Abstract
We previously showed that a prototype gel comprising zinc acetate (ZA) in carrageenan (CG) protected mice against vaginal and rectal herpes simplex virus 2 (HSV-2) challenge as well as macaques against vaginal simian-human immunodeficiency virus reverse transcriptase (SHIV-RT) challenge. In this work, we modified buffers and cosolvents to obtain a stable, nearly iso-osmolal formulation and evaluated its safety and efficacy against SHIV-RT and HSV-2. In vitro toxicity to lactobacilli and Candida albicans was determined. Macaques were given daily doses of ZA and CG (ZA/CG) or CG alone vaginally for 14 days and challenged with SHIV-RT 24 h later. Mice were challenged vaginally or rectally with HSV-2 immediately after a single gel treatment to measure efficacy or vaginally 12 h after daily gel treatment for 7 days to evaluate the gel's impact on susceptibility to HSV-2 infection. The modified ZA/CG neither affected the viability of lactobacilli or C. albicans nor enhanced vaginal HSV-2 infection after daily ZA/CG treatment. Vaginal SHIV-RT infection of macaques was reduced by 66% (P = 0.006) when macaques were challenged 24 h after the last dose of gel. We observed 60% to 80% uninfected mice after vaginal (P < 0.0001) and rectal (P = 0.008) high-dose HSV-2 challenge. The modified ZA/CG gel is safe and effective in animal models and represents a potential candidate to limit the transmission of HIV and HSV-2.
INTRODUCTION
Several recent clinical trials have shown that microbicides containing antiretroviral drugs (ARVs) reduce the sexual transmission of HIV and other sexually transmitted diseases (STIs) (1). The Centre for the AIDS Program of Research in South Africa (CAPRISA) 004 trial (CAPRISA-004) showed that pericoital use (before and after sex) of vaginally applied 1% tenofovir (TFV) gel reduced human immunodeficiency virus type 1 (HIV-1) and herpes simplex virus 2 (HSV-2) acquisition by 39% and 51%, respectively (2). Similarly, three oral pre-exposure trials (iPrex, Partners PrEP, and TDF2) targeting men who have sex with men (MSM) and transgender women, HIV-serodiscordant couples, or heterosexual men and women demonstrated between 44% and 73% efficacy (1). Conversely, a lack of efficacy was seen in two trials (FEM-PrEP and VOICE) in which women received daily oral emtricitabine-TFV and TFV (1, 3). A recent analysis of these two studies suggested that the lack of efficacy could have been due to poor regimen adherence (1, 3, 4).
Although ARV-containing topical microbicides will likely be available soon, there are important questions regarding their use. (i) Does topical administration of an ARV in people with an undiagnosed HIV infection lead to resistance development and affect their treatment outcomes? (ii) How accessible will these products be (i.e., will they be available over the counter or by prescription only)? (iii) Can the introduction of an HIV-specific microbicide increase the transmission of other STIs?
Given these unanswered questions and the potential limitations of ARV-containing topical microbicides, we are seeking to develop broad-spectrum, non-ARV-based topical microbicides that are safe, effective, and accessible (5). We recently reported the in vivo efficacy against simian-human immunodeficiency virus reverse transcriptase (SHIV-RT) vaginal challenge of a microbicide gel comprising 50 μM MIV-150 (a nonnucleoside reverse transcriptase inhibitor [NNRTI]), 14 mM zinc acetate dihydrate (ZA), and 3% carrageenan (CG) (6). This gel significantly protected macaques (89% [P < 0.0002 versus the CG placebo]) when animals were challenged 24 h after the last dose of a 14-day daily dosing regimen. Moreover, a CG gel containing only 14 mM ZA (ZA/CG) afforded 70% protection in this model (P < 0.017) (6). Additionally, we have shown that ZA and CG act synergistically to significantly protect mice (75% to 85% uninfected [P < 0.0001]) against high-dose (106 PFU) HSV-2 challenge (7).
In order to advance a non-ARV formulation that has potential activity against both HIV and HSV-2, we needed to optimize the buffers and cosolvents of the ZA/CG gel to render them appropriate for human use and confirm its safety and efficacy in preclinical models. Our results demonstrate that the modified ZA/CG gel is safe and effective and support the notion that the gel is a potential non-ARV microbicide suitable for evaluation in clinical trials.
MATERIALS AND METHODS
Cells and virus culture.
Caco-2 cells (ATCC, Rockville, MD) were grown and differentiated using a BioCoat HTS Caco-2 assay system (BD Biosciences, Bedford, MA) as previously described (7).
Lactobacillus jensenii and Lactobacillus crispatus (ATCC) stocks were prepared as recommended by the ATCC. Briefly, the stocks were grown in Lactobacilli MRS broth (Fisher Scientific, Suwanee, GA) for 24 or 48 h at 37°C and 5% CO2. The aliquots of lactobacilli were frozen at −80°C in Lactobacilli MRS broth containing 15% glycerol (Sigma, St. Louis, MO).
Candida albicans (strain SC5413; ATCC) was propagated weekly by streaking a yeast extract-peptone-dextrose (YPD) agar (Sigma) plate. A single colony was picked and incubated in 5 ml of Sabouraud dextrose broth (SB; Sigma) overnight at 30°C with shaking at 150 rpm.
HSV-2 strain G (ATCC) was propagated in Vero cells (ATCC), and titers were determined using the plaque formation assay on Vero cells as previously described (8). Aliquots of virus stock were stored at −80°C.
The original SHIV-RT stocks (provided by Disa Bottiger, Medivir AB, Sweden) were grown in phytohemagglutinin (PHA)-activated human peripheral blood mononuclear cells (PBMCs). Stock titers were redetermined using the 174xCEM cell line (NIH AIDS Research & Reference Reagent Program), and the 50% tissue culture infective dose (TCID50) was calculated using the Reed and Muench formula. The virus was stored at −80°C.
Formulation. (i) CG.
For step 1, a 4-liter double planetary mixer (Charles Ross & Son, Hauppauge, NY) was charged with 2,829 g of sterile filtered water and 3.923 g of sodium acetate trihydrate (Sigma). The solution was heated for 5 min at 69°C with stirring at 40 rpm. CG (102 g, 3.4% final concentration) was added, and the mixture was stirred for 3 h at 69°C. For step 2, after the formulation was cooled to 25°C, a solution of 6 g of methyl paraben (Spectrum, Gardena, CA)–60 ml of propylene glycol (Sigma) was added, and the solution was stirred for an additional 1 h at 40 rpm. The formulation pH was adjusted to 6.8, and the reaction volume was stirred for 15 min under vacuum conditions to remove bubbles. The following lot numbers were used in our studies: 110408A525ML and 110512A525MR.
(ii) ZA/CG.
For step 1, a protocol similar to that used to make CG was used to generate ZA/CG with the following changes: 894 ml of 10 mM sodium acetate buffer (pH 6.4) was used. CG (31 g) was added to reach a final concentration of 3%. A solution of 3 g zinc acetate dihydrate-50 ml sodium acetate buffer was added, and the solution was stirred for an additional 20 min at 40 rpm. A mixture of 2 g methyl paraben–20 ml propylene glycol was used. The following lot numbers were used in our studies: 110411A707ML, 110609A707ML, and 110609A707MR.
