Abstract
Quorum sensing (QS) in Pseudomonas aeruginosa regulates the production of many virulence factors and plays an important role in the pathogenesis of P. aeruginosa infection. N-acyl homoserine lactones (AHL) are major QS signal molecules. Recently, a novel AHL-lactonase enzyme, AiiM, has been identified. The aim of this study was to evaluate the effect of AiiM on the virulence of P. aeruginosa in a mouse model of acute pneumonia. We developed a P. aeruginosa PAO1 strain harboring an AiiM-expressing plasmid. The production of several virulence factors by the AiiM-expressing strain was examined. Mice were intratracheally infected with an AiiM-expressing PAO1 strain. Lung histopathology, bacterial burden, and bronchoalveolar lavage (BAL) fluid were assessed at 24 h postinfection. AiiM expression in PAO1 reduced production of AHL-mediated virulence factors and attenuated cytotoxicity against human lung epithelial cells. In a mouse model of acute pneumonia, AiiM expression reduced lung injury and greatly improved the survival rates. The levels of proinflammatory cytokines and myeloperoxidase activity in BAL fluid were significantly lower in mice infected with AiiM-expressing PAO1. Thus, AiiM can strongly attenuate P. aeruginosa virulence in a mammalian model and is a potential candidate for use as a therapeutic agent against P. aeruginosa infection.
INTRODUCTION
Pseudomonas aeruginosa is a Gram-negative pathogen responsible for opportunistic and health care-associated infections, such as pneumonia and urinary tract-, surgical site-, and catheter-related bloodstream infections (1). Often, these infections are difficult to treat because of biofilm formation and intrinsic resistance to many antibiotics. In addition, P. aeruginosa easily develops resistance to many currently used antibiotics; therefore, the development of novel treatment strategies is imperative.
Recently, the quorum-sensing (QS) system has attracted attention as a new therapeutic target (2). P. aeruginosa has two well-characterized QS systems (the LasR-LasI and RhlR-RhlI systems) which mainly utilize two N-acyl homoserine lactone (AHL) molecules, namely, N-3-oxododecanoyl-l-homoserine lactone (3-oxo-C12-HSL) and N-butyryl-l-homoserine lactone (C4-HSL) (3). These systems regulate the production of various virulence factors, including pyocyanin, elastase, and rhamnolipid, which play important roles in promoting the infection (4). In addition, 3-oxo-C12-HSL itself has immunomodulatory activity, stimulates cytokine production, and induces neutrophil or macrophage apoptosis (5, 6).
Previous studies showed that AHL-negative mutant P. aeruginosa had considerably lower virulence than the wild-type strain (7, 8). Thus, AHL inhibitor could be a therapeutic agent for infections by Gram-negative bacteria.
The first AHL-inactivating enzyme, AHL-lactonase AiiA, was isolated from Bacillus species (9). Recently, Wang et al. have found a novel AHL-lactonase, termed AiiM, from Microbacterium testaceum, which resides on the leaf surface of potato (10, 11). AiiM is a member of the α/β hydrolase fold family and inactivates a broad range of AHL. They have also reported that expression of the aiiM gene in the Gram-negative plant-pathogenic bacterium Pectobacterium carotovorum subsp. carotovorum resulted in significantly reduced pathogenicity to the potato slices (11). To date, although many AHL-lactonase enzymes (approximately 20) have been discovered, the efficacy of AHL-lactonase in infectious diseases has been evaluated only in nonmammalian species (9, 10). The aim of the present study was to investigate whether AHL-lactonase AiiM would attenuate P. aeruginosa pathogenicity both in vitro and in a mammalian model.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. aeruginosa reference strain PAO1 (12) was grown at 37°C in Luria-Bertani (LB) medium with shaking at 250 rpm. The AHL reporter strains Chromobacterium violaceum CV026 (13) and C. violaceum VIR07 (14) were grown at 30°C in LB medium. When required, antibiotics were added to the medium at the following final concentrations to maintain the plasmid: carbenicillin (50 μg/ml) for P. aeruginosa and kanamycin (40 μg/ml) for C. violaceum. A final concentration of 0.5% l-arabinose was used as an inducer in vitro. Since there was some residual expression of AiiM, in vivo experiments were performed in the absence of arabinose induction.
Construction of a plasmid containing aiiM and P. aeruginosa mutants.
A 1,029-bp fragment containing aiiM was PCR amplified with NheI/EcoRI ends from pUC118-aiiM (11) and cloned into pMJT1 (15), which contains an arabinose-inducible araBAD promoter, to generate pMJT1-aiiM. The constructed plasmid pMJT1-aiiM and pMJT1 (vector control) were transferred to P. aeruginosa by chemical transformation (15). The strains and plasmids used in this study are listed in Table 1. The primers used were AiiMrbsNheI_F (TACgctagcGATATGCTCGGGCAAAGCCCG) and AiiMendEcoRI_R (CTCgaattcTCGACGACGACATCCAGCTCCACG) (lowercase letters indicate restriction recognition sites).
Table 1.
