Abstract
This study assessed the safety, tolerability, and pharmacokinetic interaction between PA-824, a novel antitubercular nitroimidazo-oxazine, and midazolam, a CYP3A4 substrate, in 14 healthy adult male and female subjects. The study followed up on observations in vitro that PA-824 caused weak and time-dependent inhibition of CYP3A4. Subjects received a single oral dose of midazolam (2 mg), followed by a 2-day washout. After the washout, all subjects received PA-824 (400 mg) once daily for 14 consecutive days. On day 14, all subjects received the final PA-824 dose coadministered with a 2-mg oral dose of midazolam. The pharmacokinetic endpoints AUC0–t, AUC0–∞, and Cmax for midazolam and 1-hydroxy midazolam were compared between midazolam administered alone versus midazolam coadministered with PA-824. Statistical analysis demonstrated that the mean midazolam values of Cmax, AUC0–t, and AUC0–∞ parameters were reduced by ca. 16, 15, and 15%, respectively, when PA-824 was coadministered with midazolam. The total exposure (AUC) of 1-hydroxy midazolam was 13 to 14% greater when coadministered with PA-824 compared to midazolam administered alone. The Cmax of 1-hydroxy midazolam was similar between treatments. Based on these results, PA-824 does not inhibit or induce CYP3A4 to a clinically meaningful extent and is not likely to markedly affect the pharmacokinetics of CYP3A4 metabolized drugs.
INTRODUCTION
PA-824 is an antitubercular nitroimidazo-oxazine that possesses significant activity against replicating and nonreplicating/persistent Mycobacterium tuberculosis via a complex mechanism of action distinct from that of any currently marketed drugs for the treatment of tuberculosis (1, 2). Its mechanism of action is believed similar to that of Delamanid, a drug currently under review for market approval by the regulatory authorities (3). PA-824 interferes with M. tuberculosis cell wall biosynthesis by inhibiting the oxidation of hydroxymycolate to ketomycolate. A deazaflavin (F420)-dependent nitroreductase has also been identified whose activity in M. tuberculosis cells is involved in PA-824 activation and activity (2). Reduction of PA-824 to its des-nitroimidazole metabolite by this nitroreductase is associated with generation of reactive nitrogen species, including nitric oxide. PA-824 is active against M. tuberculosis isolates resistant to single or multiple antituberculous drugs and has proven effective in shortening treatment time of drug-sensitive M. tuberculosis in a murine model of tuberculosis (TB) as part of novel drug regimens (4). Moreover, PA-824 was highly active as monotherapy in a 14-day dose ranging early bactericidal activity (EBA) study in humans where similar efficacy profiles were observed across all doses assessed (200 to 1,200 mg/day) (5). In EBA studies, the rate of change over time in the number of M. tuberculosis CFU per ml of sputum in an overnight sputum collection is used to compare different dosing and treatment regimens. In a follow up study exploring a lower dose range (50 to 200 mg/day), a dose-response trend was detected with doses 100 mg/day and above showing similar efficacy profiles (6). In addition, in a 14-day EBA study of novel antituberculosis drug combinations, the combination of PA-824 (200 mg/day), moxifloxacin (400 mg/day), and pyrazinamide (25 mg/kg [body weight]/day) demonstrated EBA activity comparable to the current World Health Organization-recommended gold standard therapy of rifampin, isoniazid, pyrazinamide, and ethambutol (7). Based on the results of these EBA studies, the expected therapeutic doses of 100 and 200 mg/day are being evaluated in longer term trials in patients with pulmonary TB.