Methyl paraben content, zinc content, and physiochemical properties (osmolality, pH, simple viscosity) were determined using established methods (7).
Stability studies.
Aliquots (25 g) of gel were stored in 30-ml polypropylene bottles (Qorpak, Bridgeville, PA) under the following conditions: 30°C/65% relative humidity (RH), 40°C/75% RH, and 50°C/ambient humidity. Bottles were removed periodically, and the gel was analyzed for methyl paraben content, osmolality, pH, viscosity, and zinc content (7).
Transepithelial electrical resistance (TEER).
The assay was performed as previously described (9), except that the medium was replaced on the day of the assay with fresh Dulbecco's modified Eagle's medium (DMEM) without phenol red (Invitrogen, Grand Island, NY) and supplemented with MITO+ Serum Extender (BD Biosciences). All formulations were diluted 1:10 in the media described above, and 300 μl was applied in triplicate in the upper chambers. Resistance readings were performed at 0, 2, 4, and 6 h.
In vitro toxicity against lactobacilli and C. albicans.
We followed the procedure described by Moncla et al. (10) to evaluate the impact of ZA/CG on lactobacillus viability. Briefly, diluted ZA/CG and CG gels and penicillin/streptomycin (Invitrogen) were prepared in saline solution with 7.5% fetal bovine serum (FBS) (in addition to a control saline solution with 7.5% FBS only) and incubated with L. jensenii or L. crispatus at a density of 2 McFarland units (∼2 × 108 bacteria/ml) at 37°C, 5% CO2, and 98% humidity for 30 min. The samples were then plated on LBS agar (BD Biosciences) plates and incubated at 37°C, 5% CO2, and 98% humidity for 48 to 72 h. After 48 to 72 h, colonies were quantified in a Colony Doc-it Imaging station (UVP, Upland, CA). Minimum cidal dilutions that reduced viability by 99.99% (MCC99.99) were determined (11).
Washed C. albicans yeasts (1.6 × 105) were incubated with or without gels for 2 h at 30°C with shaking at 150 rpm in a total volume of 200 μl of SB. Fresh SB (2 ml) was then added to the culture, and 40 μl of the resulting mixture was added to 160 μl of fresh SB. That mixture (100 μl of a 1:10 dilution) was streaked on YPD agar plates and incubated at 37°C overnight, and the numbers of colonies were quantified in a Colony Doc-it Imaging station. The rest of the mixture was incubated overnight at 30°C with shaking at 150 rpm, and viable yeasts were quantified by trypan blue exclusion. The lack of differentiated hyphae in the yeast SB cultures (distinguished by their distinct morphologies) was also verified by light microscopy. The lactobacilli and C. albicans were considered sensitive to killing if the formulations cause a viability reduction equal to or greater than 1 log10 (11).
Macaque treatments and SHIV-RT challenge.
Housing and care of adult female Indian rhesus macaques (Macaca mulatta) complied with the regulations under the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals at the Tulane National Primate Research Center (TNPRC; Covington, LA) (12, 13). All studies were approved by the Animal Care and Use Committee of the TNPRC (OLAW Assurance no. A4499-01), complied with animal care procedures, and were performed in a facility fully accredited by the Association for Accreditation of Laboratory Animal Care (AAALAC no. 000594). Veterinarians at the TNPRC Division of Veterinary Medicine monitored animals regularly to minimize any distress or pain (14). The animals ranged in age from 4 to 12 years and weighed 4 to 10 kg. Animals tested negative for simian type D retroviruses, simian T cell leukemia virus 1, and simian immunodeficiency virus (SIV) prior to use in the efficacy studies. No more than 10 ml/kg of body weight/month EDTA blood was drawn from the animals at the indicated time points pre- and post-virus challenge. Preemptive and postprocedural analgesia were required for procedures that might cause more than momentary pain or distress in humans undergoing the same procedures. Five weeks before vaginal virus challenge, animals received a single 30 mg intramuscular (i.m.) injection of medroxyprogesterone acetate (Depo-Provera [depo]; Pfizer, New York, NY).
depo-treated macaques were atraumatically dosed vaginally with 2 ml of ZA/CG or CG using a pliable French catheter (Covidien, Mansfield, MA) for 14 consecutive days. At 24 h after the last application, they were vaginally challenged with 0.5 ml of 103 TCID50 SHIV-RT (SIVmac239 and HIV-1HxB2 RT). A supine position was maintained for all animals for 20 to 30 min postchallenge to allow absorption of virus. Monkeys were anesthetized with ketamine-HCl (10 mg/kg) or tiletamine-zolazepam (8 mg/kg) prior to all procedures. Individual animal information is summarized in Table 1. Blood samples were transported overnight from the TNPRC to our laboratories at the Population Council for processing and analysis.
Table 1.
Summary of challenged-macaque results
| Animal no. | Gel | Reactivitya |
||
|---|---|---|---|---|
| Plasma viral loadb | SIV DNA in PBMCc | SIV Abd | ||
| GE23 | CG | + | + | + |
| DD29 | CG | + | + | + |
| GM30 | CG | − | − | − |
| IR45 | CG | − | − | − |
| EF46 | CG | + | + | + |
| BC84 | ZA/CG | − | − | − |
| DE94 | ZA/CG | + | + | + |
| FT48 | ZA/CG | − | − | − |
| EE72 | ZA/CG | − | − | − |
| DR51 | ZA/CG | + | + | + |
| GC25 | ZA/CG | − | − | − |
| FH27 | ZA/CG | − | − | − |
+, reactive; −, nonreactive.
Plasma viral RNA status.
PBMC SIV DNA was tested from week 2 to week 8.
ELISA for Abs to SIV was performed at weeks 4 and 8 (compared with baseline).
Measuring SHIV-RT infection. (i) SIVgag PCR.
Peripheral blood mononuclear cells (PBMCs) were isolated as previously reported (6). Dry cell pellets (5 × 106 cells) were frozen and stored at −80°C until needed. Pellets were lysed and nested PCR was performed to determine the presence of SIV gag DNA (14).
(ii) RT-PCR.
Plasma viral RNA copy numbers were determined by quantitative RT-PCR (15). Animals were defined as infected when >103 RNA copies/ml were recorded in at least 2 consecutive samples within the 20-week postinfection follow-up period.
(iii) SIV Ab responses.
SIV-specific antibodies (Abs) were monitored by enzyme-linked immunosorbent assay (ELISA) (6, 16). Ab positivity was defined as positive OD values at 4 to 8 weeks postchallenge.
Mouse treatment and HSV-2 challenge.
All animal care procedures complied with the regulations detailed under the Animal Welfare Act (13) and the Guide for the Care and Use of Laboratory Animals (12). Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Comparative Bioscience Center (CBC) at Rockefeller University. For vaginal applications, mice were depo-treated 7 days prior to gel dosing and challenge. For rectal applications, the animals were fasted for 24 h and anesthetized (Ketamine/Xylazine) prior to gel and virus application.
Histological evaluation of the cervicovaginal and rectal mucosae after single-dose gel application.
