Abstract
Pulmonary lesions from active tuberculosis patients are thought to contain persistent, nonreplicating bacilli that arise from hypoxic stress. Metronidazole, approved for anaerobic infections, has antituberculosis activity against anoxic bacilli in vitro and in some animal models and may target persistent, nonreplicating bacilli. In this double-blind, placebo-controlled trial, pulmonary multidrug-resistant tuberculosis subjects were randomly assigned to receive metronidazole (500 mg thrice daily) or placebo for 8 weeks in addition to an individualized background regimen. Outcomes were measured radiologically (change on high-resolution computed tomography [HRCT]), microbiologically (time to sputum smear and culture conversion), and clinically (status 6 months after stopping therapy). Enrollment was stopped early due to excessive peripheral neuropathies in the metronidazole arm. Among 35 randomized subjects, 31 (15 metronidazole, 16 placebo) were included in the modified intent-to-treat analysis. There were no significant differences by arm in improvement of HRCT lesions from baseline to 2 or 6 months. More subjects in the metronidazole arm converted their sputum smear (P = 0.04) and liquid culture (P = 0.04) to negative at 1 month, but these differences were lost by 2 months. Overall, 81% showed clinical success 6 months after stopping therapy, with no differences by arm. However, 8/16 (50%) of subjects in the metronidazole group and 2/17 (12%) of those in the placebo group developed peripheral neuropathy. Subjects who received metronidazole were 4.3-fold (95% confidence interval [CI], 1.1 to 17.1) more likely to develop peripheral neuropathies than subjects who received placebo. Metronidazole may have increased early sputum smear and culture conversion but was too neurotoxic to use over the longer term. Newer nitroimidazoles with both aerobic and anaerobic activity, now in clinical trials, may increase the sterilizing potency of future treatment regimens.
INTRODUCTION
The nitroimidazole antibiotic class has been used to treat protozoan and anaerobic bacterial infections for over 50 years. Metronidazole, the most widely used nitroimidazole, is typically well tolerated at ≤2 g daily (1). Longer-term use for severe infections is often limited by the development of peripheral neuropathies (2–5), as well as optic neuropathy (6), neutropenia, and central nervous system toxicities, including cerebellar ataxia, encephalopathy, and seizures (5, 7, 8).
Nitroimidazoles have also demonstrated activity under hypoxic conditions against Mycobacterium tuberculosis. In vitro, metronidazole reduced viable counts of M. tuberculosis bacilli by 98% under anaerobic conditions but had no activity under aerobic conditions (9). In M. tuberculosis-infected animals, efficacy is species specific and linked to the presence of hypoxic necrotizing lesions. For example, metronidazole has no activity in tuberculosis (TB) models in mice (10, 11), which generally do not develop hypoxic lesions. In contrast, metronidazole has efficacy in rabbits and nonhuman primates, which develop necrotizing granulomas that are hypoxic (12, 13). One study from India compared the addition of metronidazole versus placebo to a background regimen in humans for treatment of advanced pulmonary TB. The authors reported significantly improved sputum quantity reduction and radiological outcomes at week 4, and clinical responses at weeks 4 and 8, but no significant differences in sputum culture conversion rates at week 4, 8, or 12 (14).
The objective of this study was to determine the benefit of metronidazole versus placebo added to an individualized background regimen (IBR) for subjects with pulmonary multidrug-resistant tuberculosis (MDR-TB) through changes in high-resolution computed tomography (HRCT), sputum smear and culture conversion rates, and final clinical outcomes.
MATERIALS AND METHODS
Study design.
This randomized, double-blind, placebo-controlled phase II study was conducted at the National Masan Hospital (NMH) in Changwon, South Korea, from 2005 to 2012. Subjects ≥20 years old with sputum smear- and culture-positive pulmonary MDR-TB were enrolled. Subjects were excluded if they were HIV positive, did not meet specific baseline laboratory criteria, had a prior adverse reaction to metronidazole or a similar drug, were unable to abstain from alcohol (an exclusion that was imposed due to metronidazole's disulfiram-like effect), or received ≥14 days of MDR-TB therapy before enrollment. Subjects were randomized to add oral metronidazole (500 mg thrice daily) or placebo for 8 weeks to an IBR stratified by ofloxacin resistance (yes/no). The metronidazole dose used was selected based on a literature review of studies using metronidazole over the longer term. These studies, often performed in inflammatory bowel disease patients, used a wide range of dosing but often 500 to 750 mg three times daily. The lower end of this range was selected to try to reduce toxicities. Individualized background tuberculosis regimens and durations used in this study were consistent with those recommended in World Health Organization (WHO) guidelines (15). All subjects continued their IBR for ≥18 months after sputum culture conversion and then were followed for an additional 6 months after the end of therapy (EOT).
