Abstract
Since the mid-1990s, a steady increase in the occurrence of itraconazole-resistant Aspergillus fumigatus isolates has been observed in clinical contexts, leading to therapeutic failure in the treatment of aspergillosis. This increase has been predominantly linked to a single allele of the cyp51A gene, termed TR/L98H, which is thought to have arisen through the use of agricultural azoles. Here, we investigated the current epidemiology of triazole-resistant A. fumigatus and underlying cyp51A mutations in clinical samples in Germany. From a total of 527 samples, 17 (3.2%) showed elevated MIC0 values (the lowest concentrations with no visible growth) for at least one of the three substances (itraconazole, voriconazole, and posaconazole) tested. The highest prevalence of resistant isolates was observed in cystic fibrosis patients (5.2%). Among resistant isolates, the TR/L98H mutation in cyp51A was the most prevalent, but isolates with the G54W and M220I substitutions and the novel F219C substitution were also found. The isolate with the G54W substitution was highly resistant to both itraconazole and posaconazole, while all others showed high-level resistance only to itraconazole. For the remaining six isolates, no mutations in cyp51A were found, indicating the presence of other mechanisms. With the exception of the strains carrying the F219C and M220I substitutions, many itraconazole-resistant strains also showed cross-resistance to voriconazole and posaconazole with moderately increased MIC0 values. In conclusion, the prevalence of azole-resistant A. fumigatus in our clinical test set is lower than that previously reported for other countries. Although the TR/L98H mutation frequently occurs among triazole-resistant strains in Germany, it is not the only resistance mechanism present.
INTRODUCTION
Clinical manifestations of aspergillosis range from pulmonary colonization and deep invasive mycoses of the lung and other tissues to fatal sepsis in immunocompromised patients. A steady increase in the occurrence of itraconazole-resistant Aspergillus fumigatus isolates has been observed in clinical contexts since the mid-1990s (1, 2), and the increase has been linked to therapeutic failure in the treatment of aspergillosis (2, 3).
Conidia of this soil-dwelling fungus are ubiquitously found in the environment. Its habitats include those with elevated temperatures, e.g., compost heaps, giving this species the intrinsic ability to also survive at elevated mammalian body temperatures. In contrast to endogenous infections with Candida albicans, there is no reservoir of A. fumigatus in healthy hosts: infections with A. fumigatus are therefore generally thought to be acquired exogenously from the environment.
Only a limited number of antifungal drugs are available for the therapy of such life-threatening mycoses, among which azoles are competitive inhibitors of the Cyp51A protein, a central enzyme with lanosterol-14α-demethylase activity in the ergosterol biosynthesis pathway of fungi. Several steric mutations that affect the inhibition constants of azoles toward this enzyme and lead to decreased drug susceptibility in vitro are known (4, 5). Such mutations have been thought to arise during prolonged antifungal therapy or prophylaxis in individual patients and genetically independent fungal strains.
The recent increase in itraconazole resistance, however, has been linked to a single allele of cyp51A, termed TR/L98H, and typing studies showed a close genetic relationship between early isolates, indicating a common ancestor (1, 6). The allele contains a tandem repeat in the cyp51A promoter region combined with a single amino acid exchange of leucine98 to histidine and is thought to have arisen in the 1990s, possibly through the use of agricultural azoles, which are structurally similar to clinically used drugs (6, 7). Apparently, this allele is now spreading through the A. fumigatus population, since in the past few years the TR/L98H allele has been reported to occur worldwide in patients as well as the environment (e.g., see references 2, 8, and 9). This includes two German patients for which case reports were published independently during our study period (10, 11).
In this study, we investigated the epidemiology of triazole-resistant A. fumigatus and underlying cyp51A mutations in viable clinical isolates obtained over an 18-month period in Germany during 2011 and 2012.
MATERIALS AND METHODS
Acquisition and processing of isolates.