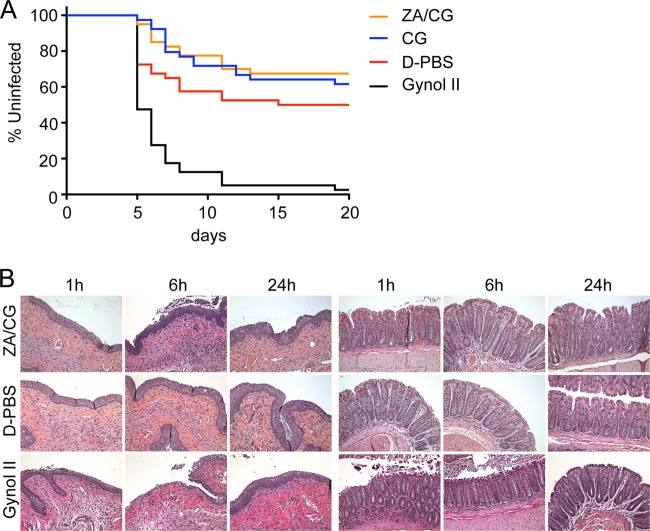
Mice received gel, Dulbecco's phosphate buffered saline (D-PBS), or Gynol II applied vaginally (10 μl) or rectally (20 μl). The mice were sacrificed 1, 6, or 24 h later, and vaginal or rectal tissue was collected, processed, and analyzed as previously described (7).
Vaginal and rectal HSV-2 challenge.
As previously described (7), gel was applied vaginally (10 μl) or rectally (20 μl) before virus challenge 10 min later with 10 μl of HSV-2 strain G (106 PFU/mouse). Placebo (D-PBS) and CG gel groups were included in all studies. Beginning 4 days after virus inoculation, mice were examined and scored daily for 20 days in total. Animals with signs of infection (e.g., hind limb paralysis, erythema, hair loss, and swelling in the vaginal area) were deemed infected and euthanized.
HSV-2 increased-susceptibility model.
As described before (7), depo-treated mice had 10 μl of gel (versus D-PBS, CG, and Gynol II) applied daily vaginally for 7 days and were challenged 12 h after the last dose with 10 μl of 2 × 103 PFU of HSV-2 strain G. Mice were examined and scored as described in the preceding section.
Statistical analyses.
Fisher's exact test was used for statistical comparison of the percentages of SHIV-RT-infected animals in the differently treated groups as well as in the comparison of signs of infection in the HSV-2 mouse models (GraphPad Prism 5.02; GraphPad Software, San Diego, CA). P values < 0.05 were taken as statistically significant. In the macaque studies, we combined real-time control data with that from historical controls as a way of increasing our power to detect a difference between control and test groups while using fewer controls in each study, as is commonly done in macaque studies (17, 18). This is possible because we consistently observe the same frequency of infection and viremia profile in the real-time control groups across studies (6, 19–22).
RESULTS
ZA/CG is safe in vitro and in vivo.
Having previously demonstrated that a prototype ZA/CG gel was safe and effective in vitro and in vivo, we developed a modified ZA/CG formulation to render it suitable for clinical testing by adjusting buffers, cosolvents, and preservatives. The modified gel contains 14 mM ZA, 3% CG, 10 mM sodium acetate, 2.0% propylene glycol, and 0.2% methyl paraben and is nearly iso-osmolal (350 to 550 mosmol/kg). Table 2 compares side by side the physicochemical properties of the modified gel versus prototype gels used in previous studies (6, 7). We examined the modified ZA/CG gel in a battery of in vitro and in vivo assays to determine any potential safety concerns that might have arisen after the optimization process.
Table 2.
Formulation attributes after long-term stability testinga
| Property | Prototype ZA/CG gel | Modified ZA/CG gel |
|---|---|---|
| Carrageenan content | 3.0% | 3.1% |
| Lambda/kappa carrageenan ratio | 95:5 | 60:40 |
| Buffer | None | 10 mM sodium acetate |
| Cosolvent | None | 2% propylene glycol |
| Zinc acetate content (13 to 15 mM) | 14 mM | 14 mM |
| Methyl paraben content (0.18 to 0.22 wt %) | 0.2 wt % | 0.2 wt % |
| pH (6.9 to 6.3) | 6.96 | 6.64 |
| Osmolality (350–500 mOs/kg) | NT | 428 |
| Viscosity, t = 0 (23,500 to 35,000 cP) | 25,300 cP | 28,800 cP |
| Viscosity (3 mos at 30°C/65% RH) | NT | 30,600 cP |
| Viscosity (6 mos at 30°C/65% RH) | NT | 33,300 cP |
| Viscosity (9 mos at 30°C/65% RH) | NT | 32,200 cP |
| Viscosity (12 mos at 30°C/65% RH) | NT | 34,300 cP |
| Viscosity (3 mos at 40°C/75% RH) | NT | 24,500 cP |
| Viscosity (6 mos at 40°C/75% RH) | NT | 24,800 cP |
| Viscosity (9 mos at 40°C/75% RH) | NT | 23,900 cP |
| Viscosity (1 mo at 50°C/ambient) | NT | 30,500 cP |
Formulations were placed in 30-ml polypropylene bottles. The bottles were capped and sealed and then stored at 30°C/65% RH, 40°C/75% RH, and 50°C/ambient humidity. The gels were analyzed at indicated times for pH, viscosity, osmolality, methyl paraben (MP) content, and zinc content. The acceptable ranges for each parameter are indicated in the Property column. All parameters stayed within specifications over 12 months at 30°C/65% RH, 9 months at 40°C/75% RH, and 1 month at 50°C/ambient humidity. NT, not tested.
Unlike Gynol II, which destroyed the Caco-2 cell monolayer and reduced TEER values within 30 min, the ZA/CG gel had no affect on TEER values for up to 6 h (Fig. 1A). Lactobacillus and C. albicans viability was not affected (<1 log10 reduction) after incubation with diluted (1:10) ZA/CG (Fig. 1B and C). Additionally, by counting the yeasts (Fig. 1C), we verified that C. albicans did not convert from the unicellular yeast-like form into the pathogenic multicellular filamentous form.
Fig 1.