Outcome measures.
Outcomes were measured radiologically, microbiologically, and clinically. The radiological outcomes measured were changes in lesions associated with active tuberculosis (16) from baseline to 2 and 6 months by pulmonary HRCT, with tuberculosis-related lesions defined as nodules (<2 mm, 2 to <4 mm, and 4 to 10 mm), consolidations, collapse, cavities, fibrosis, bronchial thickening, tree-in-bud opacities, and ground glass opacities. Based on a previous study (17), each CT scan was divided into six zones (upper, middle, and lower zones of the right and left lungs) and independently scored for the lesions listed above by three separate radiologists blinded to the treatment arm. A fourth radiologist adjudicated any discrepant scores. The HRCT score was determined by visually estimating the extent of the lesions listed above in each lung zone as follows: 0 = 0% involvement; 1 = 1% to 25% involvement; 2 = 26% to 50% involvement; 3 = 51% to 75% involvement; and 4 = 76% to 100% involvement. A composite score for each lesion type was calculated by summing the scores for each specific abnormality in each lung zone and dividing by the number of zones. Changes in composite scores measured at 2 and 6 months were compared to baseline. Microbiological outcomes included time to conversion to negative as measured by sputum smear and culture (liquid [MB/Bact] and solid [Ogawa] media). The conversion date was defined as the first date of three consecutive negative tests at least 1 month apart. The start date was the date of IBR initiation. Microbiological failure was defined as a patient being persistently culture positive while on therapy or becoming culture negative (defined by the conversion date as described above) and then reverting to being persistently culture positive while on therapy. Sporadic single positive cultures that were negative on repeat sputum culture were not considered failures. Clinical success was defined as being repeatedly culture negative on therapy and without evidence of disease 6 months after EOT by either microbiologic confirmation or a clinical report. Deaths were considered failures. Those lost to follow-up included subjects who did not complete therapy and those who successfully completed therapy but could not be contacted 6 months after EOT.
Adverse event monitoring and pharmacokinetics (PK).
Subjects underwent baseline and serial safety evaluations (complete blood count, chemistries, and liver function tests) weekly until week 12, monthly until week 24, and then every other month until EOT. Clinical exams occurred monthly until EOT. Adverse events of grade ≥ 3, as defined by the DAIDS toxicity table (http://rsc.tech-res.com/safetyandpharmacovigilance/gradingtables.aspx), were captured. For peripheral neuropathies, this was defined as follows:
Grade 1 (mild): asymptomatic with sensory alteration on examination or minimal paresthesia causing no or minimal interference with usual social and functional activities
Grade 2 (moderate): sensory alteration or paresthesia causing greater than minimal interference with usual social and functional activities
Grade 3 (severe): sensory alteration or paresthesia causing inability to perform usual social and functional activities
Grade 4 (potentially life-threatening): disabling sensory alteration or paresthesia causing inability to perform basic self-care functions
Grade 1 and 2 peripheral neuropathies were also captured due to the concern for this adverse event in metronidazole-treated subjects.
For pharmacokinetics, blood samples were collected 1 h before the 8:30 a.m. dose for trough plasma concentrations and 3 h after the 1 p.m. dose for peak plasma concentrations (the third dose was given at 6:30 p.m.) during weeks 1, 4, and 8. Metronidazole concentrations were measured by adapting and validating a previously described high-performance liquid chromatography (HPLC)-mass spectrometry method (3). The linear trapezoidal equation was used to calculate areas under the curves (AUCs) from the measured concentration-time data obtained for peak and trough concentrations as follows: AUC = ∑[(tn + 1 − tn)/2 × (Cn + Cn + 1)] where tn and Cn are the time and concentration of the nth specimen, respectively.
Study oversight.
Informed consent was collected from all participants. This study was approved by the institutional review boards (IRBs) of the NMC in South Korea and the National Institute of Allergy and Infectious Diseases (NIAID) in the United States. A Data and Safety Monitoring Board (DSMB) reviewed the study, at first quarterly and then twice a year. All serious adverse events were reported to the DSMB and both IRBs. The study was monitored by an independent clinical research organization. Metronidazole and the size, shape, and color-matched placebo were purchased from CJ Cheiljedang Pharma Corp. of South Korea.
Statistics.