Clinical isolates were obtained during routine diagnostic procedures in the respective laboratories of the MykoLabNet-D network. They were isolated from various body locations and irrespective of the clinical relevance of the material collected for further processing. Where available, pseudonymized anamnesis data, including patient age and gender, underlying disease, previous and current antifungal drug treatment, as well as outcome of treatment, were obtained. For all isolates, the species was confirmed and the antifungal drug susceptibility pattern was tested as outlined below. Conidia were archived at −70°C in Cryobank tubes (Mast Diagnostica, Reinfeld, Germany).
Species determination.
The species of all isolates in this study were confirmed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (Biotyper; Bruker Daltonics, Bremen, Germany) on extracted cells harvested from overnight shaking cultures in Sabouraud's medium (Oxoid, Wesel, Germany) using the Fungi Library database.
Susceptibility testing.
Susceptibility to the antifungal drugs itraconazole and voriconazole (both from Discovery Fine Chemicals, Bournemouth, United Kingdom) and posaconazole (MSD Sharp & Dohme, Haar, Germany) was tested by broth microdilution according to the EUCAST reference method (10). Plates were incubated at 37°C for 48 h. The MIC0 values of all drugs were determined visually as the lowest concentrations with no visible growth. To establish the tests, drug-resistant control isolates CR019, CR055, CR059, CR060, and CR061 (kindly provided by E. Mellado, ISCII, Madrid, Spain) and drug-susceptible isolates DSM819 and ATCC 46645 were used (Table 1). All isolates with elevated MIC0 values were additionally retested at least three times in parallel with the control strains. Across the entire study, drug-resistant control isolates grew over the full itraconazole concentration range, while susceptible controls produced MIC0 values of ≤0.250 mg·liter−1. Additionally, at one study site (Hamburg, Germany), isolates were prescreened by Etest, and only the resistant ones were submitted for further testing by broth dilution. For quality control purposes, eight isolates (six susceptible, two resistant) were blindly retested at a separate institution (Robert Koch-Institut, Berlin, Germany). The categorical agreement between those and our tests was 100% (data not shown).
Table 1.
Characteristics of isolates with decreased drug susceptibility
| Control type or geographical origin | Strain | MIC0 (mg·liter−1)a |
Cyp51 amino acid substitution | Previous antifungal therapyb | Specimen typeb | Cystic fibrosisb | Outcomeb | ||
|---|---|---|---|---|---|---|---|---|---|
| ITZ | VRZ | PSZ | |||||||
| Negative control | DSM819 | 0.25 | 0.5 | 0.063 | None | ||||
| Negative control | ATCC 46645 | 0.125 | 0.5 | 0.063 | None | ||||
| Positive control | CR019 | >32 | 8 | 0.5 | TR/L98H | ||||
| Positive control | CR055 | >32 | 1 | 2 | G54E | ||||
| Positive control | CR059 | >32 | 4 | 2 | M220V | ||||
| Positive control | CR060 | 32 | 4 | 0.063 | M220T | ||||
| Positive control | CR061 | >32 | 2 | 2 | G54R | ||||
| Augsburg | 273 | 8 | 8 | 1 | None | Sputum | Yes | ||
| Düsseldorf | 168 | >32 | 1 | 0.125 | TR/L98H | Caspofungin | Alveolar lavage fluid | Deceased | |
| Freiburg | 231 | >32 | 2 | 0.25 | None | Bronchial secretion | |||
