Abstract
The characterization of ABCI4, a new intracellular ATP-binding cassette (ABC) half-transporter in Leishmania major, is described. We show that ABCI4 is involved in heavy metal export, thereby conferring resistance to Pentostam, to Sb(III), and to As(III) and Cd(II). Parasites overexpressing ABCI4 showed a lower mitochondrial toxic effect of antimony by decreasing reactive oxygen species production and maintained higher values of both the mitochondrial electrochemical potential and total ATP levels with respect to controls. The ABCI4 half-transporter forms homodimers as determined by a coimmunoprecipitation assay. A combination of subcellular localization studies under a confocal microscope and a surface biotinylation assay using parasites expressing green fluorescent protein- and FLAG-tagged ABCI4 suggests that the transporter presents a dual localization in both mitochondria and the plasma membrane. Parasites overexpressing ABCI4 present an increased replication in mouse peritoneal macrophages. We have determined that porphyrins are substrates for ABCI4. Consequently, the overexpression of ABCI4 confers resistance to some toxic porphyrins, such as zinc-protoporphyrin, due to the lower accumulation resulting from a significant efflux, as determined using the fluorescent zinc-mesoporphyrin, a validated heme analog. In addition, ABCI4 has a significant ability to efflux thiol after Sb(III) incubation, thus meaning that ABCI4 could be considered to be a potential thiol-X-pump that is able to recognize metal-conjugated thiols. In summary, we have shown that this new ABC transporter is involved in drug sensitivity to antimony and other compounds by efflux as conjugated thiol complexes.
INTRODUCTION
Despite being prevalent in 98 countries spread across three continents, leishmaniasis, which is caused by the protozoan parasite Leishmania, is one of the most neglected tropical diseases (1). The estimated incidence of leishmaniasis is over 12 million people worldwide, and it is responsible for a variety of pathologies, ranging from self-healing cutaneous lesions to fatal visceral infection (1). Since there are currently no effective vaccines, treatment largely relies on chemotherapy, although its efficacy is increasingly limited by growing resistance to first-line drugs, especially antimonials, the frequent side effects associated with their use, and the high cost of treatment (1). The recommended first-line therapies for visceral leishmaniasis (VL) include: (i) pentavalent antimonials (meglumine antimoniate and sodium stibogluconate), except in some regions in the Indian subcontinent where there are significant areas of drug resistance (2); (ii) the polyene antibiotic amphotericin B; (iii) the liposomal formulation AmBisome; (iv) the aminoglycoside paromomycin; and (v) the oral drug miltefosine. Although the World Health Organization (WHO) (1, 3) recently recommended the use of either a single dose of AmBisome or combinations of antileishmanial drugs in order to reduce the duration and toxicity of treatment, prolong the therapeutic life span of existing drugs and delay the emergence of resistance, recent experimental findings have demonstrated the ability of Leishmania to develop resistance to different drug combinations (4).
One of the main problems of leishmaniasis chemotherapy is the growing antimonial resistance found in Indian VL. Studies concerning experimental resistance to antimony in Leishmania indicate that several mechanisms may occur concurrently in the same parasite and that different mechanisms may operate in field isolates compared to lab-generated resistant Leishmania lines (5, 6). One mechanism of resistance to antimony in Leishmania involves a reduction in its accumulation, either by reduced uptake or increased efflux, with the latter being mediated by the ABC transporter ABCC3 (also known as PGPA or MRPA) (7, 8). ABC transporters constitute one of the largest families of proteins described and as such play a broad variety of physiological roles with major medical consequences. These proteins are evolutionarily highly conserved, they are present in species ranging from prokaryotes to humans (9), and they use the energy provided by ATP hydrolysis to transport different compounds across biological membranes, including a variety of leishmanicidal agents such as antimonials (10), miltefosine (11), pentamidine (12), and sitamaquine (13).
Functional ABC transporters consist of two homologous halves, each of which is composed of a transmembrane domain (TMD) involved in substrate binding and a cytosolic nucleotide binding domain (NBD), which hydrolyzes ATP (9). Some ABC transporters are half-transporters with an NBD-TMD or TMD-NBD topology that requires homo-/hetero-dimerization to become functional (14). The Leishmania genome contains 42 ABC genes, with representative members from every subfamily (from ABCA to ABCH) commonly found in eukaryotes (15). To date, only ABC transporters related to the ABCA, ABCB, ABCC, and ABCG subfamilies have been described in Leishmania (10, 16).
At least two transporters from the ABCC family appear to be involved in antimony resistance (17). The Leishmania ABCC3, which is located in the intracellular vesicular membrane close to the flagellar pocket, has been reported to be involved in arsenite and antimonial resistance in the promastigotes and amastigotes of several metal-resistant Leishmania species (18, 19). In addition, this transporter presents the ability to transport thiol-conjugated metals (19).
One of the proposed mechanisms of resistance to antimony suggests that conjugation with a thiol and extrusion of the complex by an ATP-coupled pump present in the plasma membrane (PM) is required (20). However, this transport system is not found for the ABCC proteins of Leishmania since all proteins of this subfamily have an intracellular location (21). Since there is currently very little understanding of the transporter responsible for this antimony efflux system (22), further studies are required to understand the mechanism of antimony resistance in Leishmania.
The involvement of thiols in antimony resistance is supported by an increase in the levels of the enzymes responsible for thiol synthesis (γ-glutamylcysteine synthetase and ornithine decarboxylase) in experimental metal-resistant Leishmania lines and in clinical isolates resistant to Pentostam (GlaxoSmithKline) (23, 24). Furthermore, this resistant phenotype can be reverted by buthionine sulfoximine (BSO), a biosynthesis-specific glutathione inhibitor (19). Similarly, the capacity of Leishmania ABCC3 to efflux toxic metals is known to be accompanied by a decreased generation of reactive oxygen species (ROS), thus contributing to decreased antimonial toxicity (25).
The second ABC transporter involved in antimony resistance is ABCC7-PRP1 (12, 17), a gene that is overexpressed in pentamidine-resistant Leishmania lines and confers significant cross-resistance to antimony (26). However, no clear association has been found between the amplification/overexpression of PRP1 and pentamidine resistance (27). As described recently, no other ABCC proteins have been demonstrated to be amplified or overexpressed in antimony-resistant Leishmania lines (17).
Here, we report the characterization of a new ABC transporter (ABCI4) in Leishmania major that is included in the unclassified group of ABC proteins termed as “others” (15). We have renamed this group ABCI, similar to what has been proposed for plant ABC classification (53). This transporter encodes a half-transporter with a TMD-NBD topology that requires at least homodimerization, with a dual localization at the PM and mitochondria. ABCI4 has antimony and other heavy metals as potential substrates, thereby conferring resistance to antimonials due to the lower accumulation resulting from their active efflux. As such, we postulate that ABCI4 could be considered to be a potential thiol-X-pump that is able to recognize metal-conjugated thiols, thereby conferring antimony resistance in Leishmania.
MATERIALS AND METHODS
Chemical compounds.
Carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP), trivalent antimony (Sb(III); potassium antimony tartrate), pentamidine, paromomycin, amphotericin B, ketoconazole, chloroquine, quinine, mefloquine, primaquine, 4′,6-diamidino-2-phenylindole dilactate (DAPI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), BSO, CdCl2, CoCl2, CuSO4, Pb(NO3)2, NaAsO2, MnCl2, ZnCl2, n-dodecyl-β-d-maltopyranoside (DDM), pheophorbide A, and glutathione (GSH) were purchased from Sigma-Aldrich (St. Louis, MO). Miltefosine was purchased from Æterna Zentaris (Frankfurt, Germany) and daunomycin (DNM) was purchased from Pfizer (Madrid, Spain). All chemicals were of the highest quality available. Pentostam, tafenoquine, and sitamaquine dihydrochloride were provided by GlaxoSmithKline (Greenford, United Kingdom). MitoSOX Red, H2DCFDA, and JC-1 were purchased from Invitrogen (Carlsbad, CA). Zinc-protoporphyrin and zinc-mesoporphyrin were purchased from Frontier Scientific (Logan, UT).
