Abstract
Enterobacteriaceae producing the novel carbapenemase New Delhi metallo-β-lactamase (NDM-1) are emerging worldwide. While these organisms often display high levels of in vitro resistance to multiple antibiotics, in vivo efficacy data are lacking. Here, the activities of humanized ertapenem and doripenem exposures were characterized against a wild-type K. pneumoniae and its derived isogenic strains harboring either an NDM-1 or KPC-2 plasmid in immunocompetent mice. In addition, four clinical isolates expressing NDM-1 were evaluated. Human-simulated regimens of ertapenem at 1 g every 24 h and high-dose, prolonged infusion of doripenem at 2 g every 8 h as a 4-h infusion were evaluated over 24 h, and efficacy was determined by the change in bacterial density compared to that in 24-h growth controls. CFU reductions in bacterial density of greater than 1 log unit were observed against the wild-type strain as well as the derived isogenic NDM-1 strain, while no reduction was observed against the derived KPC-2 strain. Postexposure MICs confirmed the in vitro maintenance of the ertapenem resistance marker in both the NDM-1 and KPC-2 strains. Similar to the case for the isogenically derived NDM-1 strain, bacterial density was reduced at 24 h against all four clinical NDM-1 isolates showing variable levels of MICs for carbapenems, with near-maximal activity of both agents occurring when the doripenem MIC was ≤8 μg/ml. While carbapenem monotherapy does not appear to be an option against KPC-based infections, these data suggest that carbapenem monotherapy may be a viable option for treating NDM-1-producing Enterobacteriaceae under certain conditions, and this warrants further in vivo exploration.
INTRODUCTION
New Delhi metallo-β-lactamase (NDM-1) is a novel carbapenemase that has been isolated from Enterobacteriaceae worldwide (1, 2). NDM-1-producing strains have the ability to inactivate most β-lactams and display high levels of in vitro resistance to multiple antibiotic classes (1, 2). Furthermore, they have the potential to disseminate at an alarming rate, as NDM-1 has most frequently been isolated from Escherichia coli, a predominantly community-acquired pathogen (3). Moreover, the gene encoding NDM-1 is located on a highly mobile genetic element and carries growth promoters which result in an increased likelihood of gene transfer to other Gram-negative bacteria (2). Subsequently, NDM-1 has since been identified in a number of other Enterobacteriaceae isolates, including Klebsiella pneumoniae, Enterobacter spp., Morganella morganii, Citrobacter freundii, and Salmonella spp. (4, 5) Even more concerning is the fact that it has already spread to unrelated Gram-negative species, including Acinetobacter baumannii and Pseudomonas aeruginosa (6–10). Furthermore, most of these NDM-1-producing isolates also possess a number of other β-lactamase genes and additional genetic elements conferring resistance to other classes of antibiotics (2, 11). Consequently, many of these organisms are often susceptible only to tigecycline and colistin and display various levels of carbapenem susceptibility (3, 11, 12).
Currently there is a paucity of in vivo efficacy data for Enterobacteriaceae producing NDM-1. When relying on in vitro susceptibility data to guide selection of antimicrobial therapy for NDM-1-producing organisms, colistin and tigecycline are often the only two agents with which the organisms maintain reliable susceptibility (12). An in vitro time-kill study demonstrated early bactericidal activity with colistin and poor activity with tigecycline (13). Furthermore, tigecycline was found to be antagonistic when combined with colistin. Despite promising in vitro data with colistin, a number of case reports describe clinical failures with colistin-based regimens, or treatment was complicated by the onset of renal impairment (14–16). This highlights the need for exploration of additional treatment options for managing infections caused by NDM-1-producing organisms.
Since there is a lack of in vivo and clinical data evaluating carbapenem therapy for treatment of infections caused by NDM-1-producing isolates, the objective of this study was to describe the efficacy of human-simulated exposures of doripenem at 2 g every 8 h as a 4-h infusion and of ertapenem at 1 g every 24 h against NDM-1-producing Enterobacteriaceae isolates in a well-validated murine thigh infection model. The efficacy observed against NDM-1 (Ambler class B β-lactamase; metallo β-lactamase) was also compared to the efficacy observed against another contemporary carbapenemase, K. pneumoniae producing carbapenemase (KPC) (Ambler class A β-lactamase), using isogenic strains and was also confirmed using four clinical strains.