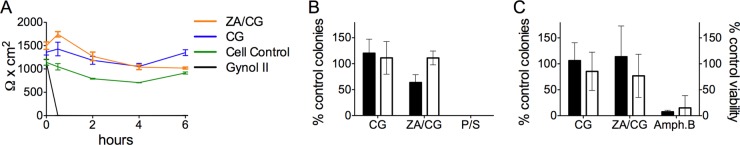
Modified ZA/CG is safe in vitro. (A) Differentiated Caco-2 cell monolayers were treated with 1:10-diluted formulations for 0 to 6 h before TEER values (triplicates for each sample at each time point) were measured. The graph shows the means ± standard deviations (SD) for each formulation (two independent experiments). (B) L. jensenii (filled bars) and L. crispatus (empty bars) were treated with 1:10-diluted CG or ZA/CG versus penicillin/streptomycin (P/S) (P, 100 U/ml; S, 100 μg/ml) and saline solution with a 7.5% FBS control (to set 100% viability) for 30 min at 37°C, 5% CO2, and 98% humidity. The samples were then plated in triplicate in LBS agar and incubated at 37°C, 5% CO2, and 98% humidity for 48 to 72 h before counting the colonies. The graph shows means ± SD of the results of three independent experiments. The SD was calculated by pooling the results of three replicates from each independent experiment. (C) C. albicans yeasts (1.6 × 105) were incubated for 2 h at 30°C in 1:10-diluted CG or ZA/CG versus 50 μg/ml amphotericin B (Amph. B) or SB alone. (Left axis [filled bars]) Diluted aliquots were plated on YPD agar plates and cultured overnight at 37°C. The number of colonies was counted for each condition and is shown as the percentage of colonies relative to the SB control (set as 100%). (Right axis [empty bars]) The samples cultured for 2 h were diluted 40-fold in SB and incubated overnight at 30°C in a 96-well flat-bottom plate. The numbers of viable yeasts were counted, and the percentages relative to the SB-treated controls are shown. No multicellular filamentous forms were seen under any condition. Means ± SD of the results of 5 independent experiments are summarized. The SD was calculated by pooling the results of three replicates from each independent experiment.
The HSV-2 susceptibility model in mice was used as an in vivo functional measurement of safety. By using a suboptimal inoculum of 2 × 103 PFU that infects only 50% of control (D-PBS-treated) mice, we can detect significant enhancement of infection as a result of the pretreatment regimen (7). Neither ZA/CG nor CG enhanced the susceptibility of mice to vaginal HSV-2 infection, whereas Gynol II did (P < 0.0001; Fig. 2A). Further support for the idea of mucosal safety was provided by the histological examination of vaginal and rectal mucosae after gel application, which revealed, at most, minor epithelial sloughing in the vaginal and rectal epithelia at 6 h after gel application, with the lamina propria remaining intact at all time points (Fig. 2B). As expected, Gynol II damaged the tissues (including exposure of the lamina propria) within 1 to 6 h of treatment (repaired by 24 h).
Fig 2.
In vivo treatment with ZA/CG does not damage the epithelia. (A) depo-treated BALB/c mice (n = 40 per group) were treated daily for 7 days with each formulation. Twelve hours after the last gel application, mice were challenged with 2 × 103 PFU of HSV-2 strain G. The percentage of uninfected mice over 21 days is shown for each formulation. There was no significant difference between the ZA/CG, CG, and D-PBS results (P > 0.12). A significant difference was observed in comparisons of Gynol II to D-PBS, ZA/CG, or CG gel (P < 0.0001). (B) depo-treated (vaginal dosing) and fasted (rectal dosing) mice had ZA/CG, D-PBS, or Gynol II applied before the mice were euthanized 1, 6, or 24 h later. The entire reproductive or rectal tract was surgically excised for morphological analyses. The panels represent a sample of the 6 sections of two or three animals that were tested per formulation for each site. The pictures were obtained with ×20 magnification.
ZA/CG significantly reduces vaginal SHIV-RT infection in macaques.
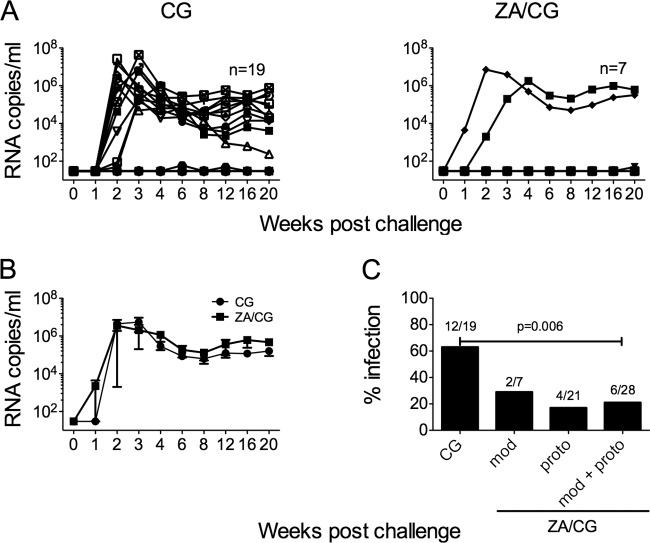
Table 3 summarizes the results obtained in the vaginal SHIV-RT microbicide efficacy macaque model using the modified ZA/CG gel compared with published efficacy data of the prototype ZA/CG gel (6). Studies with the prototype ZA/CG gel showed that repeated dosing (daily or every other day) provided significant protection against vaginal SHIV-RT infection when macaques were challenged 8 to 24 h after the last gel dose (70% protection; 4/21 infected versus 9/14 infected in the CG group; P < 0.017) (6), while a single dose appeared less effective (20). The 70% protection (4/21 infected) achieved with the prototype gel reflects the combination of different times of gel treatment versus SHIV-RT challenge. If we take into consideration only the results related to the conditions used for testing the modified gel (repeated dosing daily with virus challenge 24 h after last dose), the modified and prototype gels show the same infection frequency (2 of 7 animals; Table 3). As with previous CG- or placebo intravaginal ring-treated animals (6, 19, 21, 22), 3 of 5 animals (60%) in the real-time CG control group became infected. These real-time CG data were pooled with historical CG control data, giving a 63% infection frequency in CG-treated animals (12/19 infected). In contrast, the 2 of 7 animals infected after repeated treatment with the modified ZA/CG gel (Fig. 3A and Table 3) represent 53% protection compared to CG-treated control results.
Table 3.
Summary of the efficacy data in the SHIV-RT macaque model testing prototype and modified gelsa
| Gel | Version | No. of applications | Gel dosing relative to challenge (h) | Protection vs CG gel (%) | No. of infected macaques/no. of challenged macaques | P value vs CG gel | ||
|---|---|---|---|---|---|---|---|---|
| ZA/CGb | Prototype | 14 daily | 8 | 78 | 70 | 1/7 | 4/21 | 0.0089 |
| ZA/CGb | Prototype | 14 daily | 24 | 53 | 2/7 | |||
| ZA/CGb | Prototype | 14 EOD | 24 | 78 | 1/7 | |||
| CGb | Prototype | 14 daily | 8–24 | N/A | 9/14 | N/A | ||
| ZA/CG | Modified | 14 daily | 24 | 53 | 2/7 | 0.1354 | ||
| CG | Modified | 14 daily | 8–24 | N/A | 3/5 | N/A | ||
| ZA/CG | Poolc | 14 daily | 24 | 66 | 6/28 | 0.006 | ||
| CG | Poold | 14 daily | 8–24 | N/A | 12/19 | N/A | ||
EOD, every other day; N/A, not applicable.
Published data (6).
Pool of the results obtained with the prototype and modified ZA/CG gel (application adaily or EOD for 14 days, with SHIV-RT challenge 8 to 24 h after the last gel application).
Pool of the results obtained with the prototype and modified CG gel (application daily for 14 days, with SHIV-RT challenge 8to 24 h after the last gel application).
Fig 3.