Bacterial strains and plasmids used
| Strain or plasmid | Genotype | Source |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Wild-type prototroph | 12 |
| PAO1/pMJT1 | PAO1 with pMJT1 | 15 |
| PAO1/pMJT1-aiiM | PAO1 with pMJT1-aiiM | This study |
| C. violaceum | ||
| CV026 | ATCC 31532 derivative; cviI::Tn5xylE; Kmr, Smr | 11 |
| VIR07 | ATCC 12472 derivative; cviI::Kmr; Ampr | 11 |
| Plasmid | ||
| pUC118-aiiM | pUC118 (cloning vector; TaKaRa Bio) containing homoserine lactonase gene aiiM; Ampr | 11 |
| pMJT1 | Broad-host-range expression vector containing the araBAD promoter and the araC gene; Ampr, Carr | 15 |
| pMJT1-aiiM | pMJT1 with arabinose-inducible aiiM; Ampr, Carr | This study |
Detection of AHLs by TLC.
CV026 and VIR07 were used to detect short-chain AHLs (C4-HSL and C6-HSL) and a long-chain AHL (3-oxo-C12-HSL), respectively. AHLs were extracted from culture supernatants and separated by thin-layer chromatography (TLC) according to the methods described previously (13, 14, 16), with slight modifications. P. aeruginosa strains were grown overnight in 50 ml of LB medium, and cell-free culture supernatants were obtained by centrifugation (1,700 × g for 45 min at 4°C), followed by filtration through a 0.22-μm filter (Millipore Corp.). The supernatants were extracted twice with the same volume of acidified ethyl acetate (0.1% [vol/vol] acetic acid) in a separating funnel. The organic layers were dried over anhydrous magnesium sulfate and evaporated. The residues were resuspended in 100 μl of ethyl acetate.
Eight microliters of the solution was spotted and separated using reverse-phase TLC plates (RP-18 F245; Merck) with methanol-water (60:40, vol/vol) for the short-chain AHLs and normal-phase TLC plates (silica gel 60F254; Merck) with hexane-acetone (45:55, vol/vol) for 3-oxo-C12-HSL. TLC plates then were dried and overlaid with 120 ml of LB medium containing 0.5% (wt/vol) agar mixed with 12 ml of overnight culture of either C. violaceum CV026 or C. violaceum VIR07. After incubation for 24 h at 30°C, AHL-induced violacein production in C. violaceum appeared as purple spots. AHL standards were purchased from Cayman Chemical, MI.
Assay for pyocyanin and elastase production.
Cell-free culture supernatants were obtained by the same method as that described above for the AHL detection assay. Pyocyanin production was determined using a slightly modified procedure as previously described (17). Four milliliters of the supernatant was mixed with 3 ml of chloroform and centrifuged at 1,700 × g for 5 min. Two milliliters of the lower organic layer, containing pyocyanin, was collected and extracted with 2 ml of 0.2 M HCl. After centrifugation, the absorbance of the pink top layer was measured at 520 nm. Concentrations of pyocyanin were determined by multiplying the absorbance by 17.072 (18).
The elastase activity of the same culture supernatants was measured by the elastin Congo red (ECR) assay (19). Ten microliters of the supernatant was mixed with 250 μl of ECR buffer (30 mM Tris containing 2.5 mg of ECR, pH 7.5). After a 14-h incubation, the absorbance of the supernatant was measured at 495 nm, and the elastase activity was calculated using porcine elastase as a standard.
Assessment of cytotoxicity.
The human alveolar epithelial cell line A549 was cultured in RPMI 1640 medium with 10% fetal bovine serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml at 37°C with 5% CO2. After achieving confluence, the cells were diluted to 2.0 × 105 cells/ml, and 100-μl aliquots were dispensed in a 96-well plate. After overnight incubation, the cells were washed and infected with P. aeruginosa strains at a concentration of 4.0 × 106 CFU/well or with normal saline (negative control) for 1, 3, 6, 9, 12, and 15 h in serum-free RPMI 1640. The cytotoxicity was assessed by the amount of lactate dehydrogenase (LDH) in the culture medium by using a cytotoxicity detection kit (Roche Diagnostics, Mannheim, Germany).
Mouse model of P. aeruginosa acute lung infection.
Animal experiments were approved by the Ethics Review Committee for Animal Experimentation and performed on the basis of the Guidelines for Animal Experimentation at Nagasaki University. Pathogen-free 6-week-old male ddY mice (body weight, 30 to 35 g) were purchased from SLC Japan (Tokyo, Japan). All animals were housed under constant temperature and light conditions (12/12-h light/dark cycle) with free access to sterile food and water in the Laboratory Animal Center for Biomedical Science at Nagasaki University.