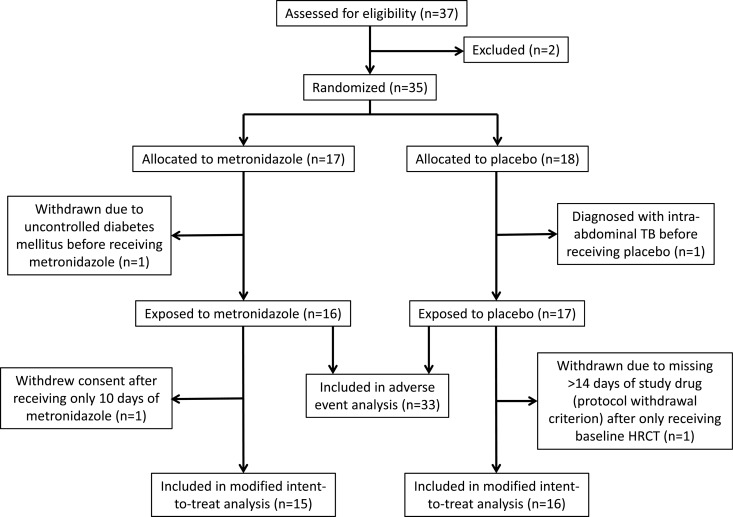
Analysis was by modified intent to treat (MITT), excluding four subjects; two were withdrawn prior to receiving study drug, and two withdrew shortly after receiving the baseline HRCT scan (Fig. 1). Treatment group comparisons were based on a Wilcoxon rank sum test. Fisher's exact test was used to compare proportions across treatment groups. Comparisons of baseline covariates and treatment outcomes, ignoring treatment assignment, were evaluated using Fisher's exact test (for binary covariates) and Wilcoxon's rank sum test (for continuous covariates). The small number of failures (n = 4) limited multivariate analyses on the effect of baseline covariates on outcomes. Time-to-smear and culture-negative analyses were conducted using Kaplan-Meier curves and log-rank statistics. Analyses were conducted in Stata version 12.0 and R version 2.15.0. Significance was defined at the P ≤ 0.05 level.
Fig 1.
Flow diagram for subjects included in the study.
RESULTS
Study subjects.
This study had a planned enrollment of 80 subjects. After 35 were enrolled, the DSMB recommended closing the study to further enrollment due to excessive peripheral neuropathies in the metronidazole arm. Among the 35 subjects, 17 were randomized to the metronidazole arm and 18 to the placebo arm (Fig. 1). Two subjects (one from each arm) were withdrawn before receiving any study drug. Two additional subjects (one from each arm) withdrew after receiving only the baseline HRCT scans: one withdrew due to missing >14 days of study drug (protocol withdrawal criterion), and the other withdrew consent after receiving 10 days of study drug. These four withdrawn subjects were excluded from the MITT analysis because the primary study endpoints could not be evaluated in them. Of the 31 included subjects, the median age was 37 years, 81% were male, and the median body-mass index was 19 (Table 1). The subjects had a median of 2 previous episodes of tuberculosis and were resistant to a median of 4 drugs at baseline. Forty-eight percent had far advanced disease (defined in Table 1) by chest X-ray, 52% had cavities, and 68% had bilateral disease. There were no significant differences in baseline characteristics between the two groups.
Table 1.
Baseline characteristicsa
| Patient characteristic | Metronidazole treatment (n = 15) | Placebo treatment (n = 16) | Total (n = 31) |
|---|---|---|---|
| Age in yrs, median (IQR) | 36 (28, 39) | 38 (31, 44) | 37 (30, 43) |
| Male, n (%) | 13 (87) | 12 (75) | 25 (81) |
| Body-mass index, median (IQR) | 19 (18, 20) | 19 (18, 22) | 19 (18, 21) |
| Diabetes mellitus, n (%) | 1 (7) | 2 (13) | 3 (10) |
| Previous TB episodes, median n (IQR) | 2 (1, 5) | 3 (2, 3) | 2 (1, 3) |
| Resistant drugs, median n (IQR) | 4 (3, 6) | 5 (4, 6) | 4 (4, 6) |
| Active drugs in regimen, median n (IQR) | 4 (4, 5) | 5 (4, 5) | 5 (4, 5) |
| Fluoroquinolone resistance, n (%) | 5 (33) | 6 (38) | 11 (35) |
| Far advanced disease,b n (%) | 5 (33) | 10 (63) | 15 (48) |
| Cavitary disease by CXR, n (%) | 6 (40) | 10 (63) | 16 (52) |
| Bilateral disease by CXR, n (%) | 9 (60) | 12 (75) | 21 (68) |
There were no statistical differences in baseline characteristics between the two arms. CXR, chest X ray.
“Far advanced tuberculosis” was defined according to the guidelines of the Korea Centers for Disease Control and Prevention as the presence of disseminated lesions of slight-to-moderate density exceeding the total volume of one lung, or dense and confluent lesions exceeding one-third the volume of one lung, or the presence of cavities greater than 4 cm in diameter.