MATERIALS AND METHODS
Antimicrobial test agents.
Clinically available ertapenem for injection (Merck & Co., Inc., Whitehouse Station, NJ) and doripenem for injection (Ortho-McNeil-Janssen Pharmaceuticals, Inc., Raritan, NJ) were used for all in vivo studies.
Antimicrobial agents were reconstituted with normal saline according to the manufacturer's instructions before each experiment and then further diluted to the requisite concentrations for dosing (17, 18). Solutions were stored under refrigeration until the time of use. Ertapenem solutions were discarded after 6 h, and doripenem solutions were discarded after 24 h, as recommended by the manufacturer.
Bacterial isolates.
A wild-type K. pneumoniae strain (454) and two derived isogenic strains harboring either an NDM-1 or a KPC-2 plasmid were utilized. The isogenic strains were obtained by conjugation and transformation, respectively, using imipenem (2 μg/ml)-containing selection plates (19, 20). Four other clinical NDM-1-producing Enterobacteriaceae were also evaluated in the in vivo model. Doripenem and ertapenem MICs were determined by Etest (AB bioMérieux, Solna, Sweden) according to the manufacturer's specifications. All isolates were stored frozen at −80°C in double-strength skim milk (Remel, Lenexa, KS). Prior to beginning each experiment, isolates were subcultured twice onto Trypticase soy agar with 5% sheep blood (BD Biosciences, Sparks, MD) and incubated in ambient air at 35°C for 18 to 24 h.
Immunocompetent mouse thigh infection model.
This study was approved by the Institutional Animal Care and Use Committee at Hartford Hospital, Hartford, CT. Pathogen-free ICR mice weighing 20 to 22 g were acquired from Harlan Laboratories (Indianapolis, IN). All animals were maintained in accordance with National Research Council recommendations and were given food and water ad libitum. An intraperitoneal injection of uranyl nitrate (5 mg/kg) was given 3 days prior to inoculation to induce a predictable degree of renal impairment by slowing drug clearance (21). Prior to the initiation of either ertapenem or doripenem therapy, both thighs of all animals were inoculated intramuscularly with 0.1 ml of a 108-CFU/ml inoculum prepared from a fresh subculture of each organism and diluted in normal saline.
Neutropenic mouse thigh infection model.
Two clinical NDM-1 isolates were also evaluated in a neutropenic mouse thigh infection model. Neutropenic mice underwent the same procedures as immunocompetent mice but with the administration of cyclophosphamide (Baxter, Deerfield, IL) (150 mg/kg) 4 days prior to inoculation and a 100-mg/kg injection given 1 day prior to inoculation to induce neutropenia (21). Additionally, neutropenic mice were inoculated intramuscularly with 0.1 ml of a 107-CFU/ml inoculum.
In vivo efficacy.
All isolates were studied in the immunocompetent mouse thigh infection model over 24 h. Following inoculation, groups of three mice were administered a regimen simulating humanized doses of either ertapenem at 1 g every 24 h (q24h) or doripenem at 2 g q8h as a 4-h infusion as 0.2-ml subcutaneous injections over 24 h. Both of these regimens utilized were developed previously by our group and have been confirmed multiple times throughout the course of several in vivo studies (22–26). While the regimen of high-dose, prolonged infusion of doripenem that was simulated is not FDA approved, this dosing regimen was selected as our best attempt to treat these multidrug-resistant pathogens, as it has the highest likelihood of achieving the necessary pharmacodynamic target for efficacy. Control animals were given normal saline with the same volume, route, and frequency as the treatment regimens. Groups of three untreated control mice were euthanized by CO2 exposure followed by cervical dislocation at 0 h and 24 h, while all treated mice were sacrificed at 24 h. Both thighs were removed from sacrificed animals and homogenized individually in normal saline. Serial dilutions of thigh homogenate were plated onto Trypticase soy agar with 5% sheep blood (BD Biosciences, Sparks, MD) for determination of bacterial density. Efficacy was determined as the change in bacterial density at each time point compared to that in the 24-h controls. One clinical NDM-1 isolate was subsequently evaluated over 72 h in the immunocompetent model, with control and treatment groups assessed at 24, 48, and 72 h to ascertain if reductions in bacterial density could be sustained when a longer duration, reflecting that of clinical practice, was employed.