Animals.
Six-week-old male BALB/c mice were purchased from Charles River Breeding Laboratories and maintained in the Animal Facility Service of our Institute under pathogen-free conditions. They were fed a regular rodent diet and given drinking water ad libitum. These mice were used to collect primary peritoneal macrophages.
Ethics statement.
All experiments were performed according to National/EU guidelines regarding the care and use of laboratory animals in research. Approval for these studies was obtained from the Ethics Committee of the Spanish National Research Council (CSIC file CEA-213-1-11).
Leishmania culture conditions.
The promastigotes of L. major (MHOM/JL/80/Friedlin) and derivative lines used in the present study were cultured at 28°C in M-199 medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (hiFBS; Invitrogen).
In vitro infection of mouse peritoneal macrophages.
Amastigotes were cultured for 24 and 72 h in infected peritoneal macrophages from BALB/c mice (Charles River, Ltd.) as described previously (28).
DNA constructs and site-directed mutagenesis.
ABCI4 from L. major (GeneDB L. major, accession code LmjF.33.3260) was isolated from the genomic DNA of L. major by PCR using sense (5′-TGCATTCTCACGCCTTCACAC) and antisense (5′-ACTAGCATTAGCTGCGAGTTCC) primers. ABCI4 was cloned into the Leishmania expression vector pXG-Hyg (29) and sequenced. The Leishmania expression vectors pXG-′GFP+ and pXG-GFP+2′ (29) were used to construct chimeric ABCI4 fusion proteins with a green fluorescent protein (GFP) at the C- and N-terminal positions, respectively. For the C-terminal position of GFP, ABCI4 was amplified by PCR using sense (5′-ATAGATCTATGAGACTCACTGATGCTCCGT) and antisense (5′-ATGATATCGCTCACTGCCTGTGCACG) primers. For the N-terminal position of GFP, ABCI4 was amplified using sense (5′-ATGCGGCCGCATGAGACTCACTGATGCTCCGT) and antisense (5′-ATAGATCTTCAGCTTCACTGCCTGTGC) primers. To obtain parasites expressing nonfunctional ABCI4, a mutation was introduced in the Walker A motif, replacing lysine 742 for methionine (K742M) using the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) and the sense (5′-GTGGGGCGGGGATGAGTACGCTGCT) and antisense (5′-AGCAGCGTACTCATCCCCGCCCCAC) primers. To incorporate an N-terminal FLAG epitope tag, ABCI4 was amplified by using sense (5′- ATGGACTACAAGGACGACGACGACAAGATGAGACTCACTGATGCTCC) and antisense (5′-TCAGCTCACTGCCTGTGCAC) primers. Similarly, sense (5′-ATGGACTACAAGGACGACGACGACAAGGACTACAAGGACGACGACGACAAGGACTAC) and antisense (5′-TCAGCTCACTGCCTGTGCAC) primers were used to add two additional FLAG epitope tags to the previous construct. These constructs were cloned into the expression vector pXG-Hyg.
Gene expression.
Total RNA was extracted from different Leishmania lines using the high pure RNA isolation kit (Roche Diagnostics GmbH, Germany) and transcribed into cDNA using the qScript cDNA synthesis kit (Quanta Biosciences, Inc.) according to the manufacturer's instructions. The cDNA obtained was diluted (1:10 and 1:50) and amplified with sense (5′-TGACGAGCATGAAGCGTACAC) and antisense (5′-GCTGTATCGATGCAGGCCT) primers for ABCI4 and with sense (5′-GAAGTACACGGTGGAGGCTG) and antisense (5′-CGCTGATCACGACCTTCTTC) primers for GADPH (as internal control) and electrophoresed on 1% agarose gel.
Cell transfection and sensitivity analysis.
Parasites transfected with the constructs obtained above were selected for hygromycin or G418 depending on the vector used in each construct, as described previously (30). In addition, the Leishmania line overexpressing ABCI4K/M was cotransfected with ABCI4 to rescue the phenotype induced by the mutant line. The sensitivity of L. major promastigote lines to different compounds was determined using the MTT colorimetric assay, as described previously (31). The 50% effective concentration (EC50) is the concentration of drug that decreases the rate of cell growth by 50%. To determine the relationship between thiol levels and the sensitivity of the different Leishmania lines, 3 mM BSO (a γ-glutamylcysteine synthetase inhibitor) was added at 28°C for 48 h before the sensitivity experiments. The sensitivity of intracellular Leishmania amastigotes to Sb(III) and Sb(V) (Pentostam) was determined after 72 h of incubation as described previously (28).
Fluorescence microscopy of Leishmania promastigotes.
Promastigotes expressing ABCI4 GFP-fused proteins were used for localization studies. Parasite mitochondria were labeled with 50 nM MitoTracker Red (Invitrogen) for 30 min at 28°C in RPMI medium without hiFBS. They were then washed with phosphate-buffered saline (PBS) and analyzed under an Axiovert confocal microscope (TCS SP5 model; Leica).
Cell surface biotinylation.
Parasite surfaces were labeled as described previously (32), using 3% DDM instead of 1% Nonidet P-40 for parasite lysis. Protein samples were fractionated by SDS-PAGE under standard conditions and then electrotransferred onto Immobilon P membranes (Millipore, Bedford, MA). Immunodetection was performed using 1:5,000 polyclonal anti-GFP or 1:3,000 polyclonal anti-LRos3 (LRos3 is a PM protein) (32), respectively, in PBS plus 0.01% Tween 20 and 0.1% bovine serum albumin (BSA). Control of PM integrity was determined by immunodetection with monoclonal anti-α-tubulin antibody (Sigma-Aldrich) at 1:5,000. After washing, membranes were incubated with horseradish peroxidase-conjugated secondary goat anti-rabbit (1:5,000) immunoglobulin G (Dako, Barcelona, Spain) for GFP and LRos3, and rabbit anti-mouse (1:5,000) immunoglobulin G (Dako) for α-tubulin. Signals were detected using the enhanced chemiluminescence (ECL) substrate (Pierce).
Coimmunoprecipitation assays.
Leishmania promastigotes (8 × 109) expressing 3FLAG-ABCI4 and both 3FLAG- and GFP-ABCI4 chimeric proteins were washed with PBS, resuspended in 1 ml of lysis buffer, and disrupted for 45 min in a prechilled, high-pressure cavitator (Ashcroft, USA) connected to a compressed nitrogen cylinder at 100 bar pressure. The parasite lysates were centrifuged at 1,000 × g to eliminate cell debris; the supernatant was then centrifuged at 100,000 × g and 4°C for 1 h to obtain parasite membrane-enriched fractions. The pellets obtained were solubilized in 550 μl of lysis buffer supplemented with 2.5% DDM, and the resulting solution clarified by centrifugation at 100,000 × g at 4°C for 30 min. Supernatants were incubated with magnetic beads covalently bound to anti-GFP for 24 h at 4°C. After incubation, the magnetic beads were washed three times with lysis buffer supplemented with 0.05% DDM and eluted with Laemmli buffer. Samples were fractionated by SDS-PAGE under standard conditions and then electrotransferred onto Immobilon P membranes (Millipore, Bedford, MA). Immunodetection was performed using 1:5,000 polyclonal anti-GFP or 1:2,000 polyclonal anti-FLAG (Pierce), respectively, in wash solution (PBS plus 0.01% Tween 20 and 0.1% BSA). Membranes were incubated with horseradish peroxidase-conjugated secondary goat anti-rabbit (1:5,000) immunoglobulin G (Dako), and signals were detected using the ECL substrate (Pierce).