Repeated dosing of the ZA/CG gel offers protection against vaginal SHIV-RT infection for up to 24 h. ZA/CG (n = 7) or CG (n = 3 real-time controls that have been added to 16 historical controls; n = 19) was applied daily for 14 days to depo-treated macaques before they were challenged vaginally with 103 TCID50 SHIV-RT 24 h later. (A) Plasma viral loads were measured over time (SIV RNA copies/ml of plasma), and each symbol denotes an individual animal. (B) Means (± SEM) of SIV RNA copies/ml for the animals that became infected in the two groups. (C) Percent infection in each of the different treatment groups: modified (mod) data, prototype (proto) data, and the combined data from the modified and prototype (mod/proto) ZA/CG groups versus the CG group are shown. The number of animals infected versus the number of animals challenged in each group is indicated above each bar.
The infected animals in all groups showed typical plasma viremia profiles, with mean peak levels of 4.5 × 106 (± 5.5 × 105) RNA copies/ml 2 to 3 weeks after SHIV-RT challenge and mean set point viral loads of 2.6 × 105 (± 1 × 105) RNA copies/ml being reached by week 12 (Fig. 3B). Animals designated uninfected showed no detectable viral RNA over the course of 20 weeks of follow-up (Table 1 and Fig. 3). Since the infection frequencies in animals treated with prototype (4/21 infected) versus modified (2/7 infected) ZA/CG were not significantly different (P = 0.5949), we combined the results from the two groups (6/28 animals infected; 21% infected). With greater power to compare the infection frequencies in the ZA/CG and CG groups (6/28 versus 12/19 infected), we confirmed that ZA/CG afforded significant (66%) protection even when we challenged macaques 24 h after the last gel dose (P = 0.006; Fig. 3C). Only infected animals developed SIV-specific Ab responses (Table 1).
Protection against high-dose vaginal and rectal HSV-2 infection in mice.
We previously showed that the prototype ZA/CG gel is safe and effectively blocks HSV-2 infection in stringent, high-viral-dose (106 PFU) vaginal and rectal mouse models (7). We used this system to determine if the modified ZA/CG was comparably effective against high-dose HSV-2 challenge across the vaginal and rectal mucosae. Modified ZA/CG significantly increased the number of animals without signs of infection after vaginal (80%, P < 0.0001 versus D-PBS or CG) and rectal (60%, P = 0.008 and P = 0.03 versus D-PBS and CG, respectively) challenge (Fig. 4).
Fig 4.
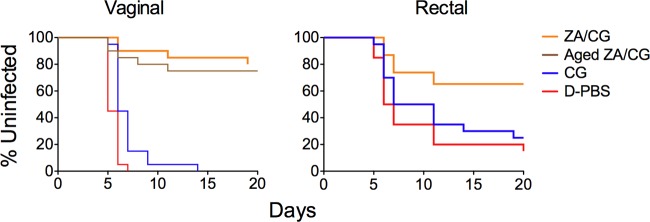
ZA/CG prevents high-dose HSV-2 infection. depo-treated (vaginal) or fasted (rectal) BALB/c mice were challenged with 106 PFU HSV-2 10 min after application of the indicated formulations (20 animals per formulation). The aged ZA/CG (7 months at 40°C) was also applied in the vaginal study. The percentages of uninfected mice over time, based on signs of infection, are shown for each treatment group. ZA/CG gels, including the sample kept at 40°C for 7 months, are significantly more protective than CG (P < 0.0001 for vaginal data and P = 0.03 for rectal data) or D-PBS (P < 0.0001 for vaginal data and P = 0.008 for rectal data).
ZA/CG gel is stable.
Having demonstrated that the (freshly prepared) modified ZA/CG gel was safe and effective, we finally verified that the formulation remained stable over time. The modified ZA/CG gel was stable for 12 months at 30°C/65% RH (relative humidity) and 9 months at 40°C/75% RH (Table 2). Temperature-dependent changes in viscosity were evident over time but remained within the acceptable ranges. Gel rheological measurements of viscosity versus shear rate coupled with computational modeling have predicted optimal vaginal spreading of gels with this range of viscosity (7). Samples stored at 30°C/65% RH became more viscous whereas samples stored at 40°C/75% RH became less viscous over time. Even after 1 month at 50°C, ZA/CG remained stable (Table 2).
To evaluate the effect of prolonged storage on gel efficacy, we tested ZA/CG aged at 40°C/75% RH for 7 months for its ability to prevent high-dose HSV-2 infection in mice. The aged ZA/CG retained its activity against high-dose vaginal HSV-2 challenge (P < 0.0001 versus CG gel; Fig. 4).
DISCUSSION
We are seeking to develop safe and effective non-ARV microbicides that target HIV and other STIs and that could be used vaginally and rectally to reduce HIV/HSV-2 transmission (5) and potentially limit the selection of ARV-resistant HIV that may compromise the treatment options. However, the full consequences of using ARV-based preventatives for the potential spread of drug-resistant viruses are not known, and HIV drug resistance in the context of prevention could be much less prevalent than in treatment settings (23, 24).
Differing from the ZA/CG prototype gel, the modified ZA/CG gel is nearly iso-osmolal and buffered and contains a cosolvent, making this formulation more suitable for clinical testing. Earlier we showed that prototype ZA/CG was effective against HSV-2 in mice and SHIV-RT in macaques. In mice, ZA/CG protected against high-dose vaginal and rectal HSV-2 infection, and even the higher dose gel (23 mM ZA) was safe (7). Notably, the ZA/CG combination was synergistic against HSV-2 (7). CG represents an excellent delivery vehicle for ZA. It has been shown to be safe and acceptable in numerous preclinical and clinical studies (7, 19, 22, 25–32) despite contradictory observations using in vitro systems that CG can enhance HIV infection (22, 33). Additionally, CG may have activity against HPV (34–36). Because the 14 mM ZA formulation (but not lower concentrations) afforded efficacy comparable to that of the 23 mM ZA formulation in mice (7), we advanced the 14 mM ZA/CG gel for macaque testing. In macaques, ZA/CG reduced vaginal SHIV-RT infection even when animals were challenged up to 24 h after the last dose of gel was applied (6, 20). Based on these data, we decided to develop a modified (i.e., safe, effective, and stable) 14 mM ZA/CG gel that we could advance to clinical testing.
The modified, nearly iso-osmolal ZA/CG formulation (14 mM ZA, 3.1% CG, 10 mM sodium acetate, 2.0% propylene glycol, 0.2% methyl paraben) is stable for at least 12 months at 30°C/65% RH (and 9 months at 40°C/75% RH). Modified ZA/CG is safe in vitro and in vivo, showing no lower TEER values after 6 h and only minor epithelial sloughing. Additionally, modified ZA/CG did not affect the viability of lactobacilli and C. albicans in vitro. Our data underscore the promise of this formulation for rectal and vaginal use. As suggested from the original mouse studies in which prototype ZA/CG gels were tested and resulted in a significant increase of mice without signs of infection (vaginal, 75% to 85%; rectal, 60% to 80%) (7), modified ZA/CG protected mice against both vaginal (75 to 80% uninfected) and rectal (60% uninfected) HSV-2 infection. Moreover, modified ZA/CG reduced vaginal SHIV-RT infection of macaques even when the animals were challenged 24 h after the last dose of gel.