Confirmation of ertapenem MIC following in vivo exposure.
Ertapenem MICs were confirmed after in vivo exposure to ensure that plasmids were retained and functionally expressed throughout the course of the experiment. For each strain utilized in the isogenic experiment, including the wild-type K. pneumoniae and constructed KPC-2 and NDM-1 strains, the ertapenem MIC was determined in triplicate for bacteria recovered from thigh homogenate samples after 24 h of doripenem therapy. Similarly, postexposure ertapenem MICs were determined for one of the clinical isolates, E. coli 389.
RESULTS
Bacterial isolates.
The wild-type K. pneumoniae 454 isolate and its derived isogenic strains harboring either an NDM-1 or a KPC-2 plasmid, as well as four clinical NDM-1-producing Enterobacteriaceae isolates, were utilized. These isolates represented a variety of phenotypic profiles, as described in Table 1.
Table 1.
Phenotypic profiles of Enterobacteriaceae isolates utilized in the efficacy studies of the carbapenems
| Isolate | β-Lactamase content | MIC (μg/ml)a |
|||
|---|---|---|---|---|---|
| ETP | DOR | IMP | MER | ||
| K. pneumoniae 454 | 0.012 | 0.03 | 0.19 | 0.06 | |
| K. pneumoniae 454 + NDM-1 plasmid | NDM-1 | 16 | 4 | 6 | 4 |
| K. pneumoniae 454 + KPC-2 plasmid | KPC-2 | 4 | 1 | 8 | 2 |
| E. coli 391 (NDM-1) | NDM-1, TEM-1, CMY-16, OXA-1, OXA-10 | 3 | 0.5 | 1 | 1 |
| E. coli 393 (NDM-1) | NDM-1, CTX-M-15, TEM-1, OXA-1, OXA-2 | >32 | 4 | 4 | 8 |
| E. coli 389 (NDM-1) | NDM-1, TEM-1, CTX-M-15 | >32 | 8 | 16 | 8 |
| K. pneumoniae 450 (NDM-1) | NDM-1, SHV-11, SHV-28, TEM-1, CTX-M-15, OXA-1, OXA-9 | >32 | >32 | >32 | >32 |
ETP, ertapenem; DOR, doripenem; IMP, imipenem; MER, meropenem.
In vivo efficacy.
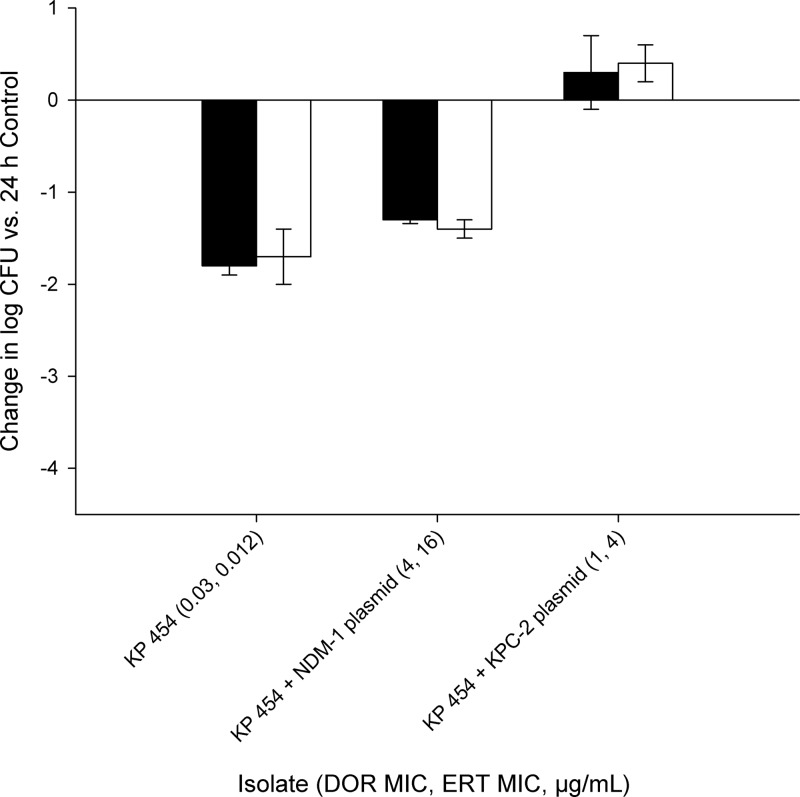
The mean (± standard deviation) bacterial density in control mice for all isolates utilized at 0 h was 6.65 ± 0.38 log10 CFU in immunocompetent mice and increased to 6.94 ± 1.45 log10 CFU after 24 h. A 1- to 2-log10 CFU reduction was observed after 24 h for both ertapenem and doripenem against the wild-type strain, K. pneumoniae 454, as well as the isogenic strain with the NDM-1 plasmid. However, an increase in bacterial density was observed for both carbapenems utilized against the isogenic KPC strain (Fig. 1).