Antimony accumulation and efflux by ICP-MS.
Promastigotes (108 per ml) were incubated with 100 μM Sb(III) in M-199 culture medium for different time points at 28°C and then centrifuged, and the resultant pellet stored at −80°C until measurement of antimony accumulation as described previously (7). To determine antimony efflux, the different lines were incubated with compensated Sb(III) concentrations (100 μM for pXG and ABCI4K/M lines and 200 μM for ABCI4 line) in culture medium at 28°C for 1 h to allow a similar labeling in the Leishmania lines. The parasites were then washed with PBS, resuspended in culture medium at 28°C, and pelleted at different time points. The cell pellet was dissolved in 200 μl of concentrated nitric acid for 24 h at room temperature. The sample was diluted to 3 ml with distilled water and then injected into an inductively coupled plasma mass spectrometer (ICP-MS; Perkin-Elmer) for quantitation.
Cadmium accumulation and efflux.
Cadmium (Cd(II)) accumulation assays were performed using the fluorimetric dye Leadmium Green (Invitrogen), as described previously for mammalian cells (33). Thus, promastigotes (107) were incubated with 100 μM Cd(II) in saline buffer (21 mM HEPES, 137 mM NaCl, 5 mM KCl, 6 mM glucose; pH 7) for different time points at 28°C. The parasites were then pelleted and washed twice with saline buffer. Sample fluorescence was measured using an Infinite F200 luminescence system (Tecan Austria GmbH). To determine Cd(II) efflux, pXG and ABCI4 lines were incubated with compensated concentrations of the metal (100 and 175 μM, respectively), according to the results obtained in the accumulation experiments. After incubation for 1 h, parasites were centrifuged, washed twice with saline buffer, and then resuspended in the same buffer. The fluorescence retained was measured at different time points as described above.
Zinc-mesoporphyrin accumulation and efflux.
Promastigotes in their logarithmic phase were washed and resuspended in HPMI medium (120 mM NaCl, 5 mM KCl, 400 μM MgCl2, 40 μM CaCl2, 10 mM HEPES, 10 mM NaHCO3, 10 mM glucose, 5 mM Na2HPO4) at a final concentration of 5 × 107 promastigotes/ml. The parasites were then incubated with 10 μM zinc-mesoporphyrin (ZnMP) at 28°C for 10 min and washed with PBS buffer plus 5% BSA. Finally, the parasites were fixed with 2% paraformaldehyde in PBS, and the fluorescence retained was measured by flow cytometry (excitation at 405 nm and emission at between 575 and 585 nm) using a FacsAria Cell Sorter III (Becton Dickinson, San Jose, CA). To determine the ZnMP efflux, the Leishmania control line (pXG) and ABCI4K/M were loaded with 10 μM ZnMP, whereas the Leishmania line overexpressing ABCI4 was loaded with 20 μM in order to allow for a similar labeling in HPMI medium at 28°C. The parasites were subsequently washed with PBS, and efflux was measured by determination of the fluorescence retained at different time points by flow cytometry as described above.
Pheophorbide A accumulation and efflux.
Determination of the accumulation and efflux of pheophorbide A, a phototoxic porphyrin catabolite of chlorophyll, was performed as described previously (34), with some modifications. Briefly, parasites (107) were resuspended in 1 ml of HPMI medium and incubated with 10 μM pheophorbide A for 30 min at 28°C. The parasites were them washed with PBS buffer and the fluorescence retained measured by flow cytometry using a FACScan flow cytometer (Becton Dickinson). For pheophorbide A efflux, after incubation with 10 μM pheophorbide A, the parasites were washed with PBS and resuspended in HPMI medium for 60 min at 28°C. The fluorescence retained was measured as described above.
Determination of nonprotein thiol.
Intracellular nonprotein thiol levels were measured by flow cytometry using CellTracker, as described previously (35). Thiol effluxed to the culture medium was determined using the thiol fluorescent detection reagent ThioStar (Luminos, Ann Arbor, MI) (36). Thus, parasites (108) were incubated with 100 μM Sb(III) for 1 h in 1 ml of HBS buffer (21 mM HEPES, 0.7 mM Na2HPO4, 137 mM NaCl, 5 mM KCl, and 6 mM glucose, pH 7), washed twice with PBS buffer and then resuspended again in Sb(III)-free HBS buffer. Parasites were collected at different time points, and both the supernatants and the pellets obtained were treated with 12% trichloroacetic acid at 4°C for 30 min to deproteinize and then centrifuged at 13,000 × g. The supernatants were diluted with HBS buffer to reduce the trichloroacetic acid concentration to 2% and were then transferred to a 96-well microplate and incubated with 3 μg of ThioStar/ml for 1 h at room temperature. Sample fluorescence (excitation, 380 nm; emission, 510 nm) was measured using an Infinite F200 luminescence system (Tecan Austria GmbH). Different GSH concentrations were used as a control of the thiol concentration dependence signal of ThioStar.
Measurement of ATP and mitochondrial membrane potential (ΔΨm).
ATP was measured using a CellTiter-Glo luminescence assay, which generates a luminescent signal proportional to the amount of ATP present, as described previously (28). ΔΨm was measured by flow cytometry using the JC-1 fluorescent marker. Depolarization of ΔΨm is indicated by a reduced accumulation of the dye and a shift from red to green, with a corresponding decrease in the red/green fluorescence ratio. The parasites (4 × 106 promastigotes/ml) were incubated with 100 μM Sb(III) for different time points at 28°C in HBS buffer and then washed twice, resuspended in PBS, and further incubated at 28°C with 5 μM JC-1 for 10 min in HBS buffer. Cellular fluorescence was quantified by calculating the ratio between FL2 and FL1 using a FACScan flow cytometer (Becton Dickinson). Parasites fully depolarized by incubation in 10 μM FCCP for 10 min at 28°C were used as controls.
Measurement of ROS production.
The generation of intracellular ROS was measured in parasites exposed to increasing concentrations of Sb(III) for 48 h using the cell-permeant H2DCFDA and MitoSOX Red probes, as described previously (37). The fluorescence retained was measured by flow cytometry using a FACScan flow cytometer (Becton Dickinson).
Statistical analysis.
Statistical comparisons between groups were performed using Student t test. Differences were considered significant at a level of P < 0.05.
RESULTS
Sequence features of ABCI4 and generation of a Leishmania line expressing an inactive version of the protein.
The present study focuses on characterization of the ABCI4 gene (GeneDB L. major, accession code LmjF.33.3260) from chromosome 33, and grouped as “others” according to the recently proposed classification (15). ABCI4 codes for a 949-amino-acid protein, with a predicted molecular mass of ∼103 kDa. ABCI4 shares a significant percentage amino acid identity just in the NBD with other Leishmania ABC transporters of ca. 30% for the ABCB subfamily. In addition, a similar percent amino acid identity in the NBD domain was observed with other eukaryotic ABC transporters, as for human ABCB6 and ABCB7 transporters.