How ZA works against both immunodeficiency viruses and HSV-2 remains to be fully elucidated. A recent publication has shown that Zn2+ may compete with Mg2+ in the active site of the HIV reverse transcriptase (RT), resulting in the formation of a stable RT-primer-template complex with diminished catalytic activity (37). We (J. A. Fernández-Romero, M. Hsu, and M. Robbiani, unpublished data), and others (6, 7, 20, 38, 39), have demonstrated that ZA has some antiviral activity in vitro and in vivo. Specifically, we have observed reduced HIV infection when virus was either pretreated with ZA or when ZA was added early in the cycle of replication, possibly supporting the notion that RT may be a target. Notably, the in vivo activity (especially for repeatedly applied ZA/CG [6]) appears more pronounced than its in vitro antiviral effects. Zinc is known to have immunomodulatory properties and, therefore, ZA may make the mucosal environment more impermeable to infection by modulating immune function (38–41). Repeated dosing of ZA may more effectively elicit this effect to sustain a putative antiviral state. Immunomodulatory effects of ZA on macaque and human mucosal tissues have been observed (L. Ouattara, G. Villegas, and N. Teleshova, personal communication), suggesting that these may contribute to the in vivo antiviral effects. Increased levels of zinc in tissues may also result from repeated application of ZA/CG, leading to greater efficacy (through antiviral and/or immunomodulatory mechanisms). Several papers have shown that zinc salts are active against HSV-2 laboratory and primary isolates (42, 43). The mode of action seems to be related to binding of zinc to the virion surface glycoproteins, a process that leads to inhibition of virus entry into target cells (43). Antiviral activity against other pathogens has been widely documented (44–46).
ZA has been generally recognized as safe (GRAS), and no toxic effects have been seen in rabbits after daily vaginal dosing with 90 mM ZA or zinc sulfate-loaded sponges for 10 days (47, 48). However, some recent data have shown adverse effects of zinc salts after nasal or vaginal administration (49, 50). Both studies show these effects only at extremely high doses (200 mM for vaginal administration) that far exceed those in our formulation. Furthermore, our 0.3% ZA/CG formulation (ZA at 3 mg/ml) may release amounts of zinc much lower than the oral dietary allowances of 8 to 13 mg per day as recommended by the Food and Nutrition Board (7, 51).
A number of non-ARV formulations are currently in preclinical or clinical testing (5): VivaGel (SPL7013 dendrimer) (52), Praneen (Polyherbal tablet) (53), Griffithsin (GRFT red algal lectin) (54, 55), aptamers (56, 57), and Mabgel (monoclonal antibodies) (58). ZA/CG, like most of the non-ARV candidates mentioned above, is a safe, nearly iso-osmolal formulation with proven activity against HIV. One advantage of ZA/CG versus some of the other candidates is the broad spectrum of the activity (HIV, HSV-2, and potentially HPV). Although VivaGel has shown a broad spectrum of activity against HIV and HSV-2 similar to that of ZA/CG, the potency and durability of the antiviral effect could be inferior to those of ZA/CG formulations (52, 59–62). VivaGel has shown anti-SHIV activity when applied only 20 min before challenge with 2,500 TCID50 SHIV89.6P (60), and the anti-HSV-2 window of protection shows 75% to 88% protection when applied up to 30 min before and 27% protection when applied 1 h before HSV-2 at 104 PFU (63). VivaGel is also claimed to exhibit anti-HPV activity, but there are no published reports available to compare its activity with the strong in vitro and in vivo anti-HPV activity of carrageenan (32–34).
The potential broad-spectrum activity of ZA/CG, coupled with the low cost, safety, and extended window of protection shown in macaque studies, positions the ZA/CG formulation as a strong, non-ARV microbicide candidate that warrants further development.
ACKNOWLEDGMENTS
We thank the veterinary staff at the TNPRC and CBC at Rockefeller University for continued support.
This study was made possible by the generous support of the American people through the U.S. Agency for International Development (USAID; GPO-A-00-04-00019-00. This work was also supported by the Swedish Ministry of Foreign Affairs, the Swedish International Development Cooperation Agency, and the TNPRC National Institutes of Health (NIH; base grant RR00164) and with federal funds from the National Cancer Institute, NIH, under contract HHSN261200800001E.
The contents of the paper are the sole responsibility of the Population Council and do not necessarily reflect the views of the funding agencies or the United States government.
M.R. is a 2002 Elizabeth Glaser Scientist.
Footnotes
Published ahead of print 10 June 2013
REFERENCES
- 1. Celum C, Baeten JM. 2012. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir. Ther. 17:1483–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. 2012. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS 26:F13–F19 [DOI] [PubMed] [Google Scholar]
- 4. Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. 2012. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 367:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Romano JW, Robbiani M, Doncel GF, Moench T. 2012. Non-specific microbicide product development: then and now. Curr. HIV Res. 10:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kenney J, Aravantinou M, Singer R, Hsu M, Rodriguez A, Kizima L, Abraham CJ, Menon R, Seidor S, Chudolij A, Gettie A, Blanchard J, Lifson JD, Piatak M, Jr, Fernández-Romero JA, Zydowsky TM, Robbiani M. 2011. An antiretroviral/zinc combination gel provides 24 hours of complete protection against vaginal SHIV infection in macaques. PLoS One 6:e15835. 10.1371/journal.pone.0015835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernández-Romero JA, Abraham CJ, Rodriguez A, Kizima L, Jean-Pierre N, Menon R, Begay O, Seidor S, Ford BE, Gil PI, Peters J, Katz D, Robbiani M, Zydowsky TM. 2012. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob. Agents Chemother. 56:358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashley R. 1995. Chapter 22: herpes simplex viruses, p. 383 In Schmidt NJ, Emmons RW. (ed) Diagnostic procedures for viral, rickettsial, and chlamydial infections, 7th ed American Public Health Association, Washington, DC [Google Scholar]
- 9. Begay O, Jean-Pierre N, Abraham CJ, Chudolij A, Seidor S, Rodriguez A, Ford BE, Henderson M, Katz D, Zydowsky T, Robbiani M, Fernández-Romero JA. 2011. Identification of personal lubricants that can cause rectal epithelial cell damage and enhance HIV type 1 replication in vitro. AIDS Res. Hum. Retroviruses 27:1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moncla BJ, Pryke K, Rohan LC, Yang H. 2012. Testing of viscous anti-HIV microbicides using Lactobacillus. J. Microbiol. Methods 88:292–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, Cost M, Huang Y, Gai F, Billitto N, Lynam JD, Pryke K, Graebing P, Hopkins N, Rooney JF, Friend D, Dezzutti CS. 2010. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One 5:e9310. 10.1371/journal.pone.0009310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Research Council 2010. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC [Google Scholar]