Overnight cultures of P. aeruginosa strains were grown and adjusted to an optical density at 600 nm (OD600) of 0.1, and 30-μl aliquots were transferred to 3 ml of fresh LB medium. Following 6 h (late log phase) of incubation, bacteria were harvested by centrifugation (13,000 × g for 1 min at 4°C) and resuspended in sterile normal saline at a final concentration of 2 × 108 CFU/ml based on optical density. Inoculated bacterial counts were confirmed by spreading the appropriately diluted suspension on LB plates. Mice were infected with an intratracheal instillation of 0.05 ml of bacterial suspension (1 × 107 CFU/mouse) under anesthesia with pentobarbital sodium. As a noninfected control, intratracheal instillation was performed with normal saline in the same manner. For survival studies, 10 to 12 mice in each group were observed at least once daily until the seventh day postinfection. Another group of infected mice was sacrificed by cervical dislocation at 24 h after the infection, and the lungs, spleen, blood, and bronchoalveolar lavage (BAL) fluid were collected for further analysis.
Bacteriological and histopathological examination.
Blood was collected by right ventricular puncture using heparin-coated syringes. Lungs and spleens were dissected under aseptic conditions. For bacteriological examination, the organs were suspended in normal saline (1 ml for the lung sample and 0.5 ml for the spleen sample) and homogenized using a homogenizer (AS One Co., Osaka, Japan). Each specimen (blood, lung, and spleen) was serially diluted and plated on LB agar, followed by incubation at 37°C for 24 h. For histopathological examination, lung specimens were fixed in 10% buffered formalin, and the paraffin-embedded sections were stained with hematoxylin-eosin.
Analysis of BAL fluid.
After the pulmonary vasculature was flushed with 5 ml of normal saline via the right ventricle, an 18-gauge plastic intravenous catheter was inserted into the trachea and the lungs were lavaged three times with 1 ml of normal saline. Total and differential cell counts were determined manually.
Hemoglobin concentrations in BAL fluid were analyzed by using previously described methods, with some modifications (20, 21). A 200-μl aliquot of the BAL fluid was mixed with 800 μl of distilled water to lyse the red blood cells. The sample then was mixed with an equal volume of Drabkin's reagent (Sigma-Aldrich, St. Louis, MO) and incubated at room temperature for 15 min. Hemoglobin content was quantified by measuring the absorbance at 540 nm using purified human hemoglobin (Sigma-Aldrich, St. Louis, MO) as the standard.
Cell-free BAL fluid supernatants were obtained by centrifugation (15,000 × g for 5 min at 4°C) of BAL fluids. Albumin concentration and LDH activity in the supernatant fluid were determined by using a mouse albumin enzyme-linked immunosorbent assay (ELISA) quantitation kit (Bethyl Laboratories, Montgomery, TX) and a cytotoxicity detection kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturers' instructions. The concentrations of myeloperoxidase (MPO), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and macrophage inflammatory protein 2 (MIP-2) in cell-free BAL fluid supernatants were quantified using ELISA kits for MPO glycoprotein (Hycult Biotech, Uden, The Netherlands) and mouse cytokine and chemokine ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturers' instructions.
Statistical analyses.
The data are expressed as means ± standard errors of the means (SEM). Differences between groups were examined using the unpaired Student's t test or the Mann-Whitney's U test as appropriate, depending on the normality of data distribution. Survival analysis was performed using the log-rank test, and the survival rate was calculated by the Kaplan-Meier method. A significant difference was defined as a P value less than or equal to 0.05.
RESULTS
Expression of AiiM in P. aeruginosa reduced AHL accumulation.
As seen in Fig. 1, short- and long-chain AHL (C4-HSL, C6-HSL, and 3-oxo-C12-HSL) were detected as purple spots on the TLC plates from the extracts of the wild-type strain PAO1 and the mutant carrying the empty vector (PAO1/pMJT1). In contrast, none of these AHLs were detected from the mutant carrying the plasmid with the aiiM gene (PAO1/pMJT1-aiiM). The growth curve of PAO1/pMJT1-aiiM and PAO1/pMJT1 showed no difference (data not shown). Therefore, the results indicated that AiiM expressed in PAO1/pMJT1-aiiM degraded AHL.
Fig 1.
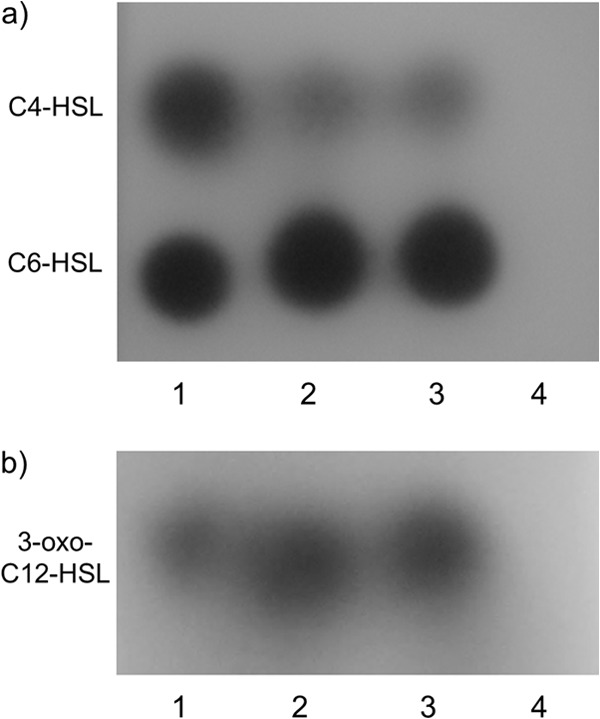
TLC analysis of AHL produced by P. aeruginosa strains. Lane 1, AHL standards (C4-HSL, C6-HSL, and 3-oxo-C12-HSL). Lane 2, PAO1. Lane 3, PAO1/pMJT1. Lane 4, PAO1/pMJT1-aiiM. Spots were visualized with the AHL reporter strains C. violaceum CV026 for short-chain AHL (a) and C. violaceum VIR07 for long-chain AHL (b). None of the three AHLs was detected from PAO1/pMJT1-aiiM.