| Hamburg | 164 | >32 | 2 | 0.25 | TR/L98H | Tracheal secretion | |||
| Hannover | 158 | >32 | 2 | 0.125 | None | None | Bronchus biopsy specimen | ||
| Hannover | 237 | >32 | 0.25 | >32 | Duplication + G54W | None | Alveolar lavage fluid | Deceased | |
| Koblenz | 243 | >32 | 4 | 0.5 | None | Nasal swab | |||
| Munich | 251 | >32 | 2 | 1 | TR/L98H | Sputum | Yes | ||
| Munich | 262 | >32 | 0.25 | 0.5 | M220I | Oral swab | Yes | ||
| Munich | 270 | >32 | 4 | 1 | TR/L98H | Sputum | Yes | ||
| Munich | 281 | >32 | 8 | 1 | TR/L98H | Sputum | Yes | ||
| Munich | 279c | 1 | 0.25 | 0.5 | None | Sputum | Yes | ||
| Tübingen | 34-1 | 32 | 1 | 1 | None | Sputum | |||
| Tübingen | 31 | 0.125 | 0.5 | 0.5 | None | Oral swab | Yes | ||
| Tübingen | 170 | >32 | 0.25 | 0.125 | F219C | Sputum | Yes | ||
| Tübingen | 248 | >32 | 2 | 0.5 | None | Sputum | Yes | ||
| Würzburg | 267 | >32 | 4 | 0.5 | TR/L98H | Sputum | |||
Numbers in boldface are MIC0 values above the EUCAST clinical breakpoints (11) used for itraconazole (ITZ) and voriconazole (VRZ), which were 2 mg·liter−1 for intermediate and >2 mg·liter−1 for resistant, and for posaconazole (PSZ), which were 0.25 mg·liter−1 for intermediate and >0.25 mg·liter−1 for resistant.
Empty fields indicate unknown.
Albino variant with sparse conidiation only.
Sequence analysis.
From isolates with MIC0 values above EUCAST breakpoints (11), the cyp51A coding region and its promoter were amplified by PCR in two overlapping fragments (the primers used were CYP51A-5 [5′-ATAATCGCAGCACCACTTCAGA-3′], CYP51A-7 [5′-CCTTGTCACCGTCAAGACGG-3′], CYP51A-6 [5′-TGGATGTGTTTTTCGACCGCTT-3′], and CYP51A-8 [5′-CGGATCGGACGTGGTGTATG-3′]), and each fragment was sequenced from both ends. Sequences from all isolates were assembled using the CAP contig assembly program and manually inspected for nucleotide changes. For isolates other than those with the TR/L98H mutation, two independent sequences for cyp51A were obtained. For control purposes, the cyp51A sequences of 12 additional random isolates with itraconazole susceptibility in the upper susceptible range were initially determined but showed no amino acid substitutions (data not shown).
RESULTS
Epidemiology of reduced A. fumigatus azole drug susceptibility in Germany.
Over a period of 18 months in 2011 and 2012, a total of 527 clinical isolates were processed. The vast majority of isolates received were obtained from pulmonary/oropharyngeal specimens (n = 353), and out of these, at least 163 were derived from cystic fibrosis patients. Other isolates were either from skin (n = 30) or from invasive/wound infections (n = 39).
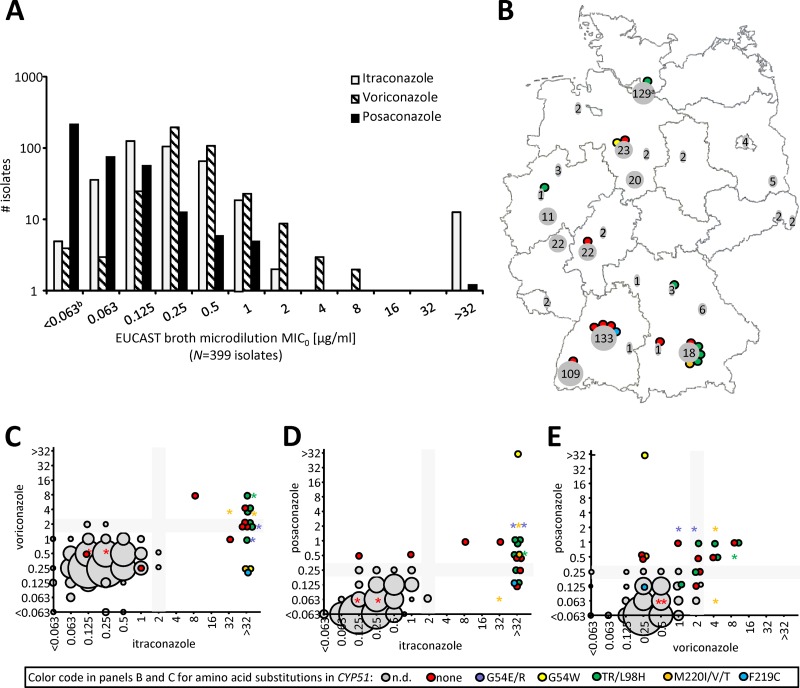
MIC0 values in the susceptible range determined by the EUCAST broth microdilution procedure for all three substances followed a Gaussian normal distribution (Fig. 1A). Posaconazole MIC0 values were the lowest, on average, itraconazole MIC0 values were intermediate, and voriconazole MIC0 values were the highest. The distributions of the itraconazole and voriconazole MIC0 values were shifted apart approximately one 2-fold dilution. These data were comparable to data obtained by the CLSI methodology (12), although differences from the data for posaconazole were not as pronounced in our test set. This, however, may reflect differences between the EUCAST and CLSI methodologies.