The current treatment of TB is greatly complicated by the human immunodeficiency virus (HIV) co-epidemic. In 2011, 13% of the estimated 8.7 million new cases of TB were coinfected with HIV and, of the 1.4 million deaths from TB, 430,000 deaths were among patients who were also HIV positive (8). The treatment of TB alone requires the use of multiple antibiotics to prevent the development of drug resistance. The use of multiple antiretroviral agents to treat HIV infection greatly increases the likelihood of drug-drug interactions for patients with both infections. Many important drugs used to treat HIV infection, such as the protease inhibitors nevirapine, lopinavir, and ritonavir, are substrates of CYP3A4 and/or p-glycoprotein and have interactions with drugs for the treatment of TB, such as rifampin, that are inducers of hepatic enzymes (9). In vitro data indicate that PA-824 is not an inducer but is a weak substrate and potential weak inhibitor of CYP3A4 (data not shown). An assessment of PA824's drug-drug interaction potential is critical to determine its place in future TB therapy.
The present study was designed to ascertain whether PA-824 affected the pharmacokinetics (PK) of midazolam, a sensitive probe substrate and representative compound for drugs metabolized by CYP3A4. Midazolam is one of the most commonly used in vivo and in vitro CYP3A4 probe substrates for drug-drug interaction studies.
MATERIALS AND METHODS
Study design.
A phase I study was conducted to evaluate the effects of the multiple-dose administration of PA-824 on the PK of orally administered midazolam and to evaluate the safety and tolerability of PA-824 when coadministered with midazolam. The study was an open-label, multidose, fixed-sequence design performed in keeping with the U.S. Food and Drug Administration's industry guidance (10). The clinical study was conducted by Celerion, Inc. (formerly MDS Pharma Services, Inc.), in Lincoln, NE, under the sponsorship of the Global Alliance for TB Drug Development.
Subjects were enrolled into the study and housed in the clinical facility from the evening of day 1 (at least 15 h before dosing) through the morning of day 18. The total study duration for each subject from check-in through study termination (including a 2-day washout) spanned 18 nights and 19 days, with follow-up evaluation over 3 months.
All subjects received an initial single oral dose of 2 mg of midazolam CIV hydrochloride syrup (Boehringer Ingelheim/Roxane Laboratories) administered with 240 ml of water, followed by a 2-day washout. After the washout, all subjects received 400 mg of PA-824 (two 200-mg tablets) administered with 240 ml of water once daily for 14 consecutive days. On the day 14 of PA-824 dosing, a single oral dose of 2 mg of midazolam was coadministered with 400 mg of PA-824 with 240 ml of water. Subjects fasted for a minimum of 8 h prior to and 4 h after dosing. Water was permitted ad libitum 1 h prior to and then again 1 h after dosing. Subjects remained in an upright position (seated or standing) for 4 h after dosing. Dietary restrictions included caffeine, alcohol, poppy seeds, and grapefruit products prior to and throughout the study. No medication was permitted without sponsor approval except acetaminophen.
Subject inclusion and exclusion criteria.
Subjects were included if they were healthy nonsmokers aged 19 to 50, with a body mass index of 18 to 29, inclusive, with negative tests for alcohol and drugs of abuse. Subjects were excluded if they had any clinically significant history or presence of a cardiovascular, pulmonary, hepatic, renal, hematologic, gastrointestinal, endocrine, immunologic, dermatologic, or neurologic disease/disorder. Subjects also were excluded if they had a psychological, psychiatric, or metabolic disorder (including eating disorders) or if they had experienced any acute illness within 4 weeks. Female subjects were excluded if they were pregnant (positive test for serum human chorionic gonadotropin at screening or check-in), breast feeding, or planning to conceive a child within 30 days of cessation of treatment. Male subjects were excluded if they were planning to father a child within 12 weeks of cessation of treatment. Finally, subjects were excluded if they had a lens opacity or evidence of lens opacity on slit-lamp ophthalmologic examination to follow up on findings that some rats developed cataracts in toxicology studies at high doses over 3 to 6 months of dosing.
All subjects provided written informed consent prior to participation in the study. Study protocols and consent forms were reviewed and approved by Celerion's Institutional Review Board, and the study was conducted in accordance with U.S. Code of Federal Regulations (21 CFR Parts 50, 56, and 312) principles and requirements and International Conference on Harmonization guidelines (ICH E6).