AiiM expression reduced virulence factor production in P. aeruginosa.
Since the production of pyocyanin and elastase, the important virulence factors of P. aeruginosa, are controlled by the QS system, we examined whether AiiM expression could inhibit the production of these virulence factors. As shown in Fig. 2, the levels of pyocyanin and elastase decreased to a larger extent in P. aeruginosa PAO1/pMJT1-aiiM than in PAO1/pMJT1.
Fig 2.
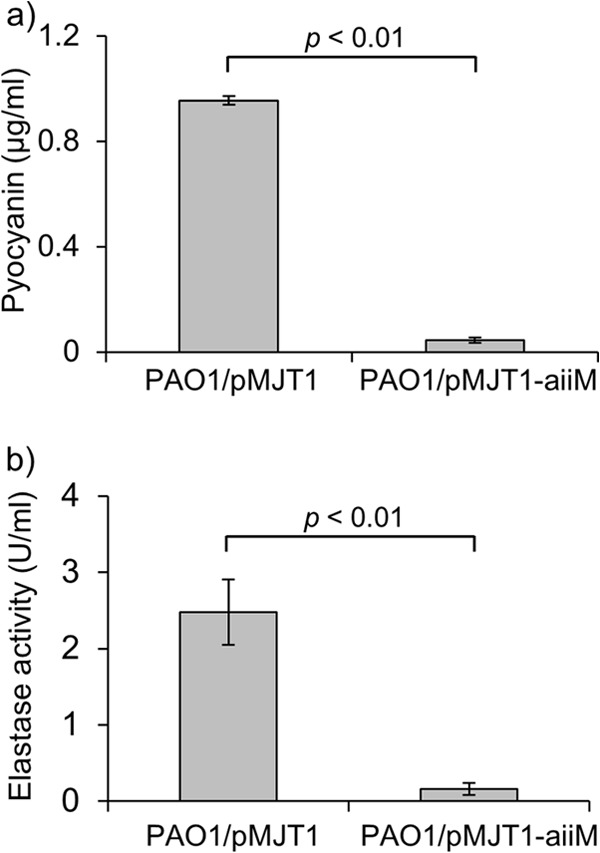
Amount of pyocyanin (a) and elastase (b) activity in the culture supernatant of PAO1/pMJT1 or PAO1/pMJT1-aiiM. Each bar represents the means from triplicates of the sample, and the error bars indicate the standard errors of the means. PAO1/pMJT1-aiiM produced very low levels of both pyocyanin and elastase.
AiiM expression attenuated cytotoxicity against human lung epithelial cells.
To test the influence of AiiM expression on the infectivity of P. aeruginosa, we evaluated the cytotoxicity of each strain against A549 cells by measuring the release of LDH following infection. P. aeruginosa PAO1/pMJT1-aiiM showed approximately half the level of cytotoxicity of PAO1/pMJT1 at 9 to 15 h postinfection (Fig. 3).
Fig 3.
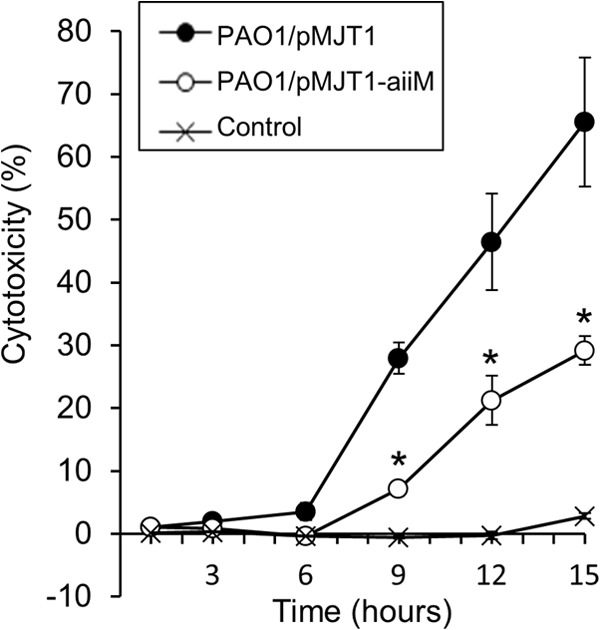
Cytotoxicity of A549 cells after infection with P. aeruginosa PAO1/pMJT1 or PAO1/pMJT1-aiiM or of the uninfected control. Cytotoxicity was determined by measuring the LDH release. Every data point represents means from triplicates of the sample, and the error bars indicate the standard errors of the means. P. aeruginosa PAO1/pMJT1-aiiM showed reduced cytotoxicity. *, P < 0.05 compared to PAO1/pMJT1.