Fig 1.
Epidemiology. (A) Distribution of MIC0 values for itraconazole, voriconazole, and posaconazole in clinical isolates. (B) Geographical origin of clinical isolates. Numbers in gray fields are the total number of isolates tested from a single city (if applicable, including multiple laboratories); colored dots represent color-coded cyp51 mutations that the sequenced isolates (see the key at the bottom) contained in total. (C to E) Cross-resistance between itraconazole, voriconazole, and posaconazole. Gray boxes in the background are zones of intermediate susceptibility according to EUCAST breakpoints (11). The sizes of the gray balls are relative to the number of isolates with that particular MIC combination; color-coded balls represent single, individual isolates. a, isolates from Hamburg were prescreened on-site by Etest, and only drug-resistant isolates (n = 1) were submitted to the broth microdilution procedure, as outlined in the text; the data for isolates susceptible by this definition are omitted from panels A and C (see the text); b, the nonnormal distribution for posaconazole and voriconazole at the lower end is explained by the fact that this category probably contains isolates with multiple MIC values; 0.063 mg·liter−1 was the lowest drug concentration tested. Asterisks, control isolates; balls, clinical isolates; n.d., not determined.
A total of 17 (3.2%) strains showed MIC0 values above the clinical breakpoints for at least one of the antifungal agents tested (Fig. 1A and Table 1). Out of these, 14 were highly resistant to itraconazole (MIC0 > 32 mg·liter−1) and 1 (strain 237) was additionally highly resistant to posaconazole (MIC0 > 32 mg·liter−1). Two other strains had moderately reduced susceptibility to posaconazole (strains 31 and 279) or to all three substances (strain 273) (Fig. 1C to E; Table 1).
No geographical hot spots of isolates with a particular resistance mechanism could be identified (Fig. 1B). For specimen subgroups, the prevalences of resistant isolates were 2.4% for non-cystic fibrosis pulmonary isolates, 2.6% for invasive/wound isolates, and 0% for skin isolates. Among the isolates from cystic fibrosis patients, the resistance rate was 5.2% (9/163).
Mutations underlying decreased azole drug susceptibility.
Among the cyp51A mutations found in the set of isolates with decreased drug susceptibility, the TR/L98H variant was the most prominent, but isolates with the G54W and M220I substitutions and the novel F219C substitution were also found. Interestingly, the G54W isolate had apparently undergone a gene duplication, since sequencing reactions of PCR products consistently showed double signals specifically at this position. Most importantly, a similar number of isolates with the wild-type allele was present among those with decreased susceptibility (Fig. 1C to E; Table 1). The MIC0 values obtained from isolates carrying the M220I, G54W, and TR/L98H substitutions were within previously reported ranges (12–15).