Sampling.
PK blood samples for midazolam (obtained once, 6 ml) were collected prior to dosing and at 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12, 16, 20, and 24 h after each 2-mg oral dose of midazolam. PK blood samples for PA-824 (obtained once, 6 ml) were collected predose on days 12 and 13 of consecutive PA-824 dosing and predose on the day 14 prior to coadministration.
Bioanalytical methods.
Blood samples were collected and centrifuged, and plasma was separated and stored at −20°C. Plasma samples were analyzed for PA-824, midazolam, and the midazolam metabolite 1-hydroxy midazolam using separately validated liquid chromatography-mass spectrometry methods developed at Covance Laboratories, Inc.
PA-824 and the internal standard, triazolam (added during sample processing), were extracted from human plasma samples using liquid-liquid extraction. After evaporation under nitrogen, the residue was reconstituted and analyzed using liquid chromatography with tandem mass spectrometric detection. The PA-824 calibration range for quantitation was 10 to 10,000 ng/ml. Accuracy ranged from 96.3 to 100%, and precision (RSD) ranged from 1.7 to 4.4%.
Midazolam, 1-hydroxy midazolam, and the internal standard α-hydroxytriazolam (added during sample processing) were extracted from human plasma samples using liquid-liquid extraction. After evaporation under nitrogen, the residue was reconstituted and analyzed using liquid chromatography with tandem mass spectrometric detection according to a validated method proprietary to Covance Laboratories, Inc. The midazolam and 1-hydroxy midazolam calibration range for quantitation was 0.1 to 100 ng/ml. Accuracy ranged from 102.0 to 102.7% and from 102 to 103.3% for midazolam and 1-hydroxy midazolam, respectively. Precision (RSD) ranged from 4.5 to 6.3% and from 3.3 to 4.7% for midazolam and 1-hydroxy midazolam, respectively.
PK analysis.
Midazolam and 1-hydroxy midazolam plasma PK parameters were calculated for each subject when midazolam was dosed alone and in combination with PA-824 by applying a noncompartmental approach using WinNonlin professional version 5.0.1 (Pharsight Corp., Mountain View, CA). The key PK parameters calculated for midazolam and 1-hydroxy midazolam included Cmax (the maximum observed concentration), Tmax (the time at which Cmax occurs), kel (the terminal elimination rate constant), t1/2 (the elimination half-life), AUC0–t (the area under the concentration-time curve during the dosing interval), and AUC0–∞ (the area under concentration-time curve extrapolated to infinity).
Plasma concentrations below the limit of quantitation (BLQ) were handled in the following manner. The lower limit of quantitation for midazolam and 1-hydroxy midazolam bioanalysis was 0.1 ng/ml. PK parameters were not calculated for subjects for whom there were insufficient data. For the calculation of the PK parameters, plasma concentrations that were BLQ prior to the first quantifiable concentration were set to zero, and plasma concentrations BLQ after the first quantifiable concentration were treated as missing. For each subject, the elimination rate constant (kel) was estimated by unweighted log-linear regression using at least three data points from the last portion of the plasma concentration profile according to the least-squares approach ending with the last concentration prior to the first assay that was below the limit of quantification. The elimination half-life (t1/2) was calculated from the kel, using the formula ln(2)/kel. The kel was not assigned if the terminal elimination phase was not apparent, if Cmax was one of the three last data points, or if the R2 value was <80%. In cases where the kel interval was not assigned, the values of kel, AUC0–∞, and t1/2 were considered not calculable and were not reported. When the resulting t1/2 was more than half as long as the sampling interval, the kel values and associated parameters (t1/2 and AUC0–∞) were not presented. AUCs were calculated by using linear trapezoidal summation from time zero to the specified time point (either the last available time point or by extrapolation to infinity).
Statistical analysis.