AiiM expression improved survival in murine models of acute lung infection and reduced systemic dissemination of bacteria.
Our preliminary results encouraged us to investigate the in vivo efficacy of AiiM expression in P. aeruginosa. Mortality was significantly lower in the mice infected with PAO1/pMJT1-aiiM than in those infected with PAO1/pMJT1 (Fig. 4a).
Fig 4.
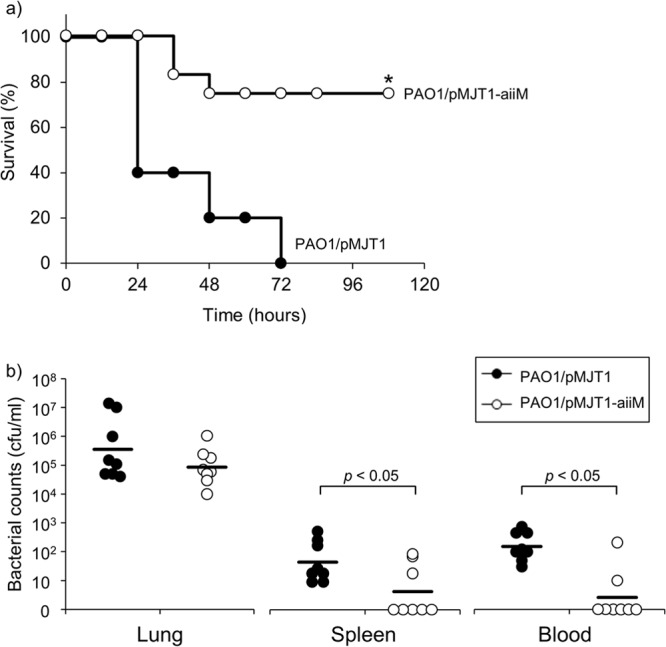
Analysis of survival data and bacterial loads. (a) Kaplan-Meier survival curve of the mice infected with P. aeruginosa PAO1/pMJT1 or PAO1/pMJT1-aiiM. Survival was monitored for 7 days (n = 10 to 12 mice per group). P. aeruginosa PAO1/pMJT1-aiiM exhibited significantly lower mortality than the wild-type control strain. *, P < 0.01 compared to PAO1/pMJT1. (b) Bacterial loads in the lungs, spleen, and blood at 24 h after infection with P. aeruginosa PAO1/pMJT1 or PAO1/pMJT1-aiiM. Each horizontal line represents mean bacterial counts (n = 8 mice per group). P. aeruginosa PAO1/pMJT1-aiiM had a decreased ability to disseminate in the infected mouse.
We further investigated whether AiiM could reduce local infection and systemic dissemination. The bacterial count in the lungs did not significantly differ between the groups. However, the bacterial counts in the blood and spleen of mice infected with PAO1/pMJT1-aiiM were significantly lower than those in the mice infected with PAO1/pMJT1 (Fig. 4b), indicating that AiiM expression contributed to reduction of systemic dissemination but did not alter the local bacterial burden.
AiiM expression decreased the lung injury induced by P. aeruginosa infection.
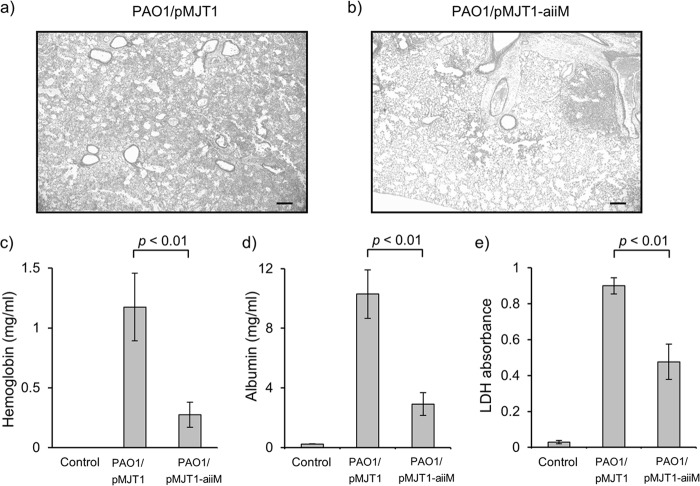
We next examined whether AiiM expression could reduce lung injury and inflammation. In spite of the identical bacterial burden in both groups, PAO1/pMJT1-aiiM-infected mice showed mild and localized inflammation, while P. aeruginosa PAO1/pMJT1-infected mice exhibited a large area of diffuse inflammation accompanied by hemorrhage into the alveolar spaces (Fig. 5a and b). In addition, the reduced levels of hemoglobin, albumin, and LDH in the cell-free BAL fluids of PAO1/pMJT1-aiiM-infected mice (Fig. 5c to e) were also observed.
Fig 5.