DISCUSSION
The prevalence of azole-resistant A. fumigatus isolates in our cohort, including isolates prescreened by Etest at one study site, was 3.2%. This rate is lower than what has been found in other studies. Prevalences ranging from 4.5% in Denmark (12) to 8% in French cystic fibrosis patients and 17% in the United Kingdom (2) have been described. In cystic fibrosis patients known to have received itraconazole prophylaxis, a prevalence of itraconazole-resistant isolates of up to 20% was found (16). The prevalence of resistant isolates in cystic fibrosis patients within our study was only 5.5%; however, the prophylaxis status for our patients was unknown. The rate of PCR-detectable DNA from resistant strains in specimens from patients with chronic pulmonary aspergillosis (CPA) or allergic bronchopulmonary aspergillosis (ABPA) was again significantly higher (up to 50 to 75% [17] in small cohorts) than what is observed in viable A. fumigatus isolates. How this relates to disease and therapeutic outcome is currently unknown, but it indicates that current prevalence rates may still be underestimated.
Our epidemiological survey shows that, among other resistance phenotypes, the allele of cyp51A is also present in isolates from German patients. Isolates of this type were highly resistant to itraconazole and showed cross-resistance to both voriconazole and posaconazole. Nevertheless, although it constituted a significant proportion of isolates (n = 6/17, 35.3%), TR/L98H was not the only azole resistance-conferring cyp51A mutation occurring: in addition to the isolates with the previously described G54W and M220I substitutions, we also found one isolate carrying the yet unobserved F219C substitution. The F-to-I substitution at position 219 has previously been implicated in azole resistance (18), indicating that F219C may also be the cause of decreased azole susceptibility in this particular isolate; however, this still needs to be confirmed on a molecular level. Furthermore, one isolate carrying the G54W substitution was resistant to both itraconazole and posaconazole. This isolate also had a potential duplication of cyp51A.
As in this study, most other epidemiological studies found a significant proportion of isolates for which no mutation in cyp51A could be identified (here, n = 8/17, 47.1%). In such isolates, other, unrelated mechanisms must be at work. This may potentially include increased production of the drug target protein Cyp51A (19–21) or increased drug efflux (22, 23).
Currently, voriconazole is still the first-line treatment of choice for pulmonary aspergillosis (24). The major high-level resistance observed within our set of isolates was directed solely against itraconazole; high-level cross-resistance was observed only in a single isolate and was observed between itraconazole and posaconazole. Nevertheless, all TR/L98H isolates showed increased MIC0 values at least partially above the clinical breakpoint for the other two substances tested. The same was true for itraconazole-resistant isolates with the wild-type cyp51A allele.
The TR/L98H allele is assumed to have been derived through the use of agricultural azoles, which are structurally similar to clinically used ones (6, 7). To what degree the different mutations are present in the German environment is unknown; this will be investigated in a future study. Whether such resistant strains are propagated within the community or hospital settings is also still unclear. Conidiation is sometimes observed within tissues where the fungus is in contact with air (25); one can hypothesize that this could allow the distribution of isolates with resistance mutations from patients; however, this has not been described yet.
Where clinically relevant, azole susceptibility testing of A. fumigatus isolates, including isolates from patients receiving azole prophylaxis, should be implemented.
ACKNOWLEDGMENTS
This study was financially supported by Pfizer Pharma GmbH (grant WS1905219 to O.B. and U.G.) and the José Carreras Leukemia Foundation (grant DJCLS R10/31f to U.R.).
Drug-resistant control isolates were kindly provided by E. Mellado (ISCII, Madrid, Spain). Posaconazole pure substance was kindly provided by MSD Sharp & Dohme (Haar, Germany; grant 103114). We thank Irmina Szymczak, Silvia Kellner, Agnieszka Goretzki, Katharina Neid, Jana Tünnermann, and Bianca Winkler for expert technical and data organization help.