All descriptive and inferential statistics were calculated in SAS version 9.1.3. Plasma concentration values for midazolam, 1-hydroxy midazolam, and PA-824 were listed and summarized using descriptive statistics. Descriptive statistics were calculated for PK parameters. The PK endpoints AUC0–t, AUC0–∞, and Cmax for midazolam and 1-hydroxy midazolam were compared between midazolam alone versus midazolam with PA-824 using an analysis of variance (ANOVA) model. The ANOVA model using SAS PROC mixed procedure included treatment, period, and sequence as fixed effects and subject within sequence as a random effect. For the present study, a potentially clinically important effect of PA-824 on midazolam pharmacokinetics, as specified in the protocol, would be concluded if the 90% confidence interval (CI) for the geometric mean ratios (GMRs) of AUC0–t, AUC0–∞, and Cmax were not contained within the prespecified “no-effect” 50 to 200% CI limit. If the GMRs were below 50% or above 200%, then a clinically important effect would be concluded. For the purposes of this study, a clinically important effect was considered to be a drug interaction of sufficient magnitude to require dose adjustment of drugs predominantly metabolized by CYP3A4 when coadministered with PA-824. If the GMRs were within the 50 to 200% limit, then sequentially narrower criteria would be tested, i.e., 70 to 143%. If these narrower criteria were met, then the standard “no-effect” or bioequivalence criteria, 80 to 125%, were to be assessed. The intended sample size was based on the known variability in the AUC and Cmax associated with midazolam. Sample size calculations were based on a study by Stoch et al. (11), who reported 90% CIs for GMRs of midazolam AUCs based on within-subject AUC variability and midazolam 2-mg oral doses in the presence of ketoconazole. The natural log-scale standard deviations (SD) computed from these CIs were 0.22 for AUC and 0.31 for Cmax. Twelve subjects provided >96% power of yielding a 90% CI for AUC or Cmax within 0.5 to 2.0 bounds if the true underlying GMR was 1.00. Midazolam and 1-hydroxy midazolam t1/2 and Tmax were compared between midazolam alone versus midazolam with PA-824 using the Student t test and Wilcoxon signed-rank test. If the t1/2 and Tmax data failed tests for normality, only the Wilcoxon signed-rank test results were reported.
RESULTS
A total of 14 healthy male (n = 10) and female (n = 4) subjects participated in this clinical study to assess the safety, tolerability, and PK parameters of midazolam when coadministered with PA-824. All subjects were 19 to 46 years old (mean, 27.2 years), had a body mass index of 18 to 29 (mean, 25.4 kg/m2), and were medically healthy as determined by the principal investigator based on their medical history, clinical laboratory results, 12-lead electrocardiograms (ECGs), and physical examination. At both screening and check-in, subjects had negative urine test results for alcohol and other drugs of abuse, such as amphetamines, cannabinoids, and cocaine metabolites. Study participants represented a racially diverse group consisting of one Asian, one black, two Hispanic, and 10 white subjects. All 14 subjects who were enrolled in the study completed all of the study procedures, and there were no dropouts.
Pharmacokinetics.
The mean plasma concentrations for midazolam and 1-hydroxy midazolam are shown in Fig. 1 and 2, respectively. PK parameters for midazolam and 1-hydroxy midazolam are provided in Table 1. The significant presence of BLQ plasma concentrations resulted in the loss of AUC0–∞ and t1/2 data for the midazolam data of one subject and the loss of 1-hydroxy midazolam data for four subjects. The predose mean PA-824 plasma concentrations ± the SD on days 12, 13, and 14 for the once-daily administration of 400 mg of PA-824 were 1,637 ± 610, 1,581 ± 539, and 1,607 ± 556 ng/ml, respectively. PA-824 plasma concentrations showed no sign of additional accumulation, indicating that steady-state had been achieved for PA-824 prior to the dosing of midazolam. These PA-824 plasma concentrations are similar to those found in earlier multiple-dose studies using similar doses.