Hydrophobicity plots of ABCI4 using the programs TMpred (http://www.ch.embnet.org/software/TMPRED_form.html) and MEMSAT-SVM (http://bioinf.cs.ucl.ac.uk/psipred/ [data not shown]) showed the expected topology for an ABC half-transporter, with a hydrophilic region, which bears the highly conserved motifs of ABC transporters (NBD) at the COOH terminus, and a hydrophobic region (TMD), containing six putative transmembrane segments at the NH2 terminus.
The dimerization requirement for ABC half-transporters to become functional led us to test a dominant-negative approach to downregulate ABCI4 function, as recently described for Leishmania LABCG5 and LABCG2 (38). To this end, we first mutated the lysine residue inside the Walker A motif in ABCI4 (742 position), which is known to be critical for ATP hydrolysis in ABC transporters (38, 39), to a methionine (K742M). The expression of other ABCG half-transporters with a similar K/M substitution produces a dominant-negative inhibition in the wild-type transporters (38, 40). The resulting construct was transfected into L. major and the expression of ABCI4K742M (ABCI4K/M), as well as other Leishmania lines overexpressing ABCI4 was verified by reverse transcription-PCR (RT-PCR) (see Fig. S1 in the supplemental material). In contrast to the phenotype observed after LABCG5K/M expression (38), parasites transfected with ABCI4K/M grew at normal rates (data not shown).
Localization of ABCI4 in mitochondria and PM using confocal microscopy and biotinylation assay.
To ascertain the subcellular localization of ABCI4, we fused ABCI4 and ABCI4K/M with an N-terminal GFP tag. These constructs were transfected into L. major promastigotes and overexpression of the corresponding proteins determined by Western blot analysis of whole parasite lysates. As expected, a band corresponding to GFP-ABCI4 was observed at ∼130 kDa (see Fig. S2 in the supplemental material). Fluorescence microscopy studies showed that GFP-ABCI4 mainly colocalizes with the mitochondrial marker MitoTracker (Fig. 1A). In addition, the protein that localizes at the PM of the parasite was observed, although with a lower fluorescence intensity (Fig. 1A). The localization pattern of the protein did not change when GFP-ABCI4K/M was expressed in Leishmania parasites (data not shown). Despite several attempts, we were unable to obtain parasites expressing GFP-tagged ABCI4 at the COOH terminus, thus suggesting that the C-terminal region is critical for maintaining ABCI4 stability. We also determined that ABCI4 is localized to mitochondria and PM using a FLAG-tagged ABCI4 (data not shown). Overall, these studies suggest that ABCI4 has a dual localization in both mitochondria and the PM of Leishmania.
Fig 1.
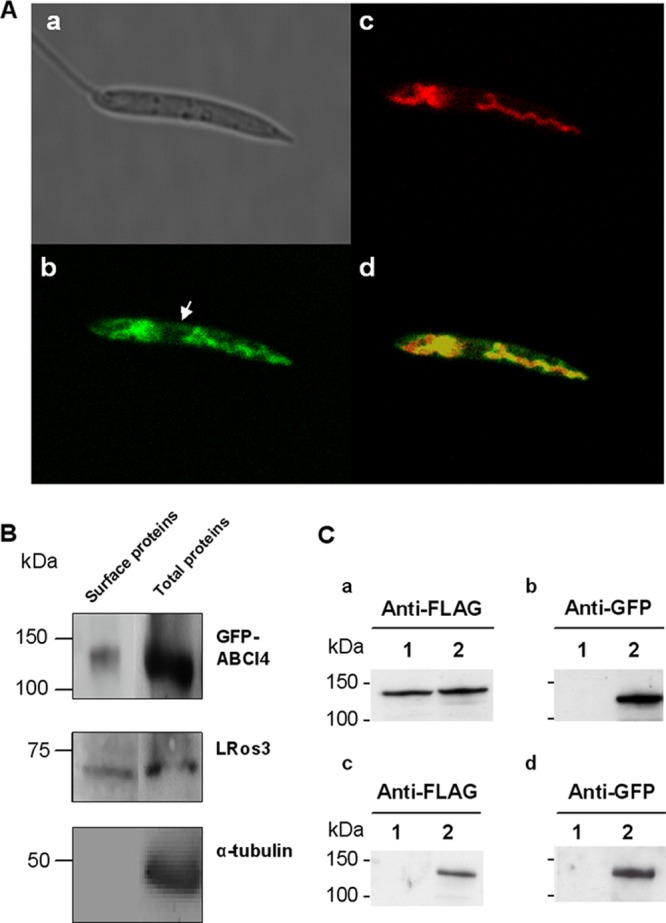
ABCI4 localizes to the mitochondria and PM of Leishmania parasites. (A) Confocal microscopy images of L. major stationary promastigotes transfected with GFP-ABCI4. (a) Nomarski image. (b) Green fluorescence associated with the GFP-ABCI4 fusion protein. (c) Red fluorescence associated with parasites incubated at 28°C with 50 nM MitoTracker Red for 30 min. (d) The merged image indicates colocalization (yellow) of ABCI4 with the mitochondrial probe. The figure illustrates a representative parasite from a total population of parasites with a similar fluorescence pattern. The arrow indicates the localization of ABCI4 in the PM. (B) ABCI4 expression levels in the PM membrane by surface biotinylation. Biotinylated proteins from the promastigote surface were analyzed by immunoblotting with anti-GFP, anti-LRos3, and anti-α-tubulin antibodies as described in Materials and Methods. Western blot assays representative of at least three independent experiments are shown. (C) Coimmunoprecipitation experiments demonstrate that ABCI4 forms homodimers. The solubilized membrane-enriched fraction of Leishmania parasites (a and b) overexpressing 3×FLAG-ABCI4 (lane 1) and 3×FLAG-ABCI4 and GFP-ABCI4 (lane 2) was incubated with magnetic beads covalently bound to anti-GFP. After incubation, the magnetic beads were washed, eluted and analyzed by immunoblotting (c and d). The positions of molecular mass markers (in kilodaltons) are indicated on the left.
Surface biotinylation experiments showed that GFP-ABCI4 localizes at the PM (Fig. 1B). ABCI4 was detected in the fraction eluted from streptavidin beads by immunoblotting with anti-GFP (Fig. 1B). LRos3, which is known to be present at the PM of Leishmania parasites (41), was used as a positive control (Fig. 1B). Furthermore, no immunoreactive bands were detected after immunoblotting of biotinylated proteins against anti-α-tubulin (Fig. 1B). This control confirms that the labeling reagent did not penetrate the PM, thereby demonstrating the specificity of the biotinylation procedure.
The dimerization requirement for ABC half-transporters to become functional led us to determine whether ABCI4 forms homodimers or heterodimers to be functionally active. The coimmunoprecipitation assay was performed using parasites coexpressing GFP- and 3×FLAG-ABCI4, and the proteins bound to magnetic bead anti-GFP fractions were visualized by immunoblotting with anti-GFP and anti-FLAG antibodies. Western blot analysis showed that anti-FLAG and anti-GFP antibodies detected a specific band of the expected size for tagged-ABCI4 in parasites overexpressing 3×FLAG-ABCI4 and coexpressing 3×FLAG-ABCI4 and GFP-ABCI4 (Fig. 1Ca and b). The coimmunoprecipitation assays revealed that anti-FLAG antibody only detected a specific band only in the eluted fraction of parasites coexpressing 3×FLAG-ABCI4 and GFP-ABCI4 after incubation of the solubilized membrane-enriched fraction with magnetic beads covalently bound to anti-GFP (Fig. 1Cc), thus suggesting that ABCI4 forms homodimers.