Outcome measures.
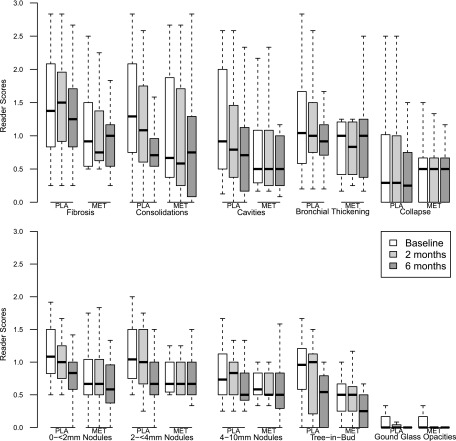
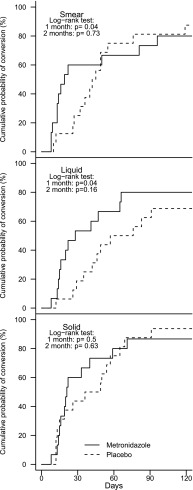
The radiologists' scores for each of the 10 lung lesions seen on the baseline and 2- and 6-month HRCT are shown in Fig. 2. Although the mean composite score across all lesions declined over time from baseline (mean, 9.1; standard error [SE], 0.84) to month 6 (mean, 7.00; SE, 0.69) (P < 0.0001), the differences were not associated with the use of metronidazole. The mean composite score declined by 19% from baseline (mean, 8.1; SE, 1.3) to month 6 (mean, 6.6; SE, 1.00) in the metronidazole arm and by 39% from baseline (mean, 10.1; SE, 1.0) to month 6 (mean, 7.2; SE, 0.9) in the placebo arm, which was not statistically significant. Time to sputum smear and culture conversion by Kaplan-Meier analysis is shown in Fig. 3. Overall, the median times to smear conversion were 19 days for the metronidazole arm and 43.5 days for the placebo arm (P = 0.76); median times to liquid-culture conversion were 28 days for the metronidazole arm and 66.5 days for the placebo arm (P = 0.51); median times to solid-culture conversion were 21 days for the metronidazole arm and 42.5 days for the placebo arm (P = 0.84). A significantly greater proportion in the metronidazole arm converted their smear (P = 0.04) and liquid cultures (P = 0.04) to negative at month 1, but these differences were lost by month 2. There was no difference in time to culture conversion by arm using solid medium. In comparing overall clinical outcomes, 80% of subjects in the metronidazole arm and 81% of subjects in the placebo arm showed clinical success 6 months after EOT (Table 2). Four subjects (three in the metronidazole arm and one in the placebo arm) were treatment failures. There were no differences in treatment outcomes by arm. In a univariate analysis of baseline characteristics associated with overall clinical outcomes, treatment-failure subjects (compared to treatment-success subjects) had significantly more previous episodes of tuberculosis (P = 0.02), had isolates resistant to a larger number of drugs at baseline (P = 0.01), had fewer active drugs used in treatment regimens (P = 0.004), and had isolates with a greater proportion with fluoroquinolone resistance (P = 0.009). Due to the small number of failure subjects (n = 4), multivariate analyses were not done.
Fig 2.
Box plots showing radiologist reader scores for each of 10 lesions on high-resolution computed tomography scans at baseline and 2 and 6 months. PLA, placebo; MET, metronidazole. There were no significant differences by arm for any lung lesion at any time point.
Fig 3.
Kaplan-Meier plots of time to conversion by sputum smear, liquid culture, and solid culture stratified by treatment arm.
Table 2.
Treatment outcomes 6 months after treatment completion
| Treatment outcome | No. (%) of patientsa |
||
|---|---|---|---|
| Metronidazole treatment (>n = 15) | Placebo treatment (n = 16) | Total (n = 31) | |
| Failure | 3 (20) | 1 (6) | 4 (13) |
| Success | 12 (80) | 13 (81) | 25 (81) |
| Lost to follow-up | 0 (0) | 2 (13) | 2 (6) |
No statistically significant differences in outcome by treatment group.
Safety.
Adverse events were stratified by days 1 to 60 and days > 60 because metronidazole was used only during the initial 2 months (Table 3). All adverse events occurred in similar numbers between arms except that more peripheral neuropathies occurred in the metronidazole arm (relative risk [RR], 4.3; 95% confidence interval [CI], 1.1 to 17.1). Half of all subjects who received metronidazole developed peripheral neuropathy. Among all 10 neuropathies, 5 were grade 2 (all in the metronidazole arm), with the remainder all being grade 1. All neuropathies either resolved completely or had only minor sequelae (tingling in toes or soles of feet) at a median of 1,006 days after starting. Twelve subjects (8 in the metronidazole arm and 4 in the placebo arm) permanently discontinued the study drug early at a median of 31 days after starting the study drug.