Fig 1.
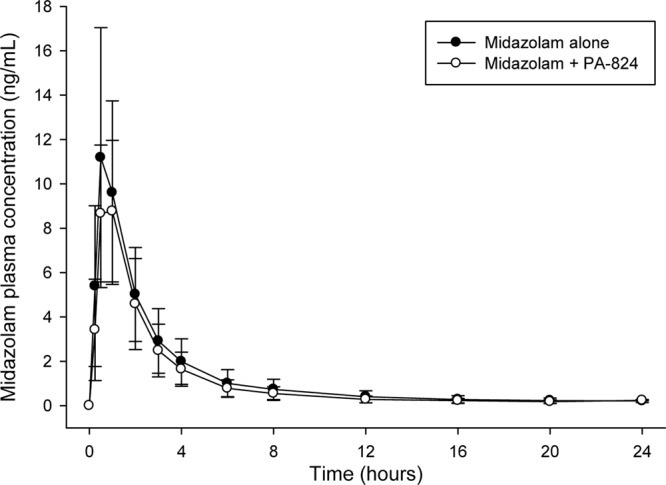
Mean plasma midazolam concentrations over time (± the SD) after a 2-mg oral dose of midazolam.
Fig 2.
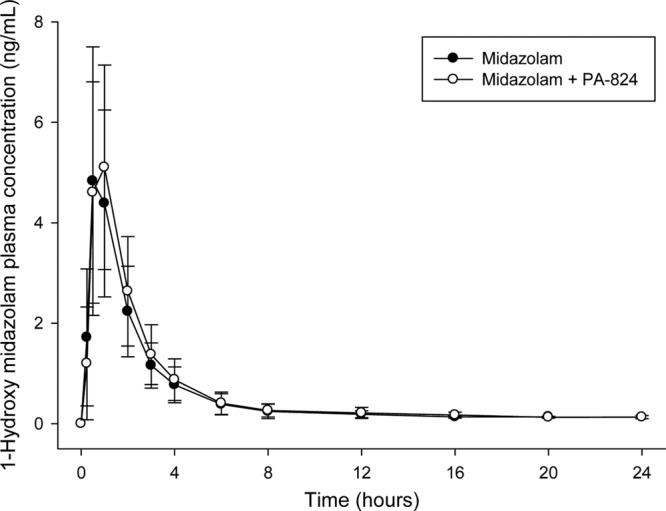
Mean plasma 1-hydroxy midazolam concentrations (± the SD) over time after a 2-mg oral dose of midazolam.
Table 1.
Midazolam and 1-hydroxy midazolam pharmacokinetic parametersa
| Pharmacokinetic parameters | Mean ± SD (n) |
|
|---|---|---|
| Treatment A (reference): midazolam alone | Treatment B (test): midazolam + PA-824 | |
| Midazolam | ||
| Cmax (ng/ml) | 11.9 ± 5.46 (14) | 9.64 ± 3.43 (14) |
| AUC0–t (ng · h/ml) | 30.7 ± 15.3 (14) | 25.3 ± 10.50 (14) |
| AUC0–∞ (ng · h/ml) | 32.1 ± 15.7 (14) | 25.0 ± 9.56 (13) |
| CL/F (liters/h) | 75.8 ± 32.0 (14) | 91.5 ± 36.2 |
| Tmax (h) | 0.505 (0.499–1.00) (14) | 1.00 (0.499–1.00) (14) |
| t1/2 (h) | 5.69 ± 2.14 (14) | 5.44 ± 2.4400 (13) |
| 1-Hydroxy midazolam | ||
| Cmax (ng/ml) | 5.32 ± 2.48 (14) | 5.42 ± 2.17 (14) |
| AUC0–t (ng · h/ml) | 12.0 ± 5.23 (14) | 13.7 ± 6.24 (14) |
| AUC0–∞ (ng · h/ml) | 12.9 ± 5.82 (13) | 14.6 ± 7.73 (10) |
| Tmax (h) | 0.505 (0.499–1.00) (14) | 1.00 (0.499–1.00) (14) |
| t1/2 (h) | 4.09 ± 2.16 (13) | 4.45 ± 2.87 (10) |
Tmax results are presented as the “median (range)”. Other values are arithmetic means ± the standard deviations. n, number of subjects contributing data; Cmax, maximum observed concentration; Tmax, time at which Cmax occurs; t1/2, elimination half-life; AUC0–t, area under the concentration-time curve during the dosing interval; AUC0–∞, area under the concentration-time curve extrapolated to infinity.