Overexpression of ABCI4 confers resistance to metal ions, antimonials, and toxic porphyrins in Leishmania.
Since several ABC transporters have been implicated in drug resistance in Leishmania (10–13), we analyzed whether overexpression of ABCI4 could confer resistance against several compounds. Different unrelated antileishmanial drugs and other unrelated compounds with potential use as antiprotozoal drugs, including Sb(III), amphotericin B, miltefosine, paromomycin, aminoquinolines (sitamaquine, tafenoquine, chloroquine, mefloquine, and primaquine), quinine, azoles (ketoconazole), daunomycin, and As(III), were tested (Table 1). Parasites overexpressing ABCI4 were ∼2-fold more resistant to Sb(III) and As(III) than the control (parasites transfected with the empty vector pXG). These results suggest that metal ions are possible substrates for ABCI4 (Table 1). Further analysis showed that overexpression of ABCI4 conferred resistance to the metal ions Cd(II) and Pb(II) but did not affect the susceptibility to Cu(II), Zn(II), Co(II), and Mn(II) (Table 2). These results suggest that some heavy metals, mainly Sb(III), are putative ABCI4 substrates. The above results were validated in a second transfection event with ABCI4 (Table 2). Promastigotes overexpressing GFP-ABCI4 showed similar behavior to parasites overexpressing untagged ABCI4, thus showing that tagging at the NH2 terminus did not interfere with the functionality of ABCI4 (Table 2). In addition, the cross-resistance profile obtained in Leishmania promastigotes overexpressing ABCI4K/M was similar to that for control parasites (Tables 1 and 2). To verify that the resistance to some heavy metals observed in parasites overexpressing ABCI4 was due to overexpression of the ABC transporter, ABCI4 parasites were cured for the plasmid pXG-Hyg (reverted line, ABCI4rev) by culturing the parasites in the absence of plasmid drug selection pressure for 1 month. This reverted line showed a similar resistance to some heavy metals as the control (pXG) Leishmania line (Table 2). In addition, parasites coexpressing ABCI4-ABCI4K/M showed similar Sb(III) sensitivity to the controls (see Fig. S3 in the supplemental material). This finding indicates that the mutated version of the transporter binds and inhibits ABCI4 activity and also suggests a low basal expression level of this transporter in wild-type parasites.
Table 1.
Drug resistance profile in L. major linesa
| Drug | Mean EC50 (μM) ± SD [RI]b |
||
|---|---|---|---|
| pXG | ABCI4 | ABCI4K/M | |
| Promastigote | |||
| Sb(III) | 20.87 ± 1.80 | 53.74 ± 3.35 [2.6]* | 21.16 ± 3.60 [1.0] |
| As(III) | 2.62 ± 0.69 | 5.14 ± 0.50 [2.0]* | 1.97 ± 0.36 [0.8] |
| Amphotericin B | 0.14 ± 0.01 | 0.11 ± 0.01 [0.8] | 0.09 ± 0.01 [0.6] |
| Miltefosine | 20.08 ± 1.30 | 16.39 ± 1.12 [0.8] | 20.79 ± 0.60 [1.0] |
| Paromomycin | 25.36 ± 2.36 | 27.54 ± 3.21 [1.1] | 26.31 ± 0.97 [1.0] |
| Tafenoquine | 4.98 ± 0.20 | 4.90 ± 0.16 [1.0] | 3.47 ± 0.28 [0.7] |
| Sitamaquine | 11.52 ± 1.23 | 11.72 ± 1.52 [1.1] | 11.09 ± 0.98 [1.0] |
| Primaquine | 3.69 ± 0.37 | 4.14 ± 0.65 [1.1] | 2.27 ± 0.24 [0.6] |
| Chloroquine | 2.82 ± 0.23 | 2.32 ± 0.14 [0.8] | 1.88 ± 0.21 [0.7] |
| Ketoconazole | 47.30 ± 3.56 | 45.07 ± 4.89 [1.0] | 39.72 ± 2.23 [0.8] |
| Daunomycin | 9.09 ± 0.36 | 9.54 ± 0.23 [1.1] | 9.71 ± 0.36 [1.1] |
| Mefloquine | 0.65 ± 0.03 | 0.66 ± 0.05 [1.0] | 0.62 ± 0.08 [1.0] |
| Quinine | 25.11 ± 1.45 | 33.23 ± 3.60 [1.3] | 22.46 ± 2.14 [0.9] |
| ZPPc | 4.35 ± 0.67 | 10.12 ± 0.23 [2.1]* | 4.85 ± 0.16 [1.1] |
| Pheophorbide Ac | 10.01 ± 0.15 | 8.61 ± 0.53 [0.9] | 8.90 ± 0.66 [0.9] |
| Amastigote | |||
| Sb(III) | 5.73 ± 0.41 | 14.97 ± 1.23 [2.6]* | 6.45 ± 0.12 [1.1] |
| Pentostam [Sb(V)] | 28.06 ± 1.98 | 53.09 ± 2.34 [1.9]* | 28.16 ± 2.30 [1.0] |
Promastigotes were grown as described in Materials and Methods for 72 h at 28°C in the presence of increasing concentrations of drugs. Cell viability was determined by using an MTT-based assay. On the other hand, intracellular amastigotes L. major lines were obtained after infection of mouse peritoneal macrophages with the different promastigote lines using a macrophage/parasite ratio of 1:10. After 72 h, drug activity was determined from the percentage of infected cells and the number of amastigotes by cells in drug-treated cultures versus nontreated cultures. The infection was determined by DAPI staining in 200 macrophages/well. Significant differences were determined by using the Student t test (*, P < 0.01).
Resistance indexes (RI) were calculated by dividing the EC50 for Leishmania line overexpressing ABCI4 or ABCI4K/M by that for Leishmania line control (pXG). Data are means from three independent experiments.
Cell densities were measured with an electronic particle counter (Coulter Electronics, United Kingdom) and by direct counting with a hemocytometer.
Table 2.
Heavy metal sensitivity in L. major promastigote linesa
| Metal | Mean EC50 (μM) ± SD [RI]b |
||||||
|---|---|---|---|---|---|---|---|
| pXG | ABCI4c | 2-ABCI4d | ABCI4rev | GFP-ABCI4e | 3FLAG-ABCI4f | ABCI4K/M | |
| Sb(III) | 20.87 ± 1.80 | 53.74 ± 3.35 [2.6]* | 40.61 ± 3.46 [2.0]* | 21.92 ± 0.36 [1.0] | 63.46 ± 2.12 [3.0]* | 50.75 ± 1.32 [2.5]* | 21.16 ± 3.60 [1.0] |
| As(III) | 2.62 ± 0.69 | 5.14 ± 0.50 [2.0]* | 4.97 ± 0.19 [1.9]* | 2.86 ± 0.27 [1.0] | 5.76 ± 0.47 [2.2]* | 5.06 ± 0.37 [2.0]* | 1.97 ± 0.36 [0.8] |
| Cd(II) | 42.36 ± 4.12 | 82.55 ± 8.84 [2.0]* | 40.96 ± 2.30 [1.0] | ||||
| Pb(II) | 825.54 ± 25.36 | 1,321.22 ± 52.14 [1.6]* | 826.15 ± 38.50 [1.0] | ||||
| Cu(II) | 164.31 ± 5.45 | 221.73 ± 4.73 [1.4] | 162.63 ± 4.50 [1.0] | ||||
| Zn(II) | 590.04 ± 0.50 | 728.95 ± 1.43 [1.2] | 555.43 ± 12.60 [0.9] | ||||
| Co(II) | 35.33 ± 1.23 | 35.89 ± 1.57 [1.0] | 38.20 ± 2.01 [1.1] | ||||
| Mn(II) | 30.27 ± 2.96 | 32.68 ± 1.25 [1.1] | 32.13 ± 2.63 [1.1] | ||||
Parasites were grown as described in Materials and Methods for 72 h at 28°C in the presence of increasing concentrations of drugs. Cell viability was determined using an MTT-based assay. Significant differences were determined by using the Student t test (*, P < 0.01).