Table 3.
All adverse events of grade 3 and above and peripheral neuropathies of grades 1 and 2 that were reported
| Adverse event(s) | During metronidazole treatment phase (days 1–60) |
After metronidazole treatment phase (days > 60) |
||||
|---|---|---|---|---|---|---|
| No. (%) of patients receiving metronidazole (n = 16) | No. (%) of patients receiving placebo (n = 17) | Relative risk (95% CI) | No. (%) of patients receiving metronidazole (n = 16) | No. (%) of patients receiving placebo (n = 17) | Relative risk (95% CI) | |
| Aspergilloma | 1 (6.3) | 0 | ||||
| Diarrhea | 1 (6.3) | 1 (5.9) | 1.1 (0.1–15.6) | |||
| Fracture | 0 | 1 (5.9) | 0 | |||
| Gastritis | 1 (6.3) | 0 | ||||
| Hemoptysis | 1 (6.3) | 0 | ||||
| Hepatitis/elevated transaminases | 1 (6.3) | 0 | 1 (6.3) | 0 | ||
| Hypercholesterolemia | 0 | 1 (5.9) | 0 | |||
| Hyperglycemia | 0 | 1 (5.9) | 0 | 1 (6.3) | 1 (5.9) | 1.1 (0.1–15.6) |
| Hyperuricemia | 3 (18.8) | 3 (17.6) | 1.1 (0.2–4.5) | 3 (18.8) | 2 (11.8) | 1.6 (0.3–8.3) |
| Hypokalemia | 1 (6.3) | 0 | ||||
| Myalgia(s) | 0 | 1 (5.9) | 0 | |||
| Nausea | 1 (6.3) | 0 | ||||
| Peripheral neuropathy | 8 (50.0) | 2 (11.8) | 4.3 (1.1–17.1) | |||
| Pneumothorax | 0 | 1 (5.9) | 0 | |||
| Rash | 0 | 1 (5.9) | 0 | |||
| Seizure | 2 (12.5) | 1 (5.9) | 2.1 (0.2–21.2) | 1 (6.3) | 0 | |
Pharmacokinetics.
For most subjects, the variability in exposure between weeks 1, 4, and 8 was low. Overall, the median peak, trough, and AUC from 0 to 24 h [AUC(0 to 24)] concentration values were 31.6 μg/ml (interquartile range [IQR], 18.0 to 36.7), 24.8 μg/ml (IQR, 14.4 to 31.2), and 527 μg/ml (IQR, 367 to 813), respectively. Clinical PK parameters reported in the literature for metronidazole administered orally at 500 mg were largely obtained from single-dose studies in healthy volunteers (8 to 13 μg/ml) (18, 19). The much higher peaks observed in this patient population could be related to accumulation of metronidazole at the steady state when administered three times daily, although the actual values for maximum concentration of drug in serum (Cmax) administered at 500 mg thrice daily are not available for direct comparison. Multiple dosing of 400 mg orally provided peak plasma concentrations of between 11 and 13 μg/ml (18). Because 12 μg/ml or 70 μM metronidazole causes a 98% reduction of viable bacillus counts under anaerobic conditions in vitro (9), therapeutic levels were maintained in the plasma in all but two patients during the entire dosing interval. Median (IQR) peak concentrations for those with and without peripheral neuropathy were 36.7 μg/ml (17.7 to 37.6) and 25.5 μg/ml (18.1 to 36.2), respectively. Median (IQR) trough concentrations for those with and without peripheral neuropathy were 29.4 μg/ml (12.4 to 34.5) and 20.2 μg/ml (14.8 to 29.4), respectively. Median (IQR) AUC(0 to 24) concentrations for those with and without peripheral neuropathy were 569 μg/ml (339.5 to 832.5) and 527 μg/ml (390 to 787), respectively. There were no statistical differences in peak, trough, or AUC(0 to 24) levels between subjects with or without peripheral neuropathy.
DISCUSSION
This study of IBR plus metronidazole versus IBR plus placebo for the initial 2 months in pulmonary MDR-TB subjects was closed to enrollment early due to an excess of peripheral neuropathies in the metronidazole arm. Among 31 subjects in the MITT analysis, there were no significant differences between arms in radiological changes by HRCT from baseline to 2 or 6 months. Microbiologically, sputum smear and liquid culture converted to negative earlier at 1 month in the metronidazole arm but equal numbers converted by 2 months and no differences in culture conversion rates were seen on solid culture. Clinically, there were no differences in outcomes between treatment arms 6 months following EOT.