Fig 1.
Change in log10 CFU/ml after 24 h observed in a wild-type K. pneumoniae strain and its derived isogenic strains harboring either an NDM-1 or a KPC-2 plasmid after treatment with human-simulated doripenem at 2 g every 8 h as a 4-h infusion (black bars) or ertapenem at 1 g every 24 h (white bars) in an immunocompetent mouse thigh infection model. Each value is the mean ± standard deviation for infected thighs for each isolate.
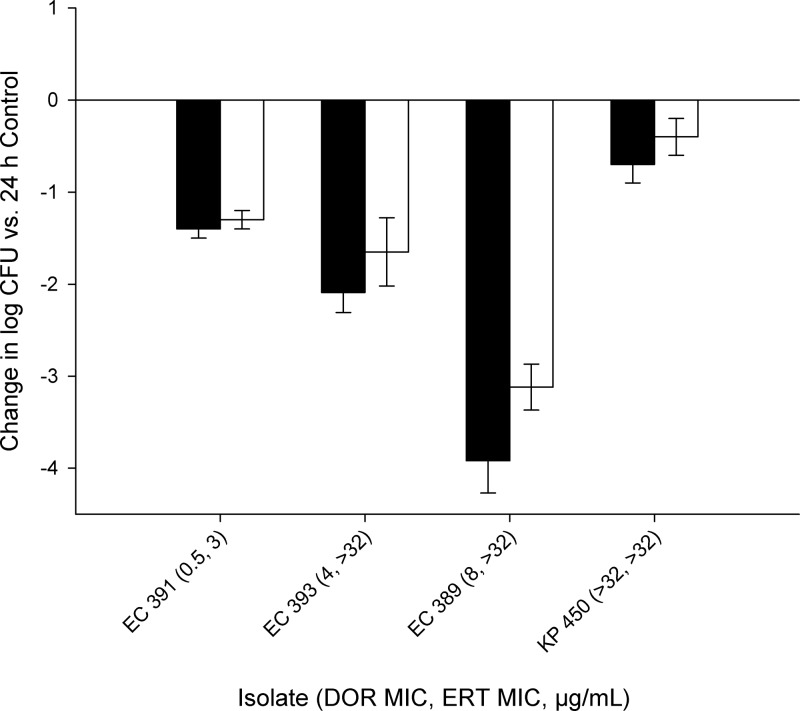
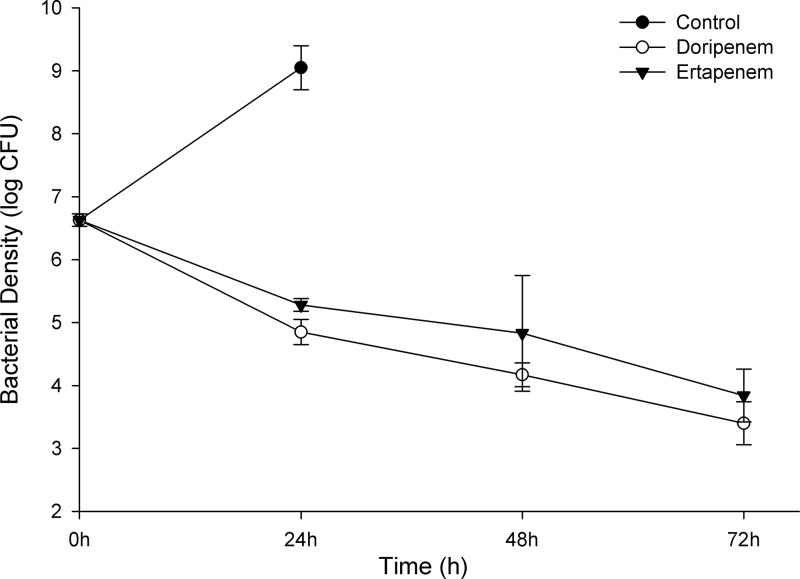
CFU reductions of greater than 1 log10 CFU were achieved with both carbapenems against the three clinical NDM-1 isolates with doripenem MICs of ≤8 μg/ml in the immunocompetent mouse model (Fig. 2), while only a modest reduction was observed against the isolate with a doripenem MIC of >32 μg/ml (K. pneumoniae 450). To confirm these findings, efficacy experiments were conducted twice for E. coli 393 and three times for E. coli 389 with similar results. Accordingly, the data from repeated experiments were combined and are reported as mean data across all experiments for these two isolates. Furthermore, two of these clinical isolates were evaluated in a neutropenic mouse model to ascertain the potential impact of host immunity on the observed activity. Similar bacterial densities were observed in neutropenic and immunocompetent mice at 24 h following treatment with doripenem and ertapenem for E. coli 389 and E. coli 393 (P > 0.05). An efficacy study was also conducted out to 72 h using an immunocompetent mouse model for E. coli 389, in which the initial bacterial reductions for both therapies observed at 24 h were maintained at both 48 and 72 h. These data are depicted in Fig. 3.
Fig 2.