The MykoLabNet-D partners involved in this project (all in Germany) were Frauke Albert, Mikrobiologisches Institut, Universitätsklinikum Erlangen, Erlangen; Wolfgang Bartelt, MEDLAB Arnold Analytik, MVZ GmbH, Würzburg; Alexander Beider, Kinderklinik auf der Bult, Hannover; Orsolya Benedek, Institut für Labordiagnostik und Transfusionsmedizin, Oberlausitz-Kliniken, Bautzen; Bettina Beyreiß, Institut für Mikrobiologie, Carl-Thiem-Klinikum Cottbus, Cottbus; Lore Draht, Bioscientia GmbH, Ingelheim; Claudia Friedrichs, Medizinisches Labor Ostsachsen, Görlitz; Moritz Fritzenwanker, Institut für Medizinische Mikrobiologie, Universitätsklinikum Gießen, Gießen; Norbert Guenther, Institut für Mikrobiologie, Immunologie und Krankenhaushygiene, Städtisches Klinikum Braunschweig, Braunschweig; Andrea Haas, Max von Pettenkofer-Institut für Hygiene und Medizinische Mikrobiologie, Klinikum Großhadern, Munich; Michael Klotz, Institut für Mikrobiologie und Hygiene, Universitätsklinikum des Saarlandes, Homburg; Edmond Kurig, Zentrales Institut des Sanitätsdienstes der Bundeswehr Koblenz, Koblenz; Roland Pfüller, Abteilung Mikrobiologie, MDI Laboratorien GmbH, Berlin; Ludwig Sedlacek, Institut für Medizinische Mikrobiologie, Medizinische Hochschule Hannover, Hannover; Michael Seibold, FG16, Mycotic and Parasitic Agents and Mycobacteria, Robert Koch-Institut, Berlin; Michaela Simon, Institut für Mikrobiologie, Universität Regensburg, Regensburg; Andreas F. Wendel, Institut für Medizinische Mikrobiologie und Krankenhaushygiene, Universitätsklinikum Düsseldorf, Düsseldorf; Max-Georg Weyer, Medizinisches Labor Münster, Münster; and Ortrud Zimmermann, Institut für Medizinische Mikrobiologie, Universitätsmedizin Göttingen, Göttingen.
Footnotes
Published ahead of print 13 May 2013
REFERENCES
- 1. Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5:e219. 10.1371/journal.pmed.0050219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15:1068–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Snelders E, Melchers WJ, Verweij PE. 2011. Azole resistance in Aspergillus fumigatus: a new challenge in the management of invasive aspergillosis? Future Microbiol. 6:335–347 [DOI] [PubMed] [Google Scholar]
- 4. Warrilow AG, Melo N, Martel CM, Parker JE, Nes WD, Kelly SL, Kelly DE. 2010. Expression, purification, and characterization of Aspergillus fumigatus sterol 14-alpha demethylase (CYP51) isoenzymes A and B. Antimicrob. Agents Chemother. 54:4225–4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Snelders E, Karawajczyk A, Schaftenaar G, Verweij PE, Melchers WJ. 2010. Azole resistance profile of amino acid changes in Aspergillus fumigatus CYP51A based on protein homology modeling. Antimicrob. Agents Chemother. 54:2425–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Snelders E, Camps SM, Karawajczyk A, Schaftenaar G, Kema GH, van der Lee HA, Klaassen CH, Melchers WJ, Verweij PE. 2012. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One 7:e31801. 10.1371/journal.pone.0031801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect. Dis. 9:789–795 [DOI] [PubMed] [Google Scholar]
- 8. Chowdhary A, Kathuria S, Xu J, Sharma C, Sundar G, Singh PK, Gaur SN, Hagen F, Klaassen CH, Meis JF. 2012. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR(34)/L98H mutations in the cyp51A gene in India. PLoS One 7:e52871. 10.1371/journal.pone.0052871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mortensen KL, Mellado E, Lass-Florl C, Rodriguez-Tudela JL, Johansen HK, Arendrup MC. 2010. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob. Agents Chemother. 54:4545–4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing 2008. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14:982–984 [DOI] [PubMed] [Google Scholar]
