bacteremia: a multicenter surveillance study in Korean hospitals. J. Korean Med. Sci. 25:992–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scheetz MH, Hoffman M, Bolon MK, Schulert G, Estrellado W, Baraboutis IG, Sriram P, Dinh M, Owens LK, Hauser AR. 2009. Morbidity associated with Pseudomonas aeruginosa bloodstream infections. Diagn. Microbiol. Infect. Dis. 64:311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El-Solh AA, Hattemer A, Hauser AR, Alhajhusain A, Vora H. 2012. Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit. Care Med. 40:1157–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. 1998. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 26:1793–1800 [DOI] [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373–383 [DOI] [PubMed] [Google Scholar]
- 23. Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 36:309–332 [DOI] [PubMed] [Google Scholar]
- 24. Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 25. El Solh AA, Akinnusi ME, Wiener-Kronish JP, Lynch SV, Pineda LA, Szarpa K. 2008. Persistent infection with Pseudomonas aeruginosa in ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 178:513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nicas TI, Iglewski BH. 1984. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect. Immun. 45:470–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 28. Hauser AR, Kang PJ, Engel JN. 1998. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27:807–818 [DOI] [PubMed] [Google Scholar]
- 29. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 30. Tam VH, Rogers CA, Chang KT, Weston JS, Caeiro JP, Garey KW. 2010. Impact of multidrug-resistant Pseudomonas aeruginosa bacteremia on patient outcomes. Antimicrob. Agents Chemother. 54:3717–3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Defez C, Fabbro-Peray P, Bouziges N, Gouby A, Mahamat A, Daures JP, Sotto A. 2004. Risk factors for multidrug-resistant Pseudomonas aeruginosa nosocomial infection. J. Hosp. Infect. 57:209–216 [DOI] [PubMed] [Google Scholar]
- 32. Vidal F, Mensa J, Almela M, Martinez JA, Marco F, Casals C, Gatell JM, Soriano E, Jimenez de Anta MT. 1996. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch. Intern. Med. 156:2121–2126 [PubMed] [Google Scholar]
- 33. Siegman-Igra Y, Ravona R, Primerman H, Giladi M. 1998. Pseudomonas aeruginosa bacteremia: an analysis of 123 episodes, with particular emphasis on the effect of antibiotic therapy. Int. J. Infect. Dis. 2:211–215 [DOI] [PubMed] [Google Scholar]
- 34. Blot S, Vandewoude K, Hoste E, Colardyn F. 2003. Reappraisal of attributable mortality in critically ill patients with nosocomial bacteraemia involving Pseudomonas aeruginosa. J. Hosp. Infect. 53:18–24 [DOI] [PubMed] [Google Scholar]
- 35. Vallés J, Alvarez-Lerma F, Palomar M, Blanco A, Escoresca A, Armestar F, Sirvent JM, Balasini C, Zaragoza R, Marin M, Study Group of Infectious Diseases of the Spanish Society of Critical Care Medicine 2011. Health-care-associated bloodstream infections at admission to the ICU. Chest 139:810–815 [DOI] [PubMed] [Google Scholar]
- 36. Tsay RW, Siu LK, Fung CP, Chang FY. 2002. Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch. Intern. Med. 162:1021–1027 [DOI] [PubMed] [Google Scholar]
- 37. Peña C, Suarez C, Gozalo M, Murillas J, Almirante B, Pomar V, Aguilar M, Granados A, Calbo E, Rodriguez-Bano J, Rodriguez F, Tubau F, Martinez-Martinez L, Oliver A, Spanish Network for Research in Infectious Diseases Research 2012. Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob. Agents Chemother. 56:1265–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schechner V, Gottesman T, Schwartz O, Korem M, Maor Y, Rahav G, Karplus R, Lazarovitch T, Braun E, Finkelstein R, Lachish T, Wiener-Well Y, Alon D, Chowers M, Bardenstein R, Zimhony O, Paz A, Potasman I, Giladi M, Schwaber MJ, Klarfeld-Lidji S, Hochman M, Marchaim D, Carmeli Y. 2011. Pseudomonas aeruginosa bacteremia upon hospital admission: risk factors for mortality and influence of inadequate empirical antimicrobial therapy. Diagn. Microbiol. Infect. Dis. 71:38–45 [DOI] [PubMed] [Google Scholar]
- 39. Lautenbach E, Weiner MG, Nachamkin I, Bilker WB, Sheridan A, Fishman NO. 2006. Imipenem resistance among Pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes. Infect. Control Hosp. Epidemiol. 27:893–900 [DOI] [PubMed] [Google Scholar]
- 40. Paramythiotou E, Lucet JC, Timsit JF, Vanjak D, Paugam-Burtz C, Trouillet JL, Belloc S, Kassis N, Karabinis A, Andremont A. 2004. Acquisition of multidrug-resistant Pseudomonas aeruginosa in patients in intensive care units: role of antibiotics with antipseudomonal activity. Clin. Infect. Dis. 38:670–677 [DOI] [PubMed] [Google Scholar]
- 41. Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. 2006. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob. Agents Chemother. 50:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mentzelopoulos SD, Pratikaki M, Platsouka E, Kraniotaki H, Zervakis D, Koutsoukou A, Nanas S, Paniara O, Roussos C, Giamarellos-Bourboulis E, Routsi C, Zakynthinos SG. 2007. Prolonged use of carbapenems and colistin predisposes to ventilator-associated pneumonia by pandrug-resistant Pseudomonas aeruginosa. Intensive Care Med. 33:1524–1532 [DOI] [PubMed] [Google Scholar]
- 43. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. 2003. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob. Agents Chemother. 47:2756–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Delden C. 2007. Pseudomonas aeruginosa bloodstream infections: how should we treat them? Int. J. Antimicrob. Agents 30(Suppl 1):S71–S75 [DOI] [PubMed] [Google Scholar]
- 45. Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. 2003. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit. Care Med. 31:2742–2751 [DOI] [PubMed] [Google Scholar]
- 46. Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767–1774 [DOI] [PubMed] [Google Scholar]