Change in log10 CFU/ml after 24 h observed in four clinical NDM-1-producing Enterobacteriaceae isolates after treatment with human-simulated doripenem at 2 g every 8 h as a 4-h infusion (black bars) or ertapenem at 1 g every 24 h (white bars) in an immunocompetent mouse thigh infection model. Each value is the mean ± standard deviation for infected thighs for each isolate.
Fig 3.
Change in log10 CFU after 24, 48, and 72 h for growth controls and human-simulated regimens of doripenem at 2 g every 8 h as a 4-h infusion and ertapenem at 1 g every 24 h against E. coli 389 (doripenem MIC, 8 μg/ml; ertapenem MIC, >32 μg/ml) in an immunocompetent murine thigh infection model. Each value is the mean ± standard deviation for infected thighs at each time point. All three animals in the 48-h and 72-h control groups expired prior to their respective time points.
Confirmation of ertapenem MIC following in vivo exposure.
Ertapenem MICs for the wild-type K. pneumoniae and the isogenic KPC-2 and NDM-1 strains, as well as the clinical strain E. coli 389, determined at the end of the in vivo efficacy experiments remained unchanged from those obtained prior to inoculation, confirming sustained in vitro resistance.
DISCUSSION
The global dissemination of NDM-1-producing Enterobacteriaceae is of great concern worldwide, as very few contemporary antimicrobial agents retain reliable susceptibility (1, 2). Furthermore, the lack of substantial in vivo and clinical efficacy data, which are limited to a small number of case reports, makes selecting therapy for these multidrug-resistant pathogens difficult. Here we sought to describe the efficacy of humanized exposures of ertapenem and doripenem against NDM-1-producing Enterobacteriaceae using an in vivo infection model.