The GMRs (90% CI) when midazolam was coadministered with PA-824 compared to when midazolam was administered alone were as follows: Cmax = 83.63% (75.11 to 93.11%), AUC0–t = 84.61% (74.21 to 96.47%), and AUC0–∞ = 84.45% (73.79 to 96.64%). The 90% CIs for the GMRs of AUCs and Cmax values were all within the predefined 50 to 200 and 70 to 143% “no-effect” limits; however, the upper limit of the CIs was <100%. The 90% CIs for the GMRs of the AUCs and Cmax values were not within the “no-effect” limit of 80 to 125%. The total extent (AUC) and peak (Cmax) exposure of midazolam decreased by ca. 15 to 16% when midazolam was coadministered with PA-824 compared to when midazolam was administered alone. Based on the nonparametric Wilcoxon signed-rank test, the midazolam Tmax (P = 0.891) and t1/2 (P = 0.893) were not different between both treatments.
As seen in Table 2, a statistical analysis of 1-hydroxy midazolam PK parameters demonstrated that the GMRs (90% CI) when midazolam was coadministered with PA-824 compared to when midazolam was administered alone were as follows: Cmax = 105.23% (93.13 to 118.9%), AUC0–t = 113.98% (105.53 to 123.09%), and AUC0–∞ = 112.68% (103.07 to 123.18%). The lower bound of the 90% CIs for the GMRs of the AUCs was >100% but all were within the “no-effect” limit of 80 to 125%. Based on the nonparametric Wilcoxon signed-rank test, the 1-hydroxy midazolam Tmax (P = 0.988) and t1/2 (P = 1.00) values were not different between both treatments.
Table 2.
Statistical comparisons of midazolam and 1-hydroxy midazolam pharmacokinetic parametersa
| Pharmacokinetic parameters | Geometric LS mean (n) |
% GMR | 90% CI | |
|---|---|---|---|---|
| Treatment A (reference): midazolam alone | Treatment B (test): midazolam + PA-824 | |||
| Midazolam | ||||
| Cmax (ng/ml) | 10.82 (14) | 9.05 (14) | 83.63 | 75.11–93.11 |
| AUC0–t (ng · h/ml) | 27.63 (14) | 23.38 (14) | 84.61 | 74.21–96.47 |
| AUC0–∞ (ng · h/ml) | 28.97 (13) | 24.47 (13) | 84.45 | 73.79–96.64 |
| 1-Hydroxy midazolam | ||||
| Cmax (ng/ml) | 4.76 (14) | 5.00 (14) | 105.23 | 93.13–118.9 |
| AUC0–t (ng · h/ml) | 10.86 (14) | 12.38 (14) | 113.98 | 105.53–123.09 |
| AUC0–∞ (ng · h/ml) | 11.57 (10) | 13.04 (10) | 112.68 | 103.07–123.18 |
n, number of subjects contributing data; Cmax, maximum observed concentration; AUC0–t, area under the concentration-time curve during the dosing interval; AUC0–∞, area under the concentration-time curve extrapolated to infinity; LS mean, least-squares mean; GMR, geometric mean ratio; CI, confidence interval.
Safety and tolerability.