- 13. U.S. Department of Agriculture 2001. Code of Federal Regulations, Animal Welfare Act and Regulation chapter 1, subchapter A: animals and animal products. U.S. Department of Agriculture, Beltsville, MD [Google Scholar]
- 14. Singer R, Derby N, Rodriguez A, Kizima L, Kenney J, Aravantinou M, Chudolij A, Gettie A, Blanchard J, Lifson JD, Piatak M, Jr, Fernández-Romero JA, Zydowsky TM, Robbiani M. 2011. The nonnucleoside reverse transcriptase inhibitor MIV-150 in carrageenan gel prevents rectal transmission of simian/human immunodeficiency virus infection in macaques. J. Virol. 85:5504–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cline AN, Bess JW, Piatak M, Jr, Lifson JD. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J. Med. Primatol. 34:303–312 [DOI] [PubMed] [Google Scholar]
- 16. Smith SM, Holland B, Russo C, Dailey PJ, Marx PA, Connor RI. 1999. Retrospective analysis of viral load and SIV antibody responses in rhesus macaques infected with pathogenic SIV: predictive value for disease progression. AIDS Res. Hum. Retroviruses 15:1691–1701 [DOI] [PubMed] [Google Scholar]
- 17. García-Lerma JG, Cong ME, Mitchell J, Youngpairoj AS, Zheng Q, Masciotra S, Martin A, Kuklenyik Z, Holder A, Lipscomb J, Pau CP, Barr JR, Hanson DL, Otten R, Paxton L, Folks TM, Heneine W. 2010. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci. Transl. Med. 2:14ra4. 10.1126/scitranslmed.3000391 [DOI] [PubMed] [Google Scholar]
- 18. Smith LM, Hensley LE, Geisbert TW, Johnson J, Stossel A, Honko A, Yen JY, Geisbert J, Paragas J, Fritz E, Olinger G, Young HA, Rubins KH, Karp CL. 2013. Interferon-beta therapy prolongs survival in rhesus macaque models of Ebola and Marburg hemorrhagic fever. J. Infect. Dis. [Epub ahead of print.] doi:10.1093/infdis/jis921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crostarosa F, Aravantinou M, Akpogheneta OJ, Jasny E, Shaw A, Kenney J, Piatak M, Lifson JD, Teitelbaum A, Hu L, Chudolij A, Zydowsky TM, Blanchard J, Gettie A, Robbiani M. 2009. A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy. PLoS One 4:e8060. 10.1371/journal.pone.0008060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kenney J, Singer R, Derby N, Aravantinou M, Abraham CJ, Menon R, Seidor S, Zhang S, Gettie A, Blanchard J, Piatak M, Jr, Lifson JD, Fernández-Romero JA, Zydowsky TM, Robbiani M. 2012. A single dose of a MIV-150/Zinc acetate gel provides 24 h of protection against vaginal simian human immunodeficiency virus reverse transcriptase infection, with more limited protection rectally 8–24 h after gel use. AIDS Res. Hum. Retroviruses 28:1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singer R, Mawson P, Derby N, Rodriguez A, Kizima L, Menon R, Goldman D, Kenney J, Aravantinou M, Seidor S, Gettie A, Blanchard J, Piatak M, Jr, Lifson JD, Fernández-Romero JA, Robbiani M, Zydowsky TM. 2012. An intravaginal ring that releases the NNRTI MIV-150 reduces SHIV transmission in macaques. Sci. Transl. Med. 4:150ra23. 10.1126/scitranslmed.3003936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turville SG, Aravantinou M, Miller T, Kenney J, Teitelbaum A, Hu L, Chudolij A, Zydowsky TM, Piatak M, Jr, Bess JW, Jr, Lifson JD, Blanchard J, Gettie A, Robbiani M. 2008. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS One 3:e3162. 10.1371/journal.pone.0003162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chateau M, Swanson MD, Garcia JV. 2013. Inefficient vaginal transmission of tenofovir-resistant HIV-1. J. Virol. 87:1274–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hurt CB, Eron JJ, Jr, Cohen MS. 2011. Pre-exposure prophylaxis and antiretroviral resistance: HIV prevention at a cost? Clin. Infect. Dis. 53:1265–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cummins JE, Jr, Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, Grohskopf LA, Paxton L, Dezzutti CS. 2007. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob. Agents Chemother. 51:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fernández-Romero JA, Thorn M, Turville SG, Titchen K, Sudol K, Li J, Miller T, Robbiani M, Maguire RA, Buckheit RW, Jr, Hartman TL, Phillips DM. 2007. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sex. Transm. Dis. 34:9–14 [DOI] [PubMed] [Google Scholar]
- 27. Kilmarx PH, Blanchard K, Chaikummao S, Friedland BA, Srivirojana N, Connolly C, Witwatwongwana P, Supawitkul S, Mock PA, Chaowanachan T, Tappero J. 2008. A randomized, placebo-controlled trial to assess the safety and acceptability of use of carraguard vaginal gel by heterosexual couples in Thailand. Sex. Transm. Dis. 35:226–232 [DOI] [PubMed] [Google Scholar]
- 28. Kilmarx PH, van de Wijgert JH, Chaikummao S, Jones HE, Limpakarnjanarat K, Friedland BA, Karon JM, Manopaiboon C, Srivirojana N, Yanpaisarn S, Supawitkul S, Young NL, Mock PA, Blanchard K, Mastro TD. 2006. Safety and acceptability of the candidate microbicide Carraguard in Thai women: findings from a phase II clinical trial. J. Acquir. Immune Defic. Syndr. 43:327–334 [DOI] [PubMed] [Google Scholar]
- 29. Martin S, Blanchard K, Manopaiboon C, Chaikummao S, Schaffer K, Friedland B, Kilmarx PH. 2010. Carraguard acceptability among men and women in a couples study in Thailand. J. Womens Health (Larchmt) 19:1561–1567 [DOI] [PubMed] [Google Scholar]
- 30. McLean CA, van de Wijgert JH, Jones HE, Karon JM, McNicoll JM, Whitehead SJ, Braunstein S, Achalapong J, Chaikummao S, Tappero JW, Markowitz LE, Kilmarx PH. 2010. HIV genital shedding and safety of Carraguard use by HIV-infected women: a crossover trial in Thailand. AIDS 24:717–722 [DOI] [PubMed] [Google Scholar]
- 31. Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, Dozier G, Maguire R, Lahteenmaki P. 2008. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet 372:1977–1987 [DOI] [PubMed] [Google Scholar]
- 32. Whitehead SJ, Kilmarx PH, Blanchard K, Manopaiboon C, Chaikummao S, Friedland B, Achalapong J, Wankrairoj M, Mock P, Thanprasertsuk S, Tappero JW. 2006. Acceptability of Carraguard vaginal gel use among Thai couples. AIDS 20:2141–2148 [DOI] [PubMed] [Google Scholar]