The efficacies of doripenem and ertapenem monotherapies were compared for two different carbapenemases, KPC and NDM-1, using isogenically constructed strains. This methodology was chosen to purely compare the impact of each enzyme on efficacy without the influence of other resistance genes, as most clinical NDM-1- or KPC-producing isolates often harbor other β-lactamases. Moreover, the use of isogenic strains also helps to standardize strain-specific virulence and pathogenicity factors that may complicate direct comparisons. A bacterial CFU reduction of greater than 1 log10 unit was observed with both doripenem and ertapenem against the parent wild-type K. pneumoniae strain, as anticipated given the phenotypic profile of this organism. However, a bacterial CFU reduction of greater than 1 log10 unit was also observed for the derived NDM-1 strain, despite it having significantly higher MICs for both doripenem and ertapenem. Furthermore, a significantly greater bacterial reduction was observed with both agents against the isogenic NDM-1-producing isolate compared to the slight increase in bacterial density that occurred during therapy with the derived KPC-producing strain, even in light of the NDM-1 producer having 2-fold-higher doripenem and ertapenem MICs. This lack of reliable efficacy with carbapenem monotherapy against KPCs is consistent with previous observations using doripenem and ertapenem against clinical KPC-producing strains in this model, which also resulted in minimal to no efficacy against three isolates with doripenem MICs ranging from 8 to 32 μg/ml and ertapenem MICs greater than 64 μg/ml (27). It should be noted that recent work with KPC-producing organisms suggests that carbapenems still have an important role in therapy, particularly when used in combination with other agents, including another carbapenem (27–30). Unlike the case for KPCs, these data suggest that carbapenems may even have a role as monotherapy in treating infections caused by NDM-1-producing Enterobacteriaceae.
Similar to what was observed with the isogenic NDM-1 strain, bacterial reductions were observed for all four clinical isolates with the use of either ertapenem or doripenem in the immunocompetent mouse model. Bacterial densities at 24 h were similar in immunocompetent and neutropenic mice, which further demonstrates that the efficacy observed with these agents truly reflects activity of the carbapenems utilized, with minimal impact from host immunity. Furthermore, sustained efficacy out to 72 h was demonstrated using one of these clinical NDM-1 strains. However, in the current study, the greatest degree of reduction in bacterial density for both carbapenems was achieved against the three isolates with doripenem MICs of ≤8 μg/ml. These results are consistent with the pharmacodynamic profile of doripenem, as a 2-g dose administered every 8 h as a 4-h infusion produces greater than 40% free time above the MIC (ƒT>MIC) for MICs up to 16 μg/ml (26). While the efficacy observed with doripenem appears to correlate with an MIC less than or equal to 8 μg/ml, ertapenem efficacy did not correlate well with MIC. CFU reductions of greater than 1 log10 unit were observed with ertapenem for the isolate with an MIC of 3 μg/ml and two of the isolates with an MIC of >32 μg/ml. A bacterial reduction of this magnitude was not anticipated, as the requisite 40% ƒT>MIC needed for bactericidal activity is achieved only for MICs of up to 0.5 μg/ml and the 20% ƒT>MIC needed for bacteriostasis is achieved only for MICs of up to 2 μg/ml when using a regimen of 1 g every 24 h (22). In two previous studies evaluating the same dosing regimen of ertapenem utilized here, bacterial reductions of approximately 2 log10 CFU were observed against extended-spectrum β-lactamase (ESBL)-producing isolates with MICs of up to 2 μg/ml (22, 24). Based on the results of these previous in vivo studies, the efficacy observed against the clinical isolate with an ertapenem MIC of 3 μg/ml is reasonable. However, these data suggest that there is discordance between ertapenem in vitro susceptibility and in vivo efficacy for the other two NDM-1-producing isolates in which efficacy was observed.
While we characterized the efficacy of doripenem and ertapenem against NDM-1-producing Enterobacteriaceae compared to KPC-producing organisms by evaluating these agents in NDM-1 and KPC-2 strains constructed from the same parent wild-type strain and confirmed the functional activity of these enzymes with repeated phenotypic profiling, several questions remain unanswered. The constructed NDM-1 and KPC-2 strains were created with two different plasmids. Since it was not possible to quantify the level of carbapenemase enzyme expression, it is unclear whether this variation in technique could have resulted in differences in the number of plasmid copies, as well as the levels of carbapenemase gene expression, in the two constructed strains.