Lung tissue damage caused by P. aeruginosa infection. Histological examination of the lungs from the mice infected with P. aeruginosa PAO1/pMJT1 (a) and PAO1/pMJT1-aiiM (b). Lung tissue sections were obtained at 24 h postinfection and stained with hematoxylin and eosin. Representative images are shown at an original magnification of ×25. Scale bar, 200 μm. The amount of hemoglobin (c), albumin (d), and LDH (e) in cell-free BAL fluids at 24 h after infection with P. aeruginosa PAO1/pMJT1, PAO1/pMJT1-aiiM, or uninfected control is also shown. Each bar represents average values, and error bars show standard errors of the means (n = 8 mice per group). Mice infected with P. aeruginosa PAO1/pMJT1-aiiM developed only small lung lesions and had significantly decreased levels of hemoglobin, albumin, and LDH in BAL fluids.
AiiM expression altered the host inflammatory response to P. aeruginosa lung infection.
The total cell counts and neutrophil percentage in the BAL fluid of both infected groups increased compared to those of the uninfected control, with no significant differences between the two groups (Fig. 6a and b); however, MPO activity, a marker of neutrophil activation, in the BAL fluid of the mice infected with P. aeruginosa PAO1/pMJT1-aiiM was significantly lower than that of the PAO1/pMJT1-infected mice (Fig. 6c).
Fig 6.
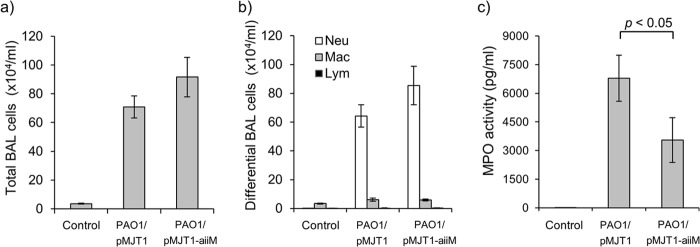
BAL fluid analysis. BAL fluids were harvested 24 h after infection with P. aeruginosa PAO1/pMJT1, PAO1/pMJT1-aiiM, or uninfected control. Total cell counts (a), differential cellular analysis (b), and MPO levels (c) in cell-free BAL fluids are shown. Each bar represents average values, and the error bars show the standard errors of the means (n = 8 mice per group). Both P. aeruginosa PAO1/pMJT1 and PAO1/pMJT1-aiiM induced a similar increase in the total BAL fluid cell and neutrophil numbers in infected mice. The levels of MPO in cell-free BAL fluids were significantly reduced in the mice infected with P. aeruginosa PAO1/pMJT1-aiiM. Neu, neutrophils; Mac, macrophages; Lym, lymphocytes.
The levels of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) in the BAL fluid were also significantly lower in the PAO1/pMJT1-aiiM-infected mice than in the PAO1/pMJT1-infected mice (Fig. 7a to c). MIP-2 levels tended to be lower in the PAO1/pMJT1-aiiM-infected group than in the PAO1/pMJT1-infected group, although the decrease was not statistically significant (Fig. 7d). Therefore, these results suggest that AiiM expression could reduce lung injury and inflammation and systemic dissemination by reducing bacterial virulence, but it does not reduce bacterial counts.
Fig 7.
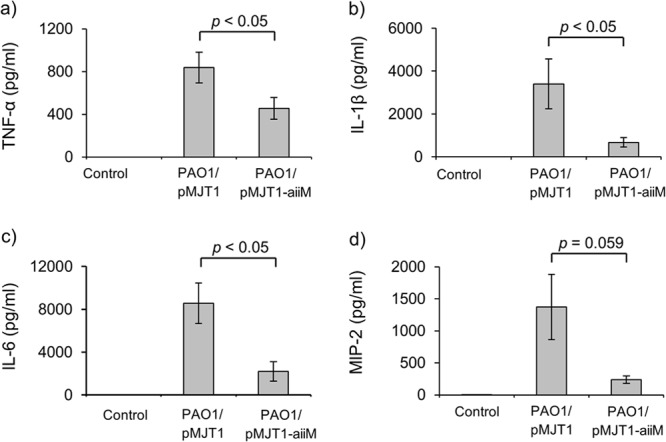
Proinflammatory cytokine and chemokine levels in cell-free BAL fluids at 24 h postinfection. Each bar represents average values, and the error bars show the standard errors of the means (n = 8 mice per group). Mice infected with P. aeruginosa PAO1/pMJT1-aiiM produced lower levels of TNF-α, IL-1β, IL-6, and MIP-2 in cell-free BAL fluid than in mice infected with PAO1/pMJT1.
DISCUSSION
Although several studies have reported the therapeutic potential of AHL-lactonases against Gram-negative pathogens in nonmammalian models (22, 23, 24), it has never been considered a therapeutic agent for mammalian infections. This is the first study demonstrating that AiiM could serve as a potent inhibitor of P. aeruginosa QS and attenuate bacterial virulence in the mouse model of P. aeruginosa lung infection.