PA-824 was well tolerated throughout the study and when coadministered with midazolam. No serious adverse events occurred. The only adverse events reported by more than one subject during any treatment period were headache, nausea, abdominal discomfort, and diarrhea. These events were reported more commonly by subjects during the periods when PA-824 was administered alone or in combination with midazolam. There was a trend toward increased serum creatinine concentrations during dosing with PA-824, a finding consistent with an earlier study that determined that higher doses of PA-824 do not affect renal function but may increase serum creatinine by inhibiting renal tubular secretion of creatinine, a clinically benign effect that has been seen with several marketed drugs (12). Mean (SD) serum creatinine concentrations were 0.785 (0.173) at check-in, 0.811 (0.184) when midazolam was dosed alone, 0.916 (0.175) after 7 days of once-daily dosing of PA-824, and 0.955 (0.189) after 14 days of once-daily dosing of PA-824 with coadministration of midazolam on day 14. All other mean laboratory parameters for serum chemistry, hematology, and urinalysis remained within reference range. There were no remarkable findings in the vital signs, ECGs, physical examinations, visual acuity tests, and slit-lamp examinations in this study.
DISCUSSION
When this study was designed, the clinical dose range in patients with TB had not been determined. The 400-mg PA-824 dose used here was chosen as the highest dose that might be used in future clinical studies. The 400-mg PA-824 dose was expected to produce the maximum change in midazolam exposure relevant to the clinic. Any effects observed at the 400-mg PA-824 dose level could be extrapolated to lower doses of PA-824, potentially requiring additional studies to appropriately characterize the drug interaction at lower clinical doses. The 50 to 200% limit for the 90% CI of the GMR was chosen as a “no-effect” criterion based on a clinical assessment that if dose adjustment of important drugs metabolized by CYP3A4 were required during coadministration of PA-824, then continued development of PA-824 as a therapy for TB would not be warranted. The 2-mg oral midazolam dose is at the lower range of that typically used in drug-drug interaction studies, but it was chosen due to concerns over the possible magnitude of CYP3A4 time-dependent inhibition after a 14-day dosing of PA-824.
The total extent (AUC) and peak (Cmax) exposure of midazolam decreased by ca. 16 and 15%, respectively, with the concomitant coadministration of midazolam and PA-824 400 mg compared to the administration of midazolam alone. The total extent (AUC) and peak (Cmax) exposure of 1-hydroxy midazolam increased 14 and 5%, respectively, with the concomitant coadministration of midazolam and PA-824 compared to the administration of midazolam alone. The 90% CI values for the GMRs of AUC0–t, AUC0−∞, and Cmax for midazolam and 1-hydroxy midazolam were all within the prespecified “no-effect” 50 to 200% limit and within the narrower prespecified 70 to 143% limit, whereas only the 1-hydroxy midazolam GMRs also fell within the default bioequivalence 80 to 125% limit. The relatively small decrease in midazolam exposure, together with the small increase in 1-hydroxy midazolam exposure observed when midazolam is coadministered, appears to be consistent with a small inductive effect by PA-824 on CYP3A4. This result appears inconsistent with in vitro data indicating that PA-824 is a weak inhibitor of CYP3A4 and exhibits metabolism-dependent inhibition. However, our in vitro metabolism data are too limited to indicate the likely mechanism. It has been reported that some metabolism-dependent inhibiters can form relatively stable PA-824 metabolite enzyme complexes resulting in decreased degradation of the enzyme itself and ultimately higher steady-state levels of active enzyme. Midazolam is known to exhibit allosteric activation of CYP3A4-mediated metabolism with some drugs. Allosteric activation involves the binding of an “activator” to the enzyme, resulting in an increased rate of metabolism. 1-Hydroxy midazolam forms a glucuronide metabolite. Glucuronide metabolites can be excreted by transporters in the kidney and other tissues. Increased serum creatinine concentrations were detected. It is possible that PA-824 inhibition of a common transporter for creatinine and 1-hydroxy midazolam might have led to the increased 1-hydroxy midazolam exposure that we observed. However, the decrease in midazolam exposure that we detected is not consistent with the inhibition of a renal transporter.