The efficacy observed with both doripenem and ertapenem against an isogenically derived NDM-1-producing isolate was similar to what was observed against its parent wild-type strain of K. pneumoniae and much greater than what was achieved against the derived KPC-producing strain. Similar to the case for the derived NDM-1-producing isolates, efficacy was achieved with the use of either doripenem or ertapenem against all clinical strains of NDM-1-producing Enterobacteriaceae, with the greatest level of efficacy attained against isolates with doripenem MICs of ≤8 μg/ml. Even though carbapenem monotherapy is not effective in treating infections caused by KPC-producing organisms, our data suggest that further evaluation of the potential utility of carbapenems in the management of NDM-1-producing Enterobacteriaceae is warranted. These observations are both unexpected and encouraging, as the current armamentarium for these burgeoning pathogens is limited. Future studies evaluating the efficacy of carbapenems, as well as additional β-lactam comparators, against isolates producing various metallo-β-lactamase enzymes, including NDM-1, NDM-4, VIM, or IMP, in both immunocompetent and immunocompromised models are needed to fully determine the potential utility of the carbapenems. Furthermore, in light of the magnitude of activity achieved with the simulated high-dose, prolonged-infusion doripenem regimen, future evaluation of the utility of standard dosing regimens of doripenem for treating NDM-1-producing organisms is warranted. Lastly, the continued development of new β-lactamase inhibitors and other novel drug targets is paramount, as the expression of these phenotypic profiles is likely to be enhanced with continued evolution of these extremely drug-resistant organisms.
ACKNOWLEDGMENTS
This study was supported by funds from the Center for Anti-Infective Research and Development, Hartford Hospital.
We acknowledge Seth Housman, Amira Bhalodi, Mao Hagihara, Debora Santini, Lindsay Tuttle, Jennifer Hull, Henry Christensen, Mary Anne Banevicius, and Pam Tessier (Center for Anti-Infective Research and Development, Hartford Hospital) for their assistance with the animal experimentation. We also thank Delphine Girlich (Department of Bacteriology-Virology, Hospital Bicetre, France) for determining MICs and constructing the isogenic strains.
Footnotes
Published ahead of print 3 June 2013
REFERENCES
- 1. Nordmann P, Poirel L, Toleman MA, Walsh TR. 2011. Does broad-spectrum beta-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J. Antimicrob. Chemother. 66:689–692 [DOI] [PubMed] [Google Scholar]
- 2. Bonomo RA. 2011. New Delhi metallo-β-lactamase and multidrug resistance: a global SOS? Clin. Infect. Dis. 52:485–487 [DOI] [PubMed] [Google Scholar]
- 3. Nordmann P, Poirel L, Walsh TR, Livermore DM. 2011. The emerging NDM carbapenemases. Trends Microbiol. 19:588–595 [DOI] [PubMed] [Google Scholar]
- 4. Poirel L, Ros A, Carricajo A, Berthelot P, Pozzetto B, Bernabeu S, Nordmann P. 2011. Extremely drug-resistant Citrobacter freundii identified in a patient returning from India and producing NDM-1 and other carbapenemases. Antimicrob. Agents Chemother. 55:447–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savard P, Gopinath R, Zhu W, Kitchel B, Rasheed JK, Tekle T, Roberts A, Ross T, Razeq J, Landrum BM, Wilson LE, Limbago B, Perl TM, Carroll KC. 2011. First NDM-positive Salmonella sp. strain indentified in the United States. Antimicrob. Agents Chemother. 55:5957–5958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karthikeyan K, Thirunarayan MA, Krishnan P. 2010. Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii in India. J. Antimicrob. Chemother. 65:2253–2254 [DOI] [PubMed] [Google Scholar]
- 7. Boulanger A, Naas T, Fortineau N, Figueiredo S, Nordmann P. 2012. NDM-1-producing Acinetobacter baumannii from Algeria. Antimicrob. Agents Chemother. 56:2214–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu H, Hu Y, Pan Y, Liang H, Wang H, Wang X, Hao Q, Yang X, Yang X, Xiao X, Luan C, Yang Y, Cui Y, Yang R, Gao G, Song Y, Zhu B. 2012. Novel plasmid and its variant harboring both a blaNDM-1 gene and type IV secretion system in clinical isolates of Acinetobacter lwoffii. Antimicrob. Agents Chemother. 56:1698–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krizova L, Bonnin RA, Nordmann P, Nemec A, Poirel L. 2012. Characterization of a multidrug-resistant Acinetobacter baumannii strain carrying the blaNDM-1 and blaOXA-23 carbapenemase genes from the Czech Republic. J. Antimicrob. Chemother. 67:1550–1552 [DOI] [PubMed] [Google Scholar]