AHL-mediated virulence factors, such as pyocyanin and elastase, and AHL itself have been reported to cause cytotoxic effects and tissue damage and to play an important role in the pathogenesis of P. aeruginosa (6, 25, 26). Expression of AiiM in P. aeruginosa PAO1 strongly suppressed AHL accumulation, including two major ones (C4-HSL and 3-oxo-C12-HSL), and the production of the QS-dependent virulence factors. Furthermore, we demonstrated that an AiiM-expressing PAO1 mutant exhibited significantly decreased virulence both in vitro and in vivo. A previous report that described less virulence in AHL-negative mutant P. aeruginosa lung infection in neonatal mice supports our results (7).
It is unclear whether systemic bacterial dissemination is directly involved with mortality in our model, although development of bacteremia has been reported to be related to the high mortality rate in acute Pseudomonas infection (27). The decreased lung injury in the mice infected with the AiiM-expressing mutant may contribute less to mortality and also minimize airway epithelial permeability, thereby resulting in reduced systemic dissemination (28).
Despite the presence of proinflammatory cytokines, chemokine and MPO activities in BAL fluid were lower in mice infected with the AiiM-carrying mutant than in the control mutant. Neutrophil counts in BAL fluid were identical in both groups. While 3-oxo-C12-HSL attracts neutrophils either directly or through the induction of chemokines (IL-8 and MIP-2) (29, 30), the proapoptotic effect of 3-oxo-C12-HSL on neutrophils is known (6). AHL-mediated virulence factors, namely, pyocyanin and rhamnolipid, also cause apoptotic or necrotic cell death of neutrophils in BAL fluid (31, 32). Thus, some of the migrated neutrophils may be killed by these factors with no change in the BAL fluid neutrophil counts.
AHL-lactonase enzymes are highly specific for AHL and are expected to have beneficial effects in patients with P. aeruginosa infection. Thus, our results indicate that a novel strategy using AHL will be useful for treating intractable Pseudomonas infections, although there are still some issues for clinical use. We consider several possibilities of AiiM for clinical applications in patients with infectious diseases. First, the aiiM gene can be used to improve probiotics to enhance their positive effects through genetic modification. There are, however, some additional issues with mutant probiotics, such as human safety or possible adverse effects on the environment; thus, this practice is not yet ethically practical. Second, the use of the purified AiiM protein will be the most convenient and practical method. Local administration of the purified AiiM protein may reduce the severity of lung infections due to P. aeruginosa and may have an efficacy in preventing infection, although it is not technically available at the present time. Therefore, further studies will be required to evaluate the efficacy and safety of purified AiiM protein. In conclusion, this study demonstrates that AiiM can disturb the QS systems of P. aeruginosa and attenuate bacterial virulence in a mouse model of acute lung infection. Although the mechanism of host immune response to QS-related factors is not fully understood, we have found that quenching QS signals by AiiM may result in the reduction of excessive inflammation and help in inciting an immune response against P. aeruginosa infection. Thus, our results are expected to supply attractive candidates to develop novel prophylactic or therapeutic strategies using AHL-lactonases against Pseudomonas infections.
ACKNOWLEDGMENTS
We thank Paul Williams (University of Nottingham, United Kingdom) for kindly providing Chromobacterium violaceum CV026.
We declare no competing financial interests.
This research is partly supported by grants from Takeda Science Foundation, the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI24791032), and the Ministry of Health, Labor and Welfare of Japan (H22shinkouippan008 and H23shinkouippan018).
Footnotes
Published ahead of print 20 May 2013
REFERENCES
- 1. Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29:996–1011 [DOI] [PubMed] [Google Scholar]
- 2. Sintim HO, Smith JAI, Wang J, Nakayama S, Yan L. 2010. Paradigm shift in discovering next-generation anti-infective agents: targeting quorum sensing, c-di-GMP signaling and biofilm formation in bacteria with small molecules. Future Med. Chem. 2:1005–1035 [DOI] [PubMed] [Google Scholar]
- 3. Pesci EC, Pearson JP, Seed PC, Iglewski BH. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith RS, Harris SG, Phipps R, Iglewski B. 2002. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol. 184:1132–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere JC, Standiford TJ, Ishiguro M, Yamaguchi K. 2003. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 71:5785–5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pearson JP, Feldman M, Iglewski BH, Prince A. 2000. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 68:4331–4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imamura Y, Yanagihara K, Tomono K, Ohno H, Higashiyama Y, Miyazaki Y, Hirakata Y, Mizuta Y, Kadota J, Tsukamoto K, Kohno S. 2005. Role of Pseudomonas aeruginosa quorum-sensing systems in a mouse model of chronic respiratory infection. J. Med. Microbiol. 54:515–518 [DOI] [PubMed] [Google Scholar]