- 11. EUCAST 2013, posting date Antifungal agents---breakpoints table for interpretation of MICs. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Antifungal_breakpoints_v_5.0.pdf
- 12. Mortensen KL, Jensen RH, Johansen HK, Skov M, Pressler T, Howard SJ, Leatherbarrow H, Mellado E, Arendrup MC. 2011. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. J. Clin. Microbiol. 49:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alcazar-Fuoli L, Mellado E, Cuenca-Estrella M, Sanglard D. 2011. Probing the role of point mutations in the cyp51A gene from Aspergillus fumigatus in the model yeast Saccharomyces cerevisiae. Med. Mycol. 49:276–284 [DOI] [PubMed] [Google Scholar]
- 14. Chowdhary A, Kathuria S, Randhawa HS, Gaur SN, Klaassen CH, Meis JF. 2012. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J. Antimicrob. Chemother. 67:362–366 [DOI] [PubMed] [Google Scholar]
- 15. Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. 2011. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob. Agents Chemother. 55:4465–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burgel PR, Baixench MT, Amsellem M, Audureau E, Chapron J, Kanaan R, Honore I, Dupouy-Camet J, Dusser D, Klaassen CH, Meis JF, Hubert D, Paugam A. 2012. High prevalence of azole-resistant Aspergillus fumigatus in adults with cystic fibrosis exposed to itraconazole. Antimicrob. Agents Chemother. 56:869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Denning DW, Park S, Lass-Florl C, Fraczek MG, Kirwan M, Gore R, Smith J, Bueid A, Moore CB, Bowyer P, Perlin DS. 2011. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin. Infect. Dis. 52:1123–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Camps SM, van der Linden JW, Li Y, Kuijper EJ, van Dissel JT, Verweij PE, Melchers WJ. 2012. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob. Agents Chemother. 56:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arendrup MC, Mavridou E, Mortensen KL, Snelders E, Frimodt-Moller N, Khan H, Melchers WJ, Verweij PE. 2010. Development of azole resistance in Aspergillus fumigatus during azole therapy associated with change in virulence. PLoS One 5:e10080. 10.1371/journal.pone.0010080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Camps SM, Dutilh BE, Arendrup MC, Rijs AJ, Snelders E, Huynen MA, Verweij PE, Melchers WJ. 2012. Discovery of a hapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS One 7:e50034. 10.1371/journal.pone.0050034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albarrag AM, Anderson MJ, Howard SJ, Robson GD, Warn PA, Sanglard D, Denning DW. 2011. Interrogation of related clinical pan-azole-resistant Aspergillus fumigatus strains: G138C, Y431C, and G434C single nucleotide polymorphisms in cyp51A, upregulation of cyp51A, and integration and activation of transposon Atf1 in the cyp51A promoter. Antimicrob. Agents Chemother. 55:5113–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slaven JW, Anderson MJ, Sanglard D, Dixon GK, Bille J, Roberts IS, Denning DW. 2002. Increased expression of a novel Aspergillus fumigatus ABC transporter gene, atrF, in the presence of itraconazole in an itraconazole resistant clinical isolate. Fungal Genet. Biol. 36:199–206 [DOI] [PubMed] [Google Scholar]
- 23. Bowyer P, Mosquera J, Anderson M, Birch M, Bromley M, Denning DW. 2012. Identification of novel genes conferring altered azole susceptibility in Aspergillus fumigatus. FEMS Microbiol. Lett. 332:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karthaus M, Buchheidt D. 2013. Invasive aspergillosis: new insights into disease, diagnostic and treatment. Curr. Pharm. Des. 19:3569–3594 [DOI] [PubMed] [Google Scholar]
- 25. De Hoog G, Guarro J, Figueras M. 2000. Atlas of clinical fungi, 2nd ed American Society for Microbiology, Washington, DC [Google Scholar]