Antituberculosis chemotherapy has traditionally targeted aerobic, replicating bacilli identified from sputum. Historically, it was assumed that these bacilli primarily represented rapidly growing organisms from the aerobic surface of cavities open to airways. However, nonreplicating M. tuberculosis bacilli are increasingly recognized as representing a subpopulation present in sputum during active disease. Acid-fast bacilli (AFB) in sputum samples have recently been reported to contain abundant lipid bodies, intracellular droplets composed of triacylglycerols, thought to be synthesized under conditions of stress, such as reduced oxygen or hypoxia (20). M. tuberculosis grown in the laboratory under hypoxic conditions enters a nonreplicating persistent state, with accumulation of triacyglycerols (21). In addition, AFB from sputum have transcriptional signatures consistent with a hypoxia-induced state of nonreplicating persistence. The proportion of lipid body-positive AFB in sputum correlated (R2 = 0.64; P < 0.03) with time to positivity in liquid cultures, showing that the more nonreplicating persistent bacteria there were, the longer the cultures took to become positive (20). Mycobacterial cells in vitro have also been shown to be longer during logarithmic growth and shorter during stationary phase (22). In another study, bacilli from sputum and bronchoalveolar lavage (BAL) fluid were compared with bacilli from cavities of active pulmonary TB patients undergoing lung surgery. The lengths of M. tuberculosis isolated from sputum and BAL fluid were consistent with mixtures of logarithmically replicating and nonreplicating bacilli in vitro, whereas bacilli from cavities of resected lung tissue were significantly shorter (P < 0.001), resembling stationary-phase organisms in vitro (23). All of these characteristics are shared by bacilli adapted to an in vitro model known as the Wayne model in which M. tuberculosis shows sensitivity to metronidazole (24). Thus, in contrast to what was previously assumed, a substantial proportion of AFB in sputum likely arises from lesions in which the bacilli are in a nonreplicating persistent state due to a local anaerobic microenvironment.
In an attempt to target more specifically these nonreplicating persistent AFB, we added metronidazole to a standard MDR-TB treatment regimen. Because we assumed that these bacilli would reside in necrotic pulmonary lesions, we carefully examined the radiologic findings in subjects by arm. Although there were no significant differences, this is, to our knowledge, the first semiquantitative, prospective examination of changes in HRCT findings in subjects being treated for active tuberculosis. A more detailed examination of these findings will be reported later. Surprisingly, our results indicated a more rapid sputum smear and liquid culture clearance at 1 month, a difference that disappeared when sputum smear and culture conversion rates in the control arm caught up. It is not clear if this early microbiologic response in the metronidazole arm would have persisted if exposure had not been limited by neurotoxicity. Although metronidazole itself is unlikely to be a clinically useful drug for the treatment of tuberculosis due to peripheral neuropathies, alternative antibiotics with anaerobic activity that are better tolerated may still increase the sterilizing potency of future treatment regimens.
In our study subjects, peripheral neuropathies improved in all subjects after stopping metronidazole, consistent with a prior report showing that symptoms improved with the reinnervation of the skin, with small nerve fibers measured by serial biopsy specimen after stopping therapy (25). Pharmacokinetic analysis of the metronidazole dose and interval used demonstrated appropriate levels, with no correlation between metronidazole peak/trough concentrations or AUC(0 to 24) and development of peripheral neuropathy.
The major limitation of our study was the small sample size, due to enrollment being closed early, limiting our power to draw definitive conclusions. Although a greater difference between arms still would not have made metronidazole a viable treatment option for MDR-TB due to the toxicity, it might have confirmed more definitively the treatment strategy of using anaerobically active drugs to treat persistent, nonreplicating bacilli.
The presence of persistent nonreplicating AFB in sputa suggests a much more dynamic environment for anaerobic lesions than previously thought and highlights the importance of including an antibiotic with anaerobic activity in antituberculosis treatment regimens. In our study, metronidazole provided an apparent early microbiological benefit but continued use of this drug was limited by neurotoxicity. Newer nitroimidazoles which have both aerobic and anaerobic activity are now in development and show promise against M. tuberculosis (26). PA-824 has demonstrated antituberculosis activity in combination with other drugs in vitro (27), in mice (28), and in human early bactericidal activity studies (29, 30), with a phase II trial now ongoing. Delamanid (OPC-67683), in combination with an optimized background regimen, has already demonstrated success compared to placebo in a phase II study in pulmonary MDR-TB subjects (31, 32). There were no reports of increased paresthesias in the delamanid arms compared to the placebo arm in this trial, and a phase III trial is now ongoing. Thus, the nitroimidazoles, particularly the newer and better-tolerated ones, have the potential to increase the sterilizing activity of antituberculosis treatment regimens by attacking more specifically persistent nonreplicating bacilli, thereby allowing a shorter total treatment duration (13). The pharmacodynamic concept of “sterilizing activity” of a regimen refers to the ability of a regimen to kill bacilli that contribute to relapse following discontinuation of therapy (33). Early clinical trials of tuberculosis included radiologic rates of change as monitored by chest X-ray but failed to come up with a quantitative measure of the sterilizing potential of regimens. In conclusion, our results highlight the potential for the newer nitroimidazoles to contribute to treatment regimens with more sterilizing potency. In future analyses, we will evaluate the role of quantitative HRCT response rates in combination with careful bacteriology as a potential radiological biomarker (radiomarker) of treatment outcomes.