- 10. Jovcic B, Lepsanovic Z, Suljagic V, Rackov G, Begovic J, Topisirovic L, Kojic M. 2011. Emergence of NDM-1 metallo-β-lactamase in Pseudomonas aeruginosa clinical isolates from Serbia. Antimicrob. Agents Chemother. 55:3929–3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lascols C, hackle M, Marshall SH, Juher AM, Bouchillon S, Badal R, Hoban D, Bonomo RA. 2011. Increasing prevalence and dissemination of NDM-1 metallo-β-lactamase in India: data from the SMART study (2009). J. Antimicrob. Chemother. 66:1992–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Albur M, Noel A, Bowker K, MacGowan A. 2012. Bactericidal activity of multiple combinations of tigecycline and colistin against NDM-1-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 56:3441–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poirel L, Ozdamar M, Ocampo-Sosa AA, Turkoglu S, Ozer UG, Nordmann P. 2012. NDM-1-producing Klebsiella pneumoniae now in Turkey. Antimicrob. Agents Chemother. 56:2784–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan HLW, Poon LM, Chan SG, Teo JWP. 2011. The perils of medical tourism: NDM-1 positive Eschericia coli causing febrile neutropenia in a medical tourist. Singapore Med. J. 52:299–302 [PubMed] [Google Scholar]
- 16. Mulvey MR, Grant JM, Plewes K, Roscoe D, Boyd DA. 2011. New Delhi metallo-β-lactamase in Klebsiella pneumoniae and Escherichia coli, Canada. Emerg. Infect. Dis. 17:103–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Merck & Co Inc 2011. Invanz (ertapenem for injection) product information. Merck & Co., Inc., Whitehouse Station, NJ [Google Scholar]
- 18. Ortho-McNeil-Janssen Pharmaceuticals Inc 2007. Doribax (doripenem for injection). Ortho-McNeil-Janssen Pharmaceuticals, Inc., Raritan, NJ [Google Scholar]
- 19. Girlich D, Poirel L, Carattoli A, Kempf I, Lartigue MF, Bertini A, Nordmann P. 2007. Extended-spectrum β-lactamase CTX-M-1 in Escherichia coli isolates from healthy poultry in France. Appl. Environ. Microbiol. 73:4681–4685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi KH, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64:391–397 [DOI] [PubMed] [Google Scholar]
- 21. Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deryke CA, Banevicius MA, Fan HW, Nicolau DP. 2007. Bactericidal activities of meropenem and ertapenem against extended-spectrum-β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a neutropenic mouse thigh model. Antimicrob. Agents Chemother. 51:1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xuan D, Banevicius MA, Capitano B, Kim MK, Nightingale C, Nicolau DP. 2002. Pharmacodynamic assessment of ertapenem (MK-0826) against Streptococcus pneumoniae in a murine neutropenic thigh infection model. Antimicrob. Agents Chemother. 46:2990–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeRyke CA, Nicolau DP. 2007. Is all free time above the minimum inhibitory concentration the same: implications for β-lactam in vivo modeling. Int. J. Antimicrob. Agents 29:341–343 [DOI] [PubMed] [Google Scholar]
- 25. Bulik CC, Nicolau DP. 2010. In vivo efficacy of simulated human dosing regimens of prolonged-infusion doripenem against carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54:4112–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crandon JL, Bulik CC, Nicolau DP. 2009. In vivo efficacy of 1- and 2-gram human simulated prolonged infusions of doripenem against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wiskirchen DE, Crandon JL, Nicolau DP. 2013. Impact of various conditions on the efficacy of dual carbapenem therapy against KPC-producing Klebsiella pneumoniae. Int. J. Antimicrob. Agents 41:582–585 [DOI] [PubMed] [Google Scholar]
- 28. Daikos GL, Markogiannakis A. 2011. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clinical Microbiol. Infect. 17:1135–1141 [DOI] [PubMed] [Google Scholar]
- 29. Tumbarello M, Pierluigi V, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by KPC-producing Klebsiella pneumoniae: importance of combination therapy. Clin. Infect. Dis. 55:943–950 [DOI] [PubMed] [Google Scholar]
- 30. Giamarellou H, Galani L, Baziaka F, Karaiskos I. 2013. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 57:2388–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]