We demonstrated that PA-824 at the 400-mg dose does not produce changes in midazolam exposure that would require dose adjustment. Any changes in midazolam exposure that occur for the 100- and 200-mg PA-824 doses are likely to be even less than that observed for the 400-mg dose. Therefore, our findings indicate that PA-824 is not likely to affect the PK of CYP3A4-metabolized drugs to a clinically meaningful extent. Most importantly, concomitant use of PA-824 is not expected to produce significant drug interactions with antiretroviral drugs that are substrates of CYP3A4, such as the protease inhibitors. Overall, single doses of oral midazolam (2 mg) administered alone and then after multiple doses of PA-824 (400 mg daily for 14 days) with a single oral dose of midazolam (2 mg on day 14 of PA-824 dosing) were well tolerated by the subjects in our study.
ACKNOWLEDGMENTS
This study was supported by grants from the Rockefeller Foundation, the Development Cooperation Ireland, the Bill & Melinda Gates Foundation, the U.S. Agency for International Development, and the Dutch Ministry of Foreign Affairs.
We thank the staff of Celerion, Inc., and the study participants.
Footnotes
Published ahead of print 20 May 2013
REFERENCES
- 1. Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, Dowd CS, Lee IY, Kim P, Zhang L, Kang S, Keller TH, Jiricek J, Barry CE. 2008. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 322:1392–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, Anderson SW, Towell JA, Yuan Y, McMurray DNBN, Kreiswirth Barry CE, Baker WR. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962–966 [DOI] [PubMed] [Google Scholar]
- 3. Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, Sasaki H, Shimokawa Y, Komatsu M. 2006. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 3:e466. 10.1371/journal.pmed.0030466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nuermberger E, Tyagi S, Tasneen R, Williams KN, Almeida D, Rosenthal I, Grosset JH. 2008. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 52:1522–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, Venter A, Donald PR, van Niekerk C, Whitney K, Rouse DJ, Laurenzi MW, Ginsberg AM, Spigelman MK. 2010. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob. Agents Chemother. 54:3402–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diacon AH, Dawson R, du Bois J, Narunsky K, Venter A, Donald P, van Niekerk C, Erondu N, Ginsberg A, Becker P, Spigelman M. 2012. A phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob. Agents Chemother. 56:3027–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diacon AH, Dawson R, von Groote-Bidlinqmaier F, Symons G, Venter A, Donald PR, van Niekerk C, Everitt D, Winter H, Becker P, Mendel CM, Spigelman M. 2012. 14-Day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 380:986–993 [DOI] [PubMed] [Google Scholar]
- 8. CDC 2012. Grand rounds: the TB/HIV syndemic. MMWR Morb. Mortal. Wkly. Rep. 61:484–489 [PubMed] [Google Scholar]
- 9. Dooley KE, Kim PS, Williams SD, Hafner R. 2012. TB and HIV therapeutics: pharmacology research priorities. AIDS Res. Treat. 2012:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Food and Drug Administration 2006. Guidance for industry: drug interaction studies—study design, data analysis, and implications for dosing and labeling. Office of Training and Communications Division of Drug Information, document HFD-240. U.S. Department of Health and Human Services, FDA Center for Drug Evaluation and Research, Bethesda, MD [Google Scholar]
- 11. Stoch SA, Friedman E, Maes A, Yee K, Xu Y, Larson P, Fitzgerald M, Chodakewitz J, Wagner JA. 2009. Effect of different durations of ketoconazole dosing on the single-dose pharmacokinetics of midazolam: shortening the paradigm. J. Clin. Pharmacol. 49:398–406 [DOI] [PubMed] [Google Scholar]
- 12. Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. 2009. Assessment of the effects of the nitroimidazo-oxazine PA-824 on renal function in healthy subjects. Antimicrob. Agents Chemother. 53:3726–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]