ACKNOWLEDGMENTS
We thank the NIAID Data and Safety Monitoring Board, the physicians and nurses of the National Masan Hospital, and the patients who volunteered to participate in this study. We also acknowledge the contributions of Hyunkyung Kwak and Eunjin Cho to the conduct of the study.
This work was supported (in part) by the Intramural Research Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health; (in part) by the Ministry of Health and Welfare, South Korea; (in part) by the Bill and Melinda Gates Foundation through the Grand Challenges in Global Health Program (to Douglas Young, Imperial College, London); and (in part) by the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services; neither does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Published ahead of print 3 June 2013
REFERENCES
- 1. Finegold SM. 1980. Metronidazole. Ann. Intern. Med. 93:585–587 [DOI] [PubMed] [Google Scholar]
- 2. Bradley WG, Karlsson IJ, Rassol CG. 1977. Metronidazole neuropathy. Br. Med. J. 2:610–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lau AH, Lam NP, Piscitelli SC, Wilkes L, Danziger LH. 1992. Clinical pharmacokinetics of metronidazole and other nitroimidazole anti-infectives. Clin. Pharmacokinet. 23:328–364 [DOI] [PubMed] [Google Scholar]
- 4. Boyce EG, Cookson ET, Bond WS. 1990. Persistent metronidazole-induced peripheral neuropathy. DICP 24:19–21 [DOI] [PubMed] [Google Scholar]
- 5. Tan CH, Chen YF, Chen CC, Chao CC, Liou HH, Hsieh ST. 2011. Painful neuropathy due to skin denervation after metronidazole-induced neurotoxicity. J. Neurol. Neurosurg. Psychiatry 82:462–465 [DOI] [PubMed] [Google Scholar]
- 6. McGrath NM, Kent-Smith B, Sharp DM. 2007. Reversible optic neuropathy due to metronidazole. Clin. Experiment Ophthalmol. 35:585–586 [DOI] [PubMed] [Google Scholar]
- 7. Kuriyama A, Jackson JL, Doi A, Kamiya T. 2011. Metronidazole-induced central nervous system toxicity: a systematic review. Clin. Neuropharmacol. 34:241–247 [DOI] [PubMed] [Google Scholar]
- 8. Patel K, Green-Hopkins I, Lu S, Tunkel AR. 2008. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int. J. Infect. Dis. 12:e111–e114 [DOI] [PubMed] [Google Scholar]
- 9. Wayne LG, Hayes LG. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klinkenberg LG, Sutherland LA, Bishai WR, Karakousis PC. 2008. Metronidazole lacks activity against Mycobacterium tuberculosis in an in vivo hypoxic granuloma model of latency. J. Infect. Dis. 198:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brooks JV, Furney SK, Orme IM. 1999. Metronidazole therapy in mice infected with tuberculosis. Antimicrob. Agents Chemother. 43:1285–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE. 2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76:2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin PL, Dartois V, Johnston PJ, Janssen C, Via L, Goodwin MB, Klein E, Barry CE, III, Flynn JL. 2012. Metronidazole prevents reactivation of latent Mycobacterium tuberculosis infection in macaques. Proc. Natl. Acad. Sci. U. S. A. 109:14188–14193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desai CR, Patel HSA, Babrekar AB, Mahashur AA, Kamat SR. 1989. Role of metronidazole in improving response and specific drug sensitivity in advanced pulmonary tuberculosis. J. Assoc. Physicians India 37:694–697 [PubMed] [Google Scholar]
- 15. Falzon D, Jaramillo E, Schünemann HJ, Arentz M, Bauer M, Bayona J, Blanc L, Caminero JA, Daley CL, Duncombe C, Fitzpatrick C, Gebhard A, Getahun H, Henkens M, Holtz TH, Keravec J, Keshavjee S, Khan AJ, Kulier R, Leimane V, Lienhardt C, Lu C, Mariandyshev A, Migliori GB, Mirzayev F, Mitnick CD, Nunn P, Nwagboniwe G, Oxlade O, Palmero D, Pavlinac P, Quelapio MI, Raviglione MC, Rich ML, Royce S, Rüsch-Gerdes S, Salakaia A, Sarin R, Sculier D, Varaine F, Vitoria M, Walson JL, Wares F, Weyer K, White RA, Zignol M. 2011. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur. Respir. J. 38:516–528 [DOI] [PubMed] [Google Scholar]