- 9. Dong YH, Xu JL, Li XZ, Zhang LH. 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. U. S. A. 97:3526–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morohoshi T, Someya N, Ikeda T. 2009. Novel N-acylhomoserine lactone-degrading bacteria isolated from the leaf surface of Solanum tuberosum and their quorum-quenching properties. Biosci. Biotechnol. Biochem. 73:2124–2127 [DOI] [PubMed] [Google Scholar]
- 11. Wang W-Z, Morohoshi T, Ikenoya M, Someya N, Ikeda T. 2010. AiiM, a novel class of N-acylhomoserine lactonase from the leaf-associated bacterium Microbacterium testaceum. Appl. Environ. Microbiol. 76:2524–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964 [DOI] [PubMed] [Google Scholar]
- 13. McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703–3711 [DOI] [PubMed] [Google Scholar]
- 14. Morohoshi T, Kato M, Fukamachi K, Kato N, Ikeda T. 2008. N-Acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC 12472. FEMS Microbiol. Lett. 279:124–130 [DOI] [PubMed] [Google Scholar]
- 15. Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. 2007. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 117:877–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ravn L, Christensen AB, Molin S, Givskov M, Gram L. 2001. Methods for detecting acylated homoserine lactones produced by Gram-negative bacteria and their application in studies of AHL-production kinetics. J. Microbiol. Methods 44:239–251 [DOI] [PubMed] [Google Scholar]
- 17. Li C, Wally H, Miller SJ, Lu C-D. 2009. The multifaceted proteins MvaT and MvaU, members of the H-NS family, control arginine metabolism, pyocyanin synthesis, and prophage activation in Pseudomonas aeruginosa PAO1. J. Bacteriol. 191:6211–6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurachi M. 1958. Studies of the biosynthesis of pyocyanine. (II) Isolation and determination of pyocyanine. Bull. Inst. Chem. Res. Kyoto Univ. 36:174–187 [Google Scholar]
- 19. Rust L, Messing CR, Iglewski BH. 1994. Elastase assays. Methods Enzymol. 235:554–562 [DOI] [PubMed] [Google Scholar]
- 20. Battistini B, Steil AA, Jancar S, Sirois P. 1998. Roles of endothelins and their receptors in immune complex-induced/polymorphonuclear-mediated lung injury (reversed passive arthus reaction) in CD-1 mice. Pulm. Pharmacol. Ther. 11:165–172 [DOI] [PubMed] [Google Scholar]
- 21. Wesselkamper SC, McDowell SA, Medvedovic M, Dalton TP, Deshmukh HS, Sartor MA, Case LM, Henning LN, Borchers MT, Tomlinson CR, Prows DR, Leikauf GD. 2006. The role of metallothionein in the pathogenesis of acute lung injury. Am. J. Respir. Cell Mol. Biol. 34:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asad S, Opal SM. 2008. Bench-to-bedside review: quorum sensing and the role of cell-to-cell communication during invasive bacterial infection. Crit. Care 12:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang L-H, Weng L-X, Dong Y-H, Zhang L-H. 2004. Specificity and enzyme kinetics of the quorum-quenching N-acyl homoserine lactone lactonase (AHL-lactonase). J. Biol. Chem. 279:13645–13651 [DOI] [PubMed] [Google Scholar]
- 24. Huang W, Lin Y, Yi S, Liu P, Shen J, Shao Z, Liu Z. 2012. QsdH, a novel AHL lactonase in the RND-type inner membrane of marine Pseudoalteromonas byunsanensis strain 1A01261. PLoS One 7:e46587. 10.1371/journal.pone.0046587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee K-M, Yoon MY, Park Y, Lee J-H, Yoon SS. 2011. Anaerobiosis-induced loss of cytotoxicity is due to inactivation of quorum sensing in Pseudomonas aeruginosa. Infect. Immun. 79:2792–2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sadikot RT, Blackwell TS, Christman JW, Prince AS. 2005. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Resp. Crit. Care Med. 171:1209–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sawa T, Ohara M, Kurahashi K, Twining SS, Frank DW, Doroques DB, Long T, Gropper MA, Wiener-Kronish JP. 1998. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect. Immun. 66:3242–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Azghani AO. 1996. Pseudomonas aeruginosa and epithelial permeability: role of virulence factors elastase and exotoxin A. Am. J. Resp. Cell Mol. Biol. 15:132–140 [DOI] [PubMed] [Google Scholar]
- 29. Smith RS, Fedyk ER, Springer TA, Mukaida N, Iglewski BH, Phipps RP. 2001. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa B and activator protein-2. J. Immunol. 167:366–374 [DOI] [PubMed] [Google Scholar]
- 30. Wagner C, Zimmermann S, Brenner-Weiss G, Hug F, Prior B, Obst U, Hänsch GM. 2007. The quorum-sensing molecule N-3-oxododecanoyl homoserine lactone (3OC12-HSL) enhances the host defence by activating human polymorphonuclear neutrophils (PMN). Anal. Bioanal. Chem. 387:481–487 [DOI] [PubMed] [Google Scholar]
- 31. Allen L, Dockrell DH, Pattery T, Lee DG, Cornelis P, Hellewell PG, Whyte MKB. 2005. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J. Immunol. 174:3643–3649 [DOI] [PubMed] [Google Scholar]
- 32. Jensen PØ, Bjarnsholt T, Phipps R, Rasmussen TB, Calum H, Christoffersen L, Moser C, Williams P, Pressler T, Givskov M, Høiby N. 2007. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 153:1329–1338 [DOI] [PubMed] [Google Scholar]