Resistance indexes (RI) were calculated by dividing the EC50 for Leishmania lines overexpressing ABCI4, GFP-ABCI4, 3FLAG-ABCI4, ABCI4K/M, or reverted line (ABCI4rev) by that for Leishmania control line (pXG). Data are means from three independent experiments.
First ABCI4 transfection event in L. major.
Second ABCI4 transfection event in L. major.
L. major overexpressing ABCI4 fused to GFP in the N-terminal position.
L. major overexpressing ABCI4 fused to 3×FLAG epitope tag in the N-terminal position.
The Sb(III) resistance shown by the promastigote forms of Leishmania was maintained in intracellular amastigotes obtained after infection of mouse peritoneal macrophages (Table 1). Furthermore, amastigotes overexpressing ABCI4 were also resistant to Pentostam, a leishmanicidal drug containing pentavalent antimony, which is reduced to Sb(III) in the amastigote forms. These findings indicated that the resistance indices to antimonials in the intracellular amastigotes overexpressing ABCI4 were very similar to those observed in their promastigote counterparts (Tables 1 and 2).
As far as the mitochondrial localization of ABCI4 and the possibility of its involvement in heme transport is concerned, as described for other ABC transporters, we determined whether porphyrins are a substrate for ABCI4, and consequently, whether the overexpression of ABCI4 confers resistance to some toxic porphyrins such as zinc-protoporphyrin (ZPP), a validated heme analog, and pheophorbide A, which is structurally related to protoporphyrins. As can be seen from Table 1, ABCI4 overexpression ABCI4 confers a significant resistance to ZPP (2-fold) but not to pheophorbide A. The mutant ABCI4K/M showed the same sensitivity levels as observed in control parasites.
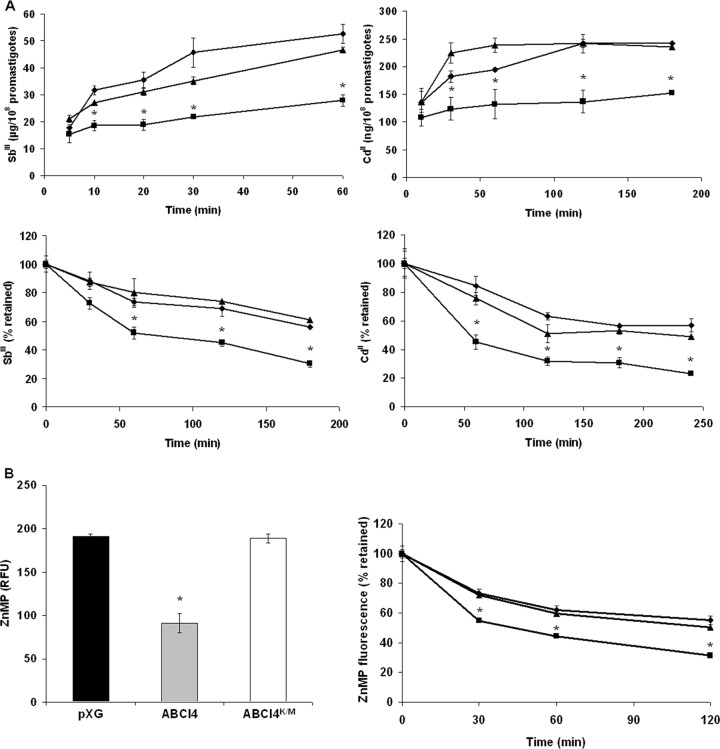
Reduction in the accumulation of Sb(III), Cd(II) and porphyrins due to an increased efflux in L. major overexpressing ABCI4.
To corroborate that ABCI4 has Sb(III) and Cd(II) as potential substrates, the intracellular accumulation of these metal ions was measured in L. major lines as a function of time by ICP-MS and using the fluorimetric dye Leadmium Green, respectively. Accumulation of Sb(III) and Cd(II) was found to be time-dependent (Fig. 2A), with parasites overexpressing ABCI4 accumulating 52% of the Sb(III) and 63% of the Cd(II) of control parasites at the final incubation time (Fig. 2A). The lower Sb(III) accumulation could explain the resistance to antimonials observed in parasites overexpressing the ABCI4 transporter. To determine whether the reduced level of accumulation of heavy metals in the resistant parasites was due to an increased efflux, L. major lines were loaded under conditions that yielded similar amounts of intracellular compounds and the amount of heavy metals retained in the parasites measured at different time points. The efflux of Sb(III) and Cd(II) was found to be time-dependent in the Leishmania lines (Fig. 2A) and was faster for parasites overexpressing ABCI4, thus confirming that the differences in Sb(III) and Cd(II) accumulation are due to an increased ABCI4-mediated efflux activity.
Fig 2.
Time-dependent accumulation and efflux of Sb(III), Cd(II), and ZnMP in Leishmania lines. (A) L. major pXG (control; ◆), overexpressing ABCI4 (■) and ABCI4K/M (▲) were incubated with 100 μM Sb(III) (upper left graphic) or Cd(II) (upper right graphic), and samples were taken at different time points as described in Materials and Methods. Antimony accumulation was measured by ICP-MS. For Cd(II) accumulation, samples were taken at different time points, mixed with Leadmium Green, and the fluorescence determined (upper right graphic) as described in Materials and Methods. The efflux assay was performed after incubation of Leishmania lines with compensated concentrations of Sb(III) (lower left graphic) or Cd(II) (lower right graphic) for 1 h to ensure similar labeling in the different lines. The parasites were then washed and resuspended in PBS buffer without Sb(III) or Cd(II) and pelleted at different time points. (B) Promastigotes of L. major lines pXG (control; black histogram), ABCI4 (gray histogram), and ABCI4K/M (white histogram) were incubated with 10 μM ZnMP for 10 min at 28°C, as described in Materials and Methods (left graphic). Relative fluorescence units (RFU). The efflux assay was performed in L. major lines loaded with different compensated ZnMP concentrations in order to ensure similar labeling, as described in Materials and Methods (right graphic). In both graphics, the accumulated fluorescence at different time points was measured by flow cytometry using a FacsAria Cell Sorter III. The data are the means ± the SD of three independent experiments. Significant differences versus the control line were determined by using the Student t test (*, P < 0.01).
We also determined accumulation of the fluorescent zinc-mesoporphyrin (ZnMP), as a validated heme analog, and found that Leishmania parasites overexpressing ABCI4 present a lower accumulation of ZnMP (2.1-fold) than controls (Fig. 2B) due to a significant efflux (Fig. 2B). Parasites overexpressing ABCI4K/M present a similar accumulation and efflux to control parasites (Fig. 2B). In contrast, the accumulation/efflux of pheophorbide A did not differ between the Leishmania lines (data not shown), thus supporting the sensitivity studies and suggesting that pheophorbide A does not interact with ABCI4.
Involvement of thiol in ABCI4-mediated drug resistance.