- 16. Hatipoğlu ON, Osma E, Manisali M, Uçan ES, Balci P, Akkoçlu A, Akpinar O, Karlikaya C, Yüksel C. 1996. High resolution computed tomographic findings in pulmonary tuberculosis. Thorax 51:397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casarini M, Ameglio F, Alemanno L, Zangrilli P, Mattia P, Paone G, Bisetti A, Giosuè S. 1999. Cytokine levels correlate with a radiologic score in active pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 159:143–148 [DOI] [PubMed] [Google Scholar]
- 18. Lamp KC, Freeman CD, Klutman NE, Lacy MK. 1999. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin. Pharmacokinet. 36:353–373 [DOI] [PubMed] [Google Scholar]
- 19. Gatchev E, Bräter M, de Mey C. 2006. Bioequivalence of a novel oral metronidazole formulation. Arzneimittelforschung 56:612–616 [DOI] [PubMed] [Google Scholar]
- 20. Garton NJ, Waddell SJ, Sherratt AL, Lee SM, Smith RJ, Senner C, Hinds J, Rajakumar K, Adegbola RA, Besra GS, Butcher PD, Barer MR. 2008. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 5:e75. 10.1371/journal.pmed.0050075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, Kolattukudy PE. 2004. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J. Bacteriol. 186:5017–5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thanky NR, Young DB, Robertson BD. 2007. Unusual features of the cell cycle in mycobacteria: polar-restricted growth and the snapping-model of cell division. Tuberculosis (Edinb) 87:231–236 [DOI] [PubMed] [Google Scholar]
- 23. Eum SY, Kong JH, Hong MS, Lee YJ, Kim JH, Hwang SH, Cho SN, Via LE, Barry CE., III 2010. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137:122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wayne LG, Sohaskey CD. 2001. Nonreplicating persistence of mycobacterium tuberculosis. Annu. Rev. Microbiol. 55:139–163 [DOI] [PubMed] [Google Scholar]
- 25. Tan C-H, Chen Y-F, Chen C-C, Chao C-C, Liou H-H, Hsieh S-T. 2011. Painful neuropathy due to skin denervation after metronidazole-induced neurotoxicity. J. Neurol. Neurosurg. Psychiatry 82:462–466 [DOI] [PubMed] [Google Scholar]
- 26. Mukherjee T, Boshoff H. 2011. Nitroimidazoles for the treatment of TB: past, present and future. Future Med. Chem. 3:1427–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piccaro G, Giannoni F, Filippini P, Mustazzolu A, Fattorini L. 2013. Activity of drug combinations against Mycobacterium tuberculosis grown in aerobic and hypoxic acidic conditions. Antimicrob. Agents Chemother. 57:1428–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL. 2012. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 56:3114–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diacon AH, Dawson R, du Bois J, Narunsky K, Venter A, Donald PR, van Niekerk C, Erondu N, Ginsberg AM, Becker P, Spigelman MK. 2012. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob. Agents Chemother. 56:3027–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, van Niekerk C, Everitt D, Winter H, Becker P, Mendel CM, Spigelman MK. 2012. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 380:986–993 [DOI] [PubMed] [Google Scholar]
- 31. Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, Gao M, Awad M, Park SK, Shim TS, Suh GY, Danilovits M, Ogata H, Kurve A, Chang J, Suzuki K, Tupasi T, Koh WJ, Seaworth B, Geiter LJ, Wells CD. 2012. Delamanid for multidrug-resistant pulmonary tuberculosis. N. Engl. J. Med. 366:2151–2160 [DOI] [PubMed] [Google Scholar]
- 32. Skripconoka V, Danilovits M, Pehme L, Tomson T, Skenders G, Kummik T, Cirule A, Leimane V, Kurve A, Levina K, Geiter LJ, Manissero D, Wells CD. 2013. Delamanid improves outcomes and reduces mortality for multidrug-resistant tuberculosis. Eur. Respir. J. 41:1393–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davies GR. 2010. Early clinical development of anti-tuberculosis drugs: science, statistics and sterilizing activity. Tuberculosis (Edinb) 90:171–176 [DOI] [PubMed] [Google Scholar]