- 33. Pirrone V, Passic S, Wigdahl B, Krebs FC. 2012. Application and removal of polyanionic microbicide compounds enhances subsequent infection by HIV-1. Virol. J. 9:33. 10.1186/1743-422X-9-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buck CB, Thompson CD, Roberts JN, Muller M, Lowy DR, Schiller JT. 2006. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2:e69. 10.1371/journal.ppat.0020069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marais D, Gawarecki D, Allan B, Ahmed K, Altini L, Cassim N, Gopolang F, Hoffman M, Ramjee G, Williamson AL. 2011. The effectiveness of Carraguard, a vaginal microbicide, in protecting women against high-risk human papillomavirus infection. Antivir. Ther. 16:1219–1226 [DOI] [PubMed] [Google Scholar]
- 36. Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 13:857–861 [DOI] [PubMed] [Google Scholar]
- 37. Fenstermacher KJ, DeStefano JJ. 2011. Mechanism of HIV reverse transcriptase inhibition by zinc: formation of a highly stable enzyme-(primer-template) complex with profoundly diminished catalytic activity. J. Biol. Chem. 286:40433–40442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mocchegiani E., Muzzioli M. 2000. Therapeutic application of zinc in human immunodeficiency virus against opportunistic infections. J. Nutr. 130:1424S–1431S [DOI] [PubMed] [Google Scholar]
- 39. Shankar A. H., Prasad A. S. 1998. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 68:447S–463S [DOI] [PubMed] [Google Scholar]
- 40. Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, Suzuki T. 2008. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv. Immunol. 97:149–176 [DOI] [PubMed] [Google Scholar]
- 41. Rink L, Gabriel P. 2000. Zinc and the immune system. Proc. Nutr. Soc. 59:541–552 [DOI] [PubMed] [Google Scholar]
- 42. Arens M, Travis S. 2000. Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. J. Clin. Microbiol. 38:1758–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kümel G, Schrader S, Zentgraf H, Daus H, Brendel M. 1990. The mechanism of the antiherpetic activity of zinc sulphate. J. Gen. Virol. 71:2989–2997 [DOI] [PubMed] [Google Scholar]
- 44. Andrews BJ, Mylvaganam H, Yule A. 1994. Sensitivity of Trichomonas vaginalis, Tritrichomonas foetus and Giardia intestinalis to bacitracin and its zinc salt in vitro. Trans. R. Soc. Trop. Med. Hyg. 88:704–706 [DOI] [PubMed] [Google Scholar]
- 45. Prasad AS, Beck FW, Bao B, Snell D, Fitzgerald JT. 2008. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecules in patients with common cold treated with zinc acetate. J. Infect. Dis. 197:795–802 [DOI] [PubMed] [Google Scholar]
- 46. Suara RO, Crowe JE., Jr 2004. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob. Agents Chemother. 48:783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chvapil M, Chvapil TA, Owen JA, Kantor M, Ulreich JB, Eskelson C. 1979. Reaction of vaginal tissue of rabbits to inserted sponges made of various materials. J. Biomed. Mater Res. 13:1–13 [DOI] [PubMed] [Google Scholar]
- 48. Fahim MS, Wang M. 1996. Zinc acetate and lyophilized aloe barbadensis as vaginal contraceptive. Contraception. 53:231–236 [DOI] [PubMed] [Google Scholar]
- 49. Alexander TH, Davidson TM. 2006. Intranasal zinc and anosmia: the zinc-induced anosmia syndrome. Laryngoscope 116:217–220 [DOI] [PubMed] [Google Scholar]
- 50. Bourne N, Stegall R, Montano R, Meador M, Stanberry LR, Milligan GN. 2005. Efficacy and toxicity of zinc salts as candidate topical microbicides against vaginal herpes simplex virus type 2 infection. Antimicrob. Agents Chemother. 49:1181–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trumbo P, Yates AA, Schlicker S, Poos M. 2001. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet Assoc. 101:294–301 [DOI] [PubMed] [Google Scholar]
- 52. Rupp R, Rosenthal SL, Stanberry LR. 2007. VivaGel (SPL7013 Gel): a candidate dendrimer-microbicide for the prevention of HIV and HSV infection. Int. J. Nanomedicine 2:561–566 [PMC free article] [PubMed] [Google Scholar]
- 53. Talwar GP, Raghuvanshi P, Mishra R, Banerjee U, Rattan A, Whaley KJ, Zeitlin L, Achilles SL, Barré-Sinoussi F, David A, Doncel GF. 2000. Polyherbal formulations with wide spectrum antimicrobial activity against reproductive tract infections and sexually transmitted pathogens. Am. J. Reprod. Immunol. 43:144–151 [DOI] [PubMed] [Google Scholar]
- 54. Emau P, Tian B, O'Keefe BR, Mori T, McMahon JB, Palmer KE, Jiang Y, Bekele G, Tsai CC. 2007. Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. J. Med. Primatol. 36:244–253 [DOI] [PubMed] [Google Scholar]
- 55. O'Keefe BR, Vojdani F, Buffa V, Shattock RJ, Montefiori DC, Bakke J, Mirsalis J, d'Andrea AL, Hume SD, Bratcher B, Saucedo CJ, McMahon JB, Pogue GP, Palmer KE. 2009. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. U. S. A. 106:6099–6104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moore MD, Cookson J, Coventry VK, Sproat B, Rabe L, Cranston RD, McGowan I, James W. 2011. Protection of HIV neutralizing aptamers against rectal and vaginal nucleases: implications for RNA-based therapeutics. J. Biol. Chem. 286:2526–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wheeler LA, Trifonova R, Vrbanac V, Basar E, McKernan S, Xu Z, Seung E, Deruaz M, Dudek T, Einarsson JI, Yang L, Allen TM, Luster AD, Tager AM, Dykxhoorn DM, Lieberman J. 2011. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J. Clin. Invest. 121:2401–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brinckmann S, da Costa K, van Gils MJ, Hallengard D, Klein K, Madeira L, Mainetti L, Palma P, Raue K, Reinhart D, Reudelsterz M, Ruffin N, Seifried J, Schäfer K, Sheik-Khalil E, Sköld A, Uchtenhagen H, Vabret N, Ziglio S, Scarlatti G, Shattock R, Wahren B, Gotch F. 2011. Rational design of HIV vaccines and microbicides: report of the EUROPRISE network annual conference 2010. J. Transl. Med. 9:40. 10.1186/1479-5876-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gong E, Matthews B, McCarthy T, Chu J, Holan G, Raff J, Sacks S. 2005. Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses. Antiviral Res. 68:139–146 [DOI] [PubMed] [Google Scholar]
- 60. Jiang YH, Emau P, Cairns JS, Flanary L, Morton WR, McCarthy TD, Tsai CC. 2005. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retroviruses 21:207–213 [DOI] [PubMed] [Google Scholar]
- 61. Price CF, Tyssen D, Sonza S, Davie A, Evans S, Lewis GR, Xia S, Spelman T, Hodsman P, Moench TR, Humberstone A, Paull JR, Tachedjian G. 2011. SPL7013 Gel (VivaGel(R)) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS One 6:e24095. 10.1371/journal.pone.0024095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tyssen D, Henderson SA, Johnson A, Sterjovski J, Moore K, La J, Zanin M, Sonza S, Karellas P, Giannis MP, Krippner G, Wesselingh S, McCarthy T, Gorry PR, Ramsland PA, Cone R, Paull JR, Lewis GR, Tachedjian G. 2010. Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PLoS One 5:e12309. 10.1371/journal.pone.0012309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bernstein DI, Stanberry LR, Sacks S, Ayisi NK, Gong YH, Ireland J, Mumper RJ, Holan G, Matthews B, McCarthy T, Bourne N. 2003. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob. Agents Chemother. 47:3784–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]