An increase in thiol levels has been considered to be one of the main detoxification mechanisms observed in Leishmania lines selected for resistance to Sb(III) (23). In light of this, we determined the drug sensitivity profile for L. major lines overexpressing ABCI4 in the presence of BSO (a γ-glutamylcysteine synthetase inhibitor). After incubation with BSO (3 mM) for 48 h, Leishmania parasites presented a decrease in thiol levels, as measured by flow cytometry using CellTracker (data not shown). The drug sensitivity profile for Leishmania lines treated with BSO showed a significant decrease in the EC50s for Sb(III) and Cd(II), with higher rates in parasites overexpressing ABCI4 than ABCI4K/M or control (Table 3). Consequently, the Sb(III) and Cd(II) resistance of L. major overexpressing ABCI4 is associated with the thiol levels in the parasites, probably due to the ability of the transporter to efflux thiol-conjugated complexes.
Table 3.
Metal sensitivity profile of L. major lines in the presence of BSOa
| Compound | Mean EC50 (μM) ± SD [EC50 decrease]b |
||
|---|---|---|---|
| pXG | ABCI4 | ABCI4K/M | |
| Sb(III) | 20.87 ± 1.80 | 53.74 ± 3.35 | 21.16 ± 3.60 |
| Sb(III) + BSOc | 9.28 ± 0.48 [2.2]* | 15.30 ± 1.23 [3.6]* | 11.48 ± 0.96 [1.9]* |
| Cd(II) | 42.36 ± 4.12 | 82.55 ± 8.84 | 40.96 ± 2.30 |
| Cd(II) + BSO | 6.30 ± 0.23 [6.7]* | 6.85 ± 0.45 [13.7]* | 6.36 ± 0.37 [6.4]* |
Parasites were grown as described in Materials and Methods for 72 h at 28°C in the presence of increasing concentrations of drugs. Cell viability was determined by using an MTT-based assay. Data are means from three independent experiments. Significant differences were determined by the Student t test (*, P < 0.01).
The EC50 decrease, indicated between brackets, was calculated by dividing the EC50s after metal treatment by that for metal plus BSO in each Leishmania line.
3 mM BSO (a γ-glutamylcysteine synthetase inhibitor) was added to the culture medium 48 h before the sensitivity experiment.
Since both conjugation and extrusion of thiol adducts of Sb(III) are required for resistance to antimonials (23), we analyzed the non-protein thiol efflux in L. major lines using ThioStar. In the presence of Sb(III), L. major lines overexpressing ABCI4 showed significantly higher thiol efflux levels than control and mutant lines (Fig. 3A). As expected, no differences in thiol efflux levels were observed in the absence of Sb(III) (Fig. 3B). However, we found that in parasites overexpressing ABCI4, the efflux of ZnMP in Leishmania lines is not thiol-dependent (data not shown). As a result, we suggest that ABCI4 confers resistance to Sb(III) via an efflux of Sb(III)-thiol complex and that this transporter could be considered to be a potential thiol-X-pump.
Fig 3.
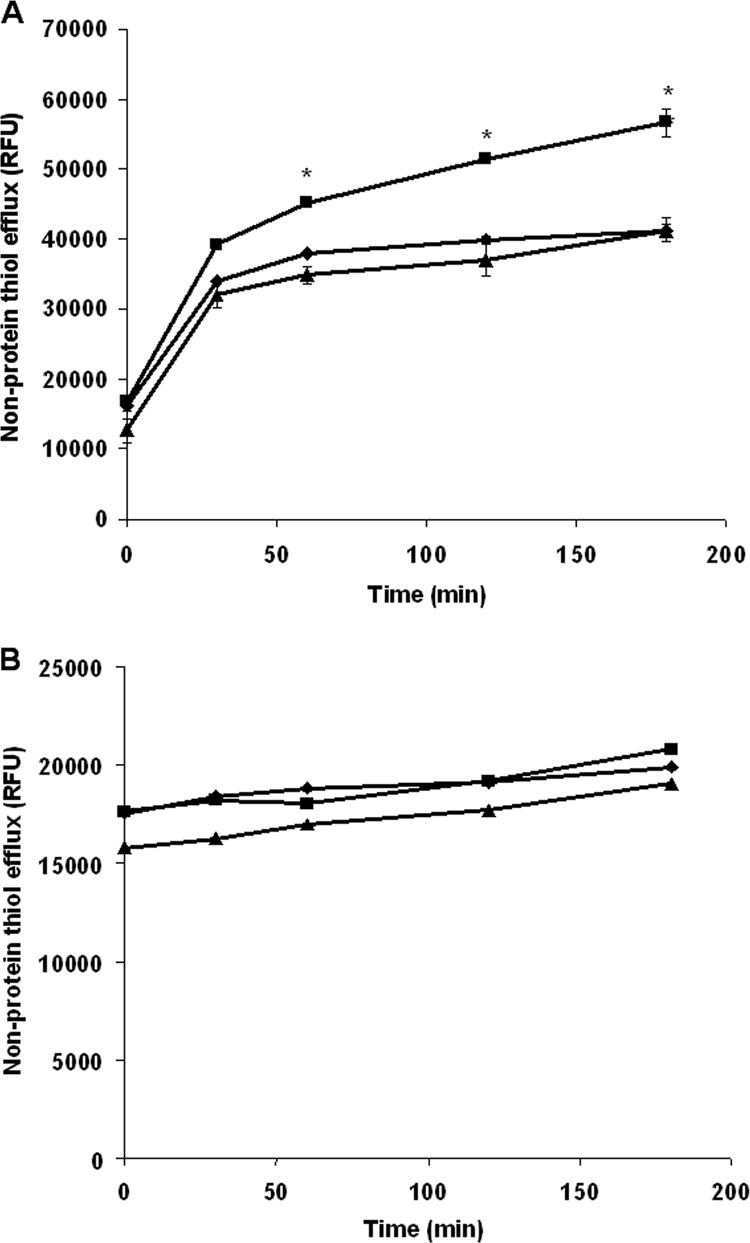
Nonprotein thiol efflux in Leishmania lines. L. major lines pXG (control; ◆), ABCI4 (■), and ABCI4K/M (▲) were incubated with (A) or without (B) 100 μM Sb(III) for 1 h. The promastigotes were then washed with PBS, and the supernatants processed at different time points, as described in Materials and Methods. Sample fluorescence (excitation, 380 nm; emission, 510 nm) was collected using an Infinite F200 luminescence system (Tecan Austria GmbH) and expressed as relative fluorescence units (RFU). The data are the means ± the standard deviation (SD) of three independent experiments. Significant differences versus the control line (*, P < 0.01) were determined by using the Student t test.
ATP and ΔΨm levels in Leishmania lines overexpressing ABCI4.
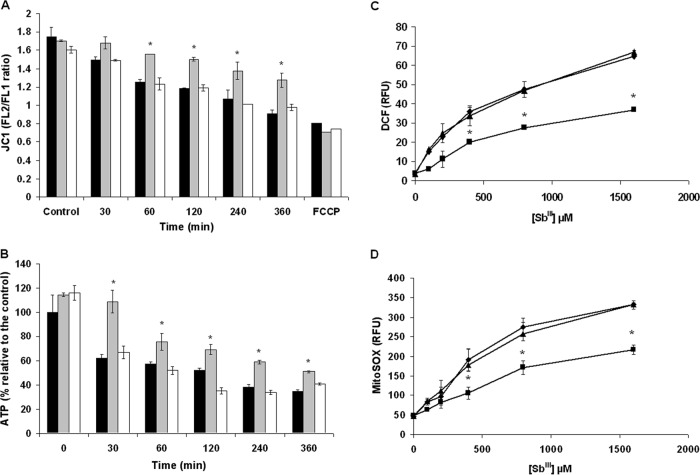
It has previously been reported that antimony induces mitochondrial dysfunction in Leishmania, as demonstrated by a decrease in ΔΨm and ATP loss (41). As a result, we determined whether ABCI4 overexpression confers a protective effect against antimony-induced mitochondrial dysfunction in Leishmania parasites. A decrease in ΔΨm was observed within the first 360 min post-Sb(III) exposure in both control and ABCI4K/M lines (Fig. 4A); however, ΔΨm was altered to a lesser extent after Sb(III) exposure in parasites overexpressing ABCI4 (Fig. 4A).
Fig 4.
Changes of ΔΨm, ATP, and ROS generation in Leishmania lines exposed to Sb(III). L. major lines pXG (control; black histograms), ABCI4 (gray histograms), and ABCI4K/M (white histograms) were preincubated with 100 μM Sb(III) for different times (30, 60, 120, 240, and 360 min) and then incubated with 5 μM JC1 for 10 min for ΔΨm determination (A) or mixed with the same volume of CellTiter-Glo (Promega) for ATP measurement (B), as described in Materials and Methods. The FL2/FL1 fluorescence ratio was measured by flow cytometric analysis. Parasites were pretreated with 10 μM FCCP for depolarization control. Intracellular ROS levels were measured using the fluorescent probes H2DCFDA (C) and MitoSOX Red (D). L. major lines pXG (controls; ◆) and overexpressing ABCI4 (■) and ABCI4K/M (▲) were incubated with different concentrations of Sb(III), as described in Materials and Methods. (C) After Sb(III) treatment, parasites were incubated with 40 nM H2DCFDA for 30 min at 28°C. (D) Prior to the addition of Sb(III), parasites were incubated with 5 μM MitoSOX for 2 h at 28°C. The fluorescence intensity was determined by flow cytometric analysis and expressed as relative fluorescence units (RFU). The data are the means ± the SD of at least three independent experiments. Significant differences versus the control line were determined by using the Student t test (*, P < 0.01).
Since ΔΨm is essential for mitochondrial ATP synthesis, its variation with Sb(III) exposure was monitored in Leishmania lines. Thus, although a progressive decrease in ATP levels was observed after Sb(III) treatment (Fig. 4B), parasites overexpressing ABCI4 maintained significantly higher ATP values than control and ABCI4K/M parasites (Fig. 4B). The above data therefore provide clear evidence for the ability of ABCI4 to protect against the mitochondrial toxicity of antimony as manifested by the maintenance of ΔΨm and ATP levels, probably through reduction of antimony accumulation.
ROS production induced by Sb(III) exposure in Leishmania lines.
The decrease of ΔΨm induced in Leishmania by a variety of drug treatments, including antimonials (42), has been associated with ROS production, which induces damage to the components of the electron transport chain, disrupts mitochondrial function, decreases cellular ATP levels, and produces cell death. We therefore explored the ability of ABCI4 to protect parasites from ROS production after treatment with Sb(III). ROS levels were measured using the cell-permeable probes H2DCFDA and MitoSOX Red, the latter of which selectively targets mitochondria. After treatment with Sb(III) for 48 h, it was observed that ROS generation increased significantly with Sb(III) concentration, in a time-dependent manner, at both cytosolic and mitochondrial levels (Fig. 4C and D). The results showed that Leishmania line overexpressing ABCI4 generated lower ROS levels than control and mutant lines (Fig. 4C and D), probably due to a decreased intracellular accumulation of Sb(III). Remarkably, ROS generation occurred 48 h after treatment with Sb(III), whereas a marked decrease in ΔΨm was observed at 60 min (Fig. 4A), thus showing that the Sb(III)-induced ROS increase is a postmitochondrial event that is significantly reduced by ABCI4 overexpression.
Infectivity and surveillance of Leishmania lines in mouse peritoneal macrophages.
We analyzed whether ABCI4 overexpression confers an advantage in terms of the survival ability of intracellular amastigotes of Leishmania. Specifically, we determined the capacity of Leishmania lines to maintain their infectivity and intracellular replication in mouse peritoneal macrophages after 72 h postinfection, as a measure of the ability to resist stress conditions encountered inside the host mammalian cells. Thus, mouse peritoneal macrophages were infected and the infection index evaluated at 24 and 72 h postinfection to assess their infectivity and the mean number of amastigotes per infected macrophage. In the early stages of infection (24 h postinfection), the different Leishmania lines showed a similar percentage of infection in macrophages (Fig. 5A); similarly, no statistically significant differences were observed in the percentage of infection after 72 h of infection (Fig. 5A). In addition, the study of the ability of intracellular amastigotes to replicate inside the host peritoneal macrophages showed an increased replication of Leishmania lines overexpressing ABCI4 versus control and ABCI4K/M parasites after 72 h of infection (Fig. 5B). Consequently, these results lead to the conclusion that Leishmania lines overexpressing ABCI4 are better able to survival inside host mammalian cells than the other Leishmania lines, although the impact of ABCI4 overexpression on the biological fitness of these parasites still needs to be explored.
Fig 5.
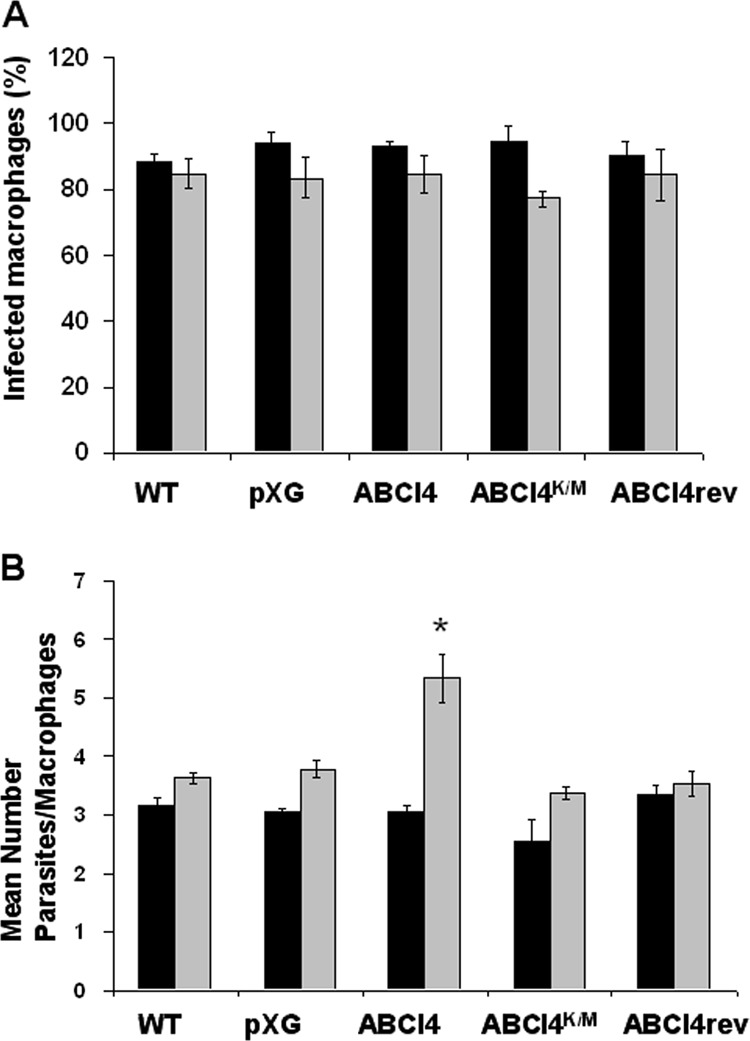
Infectivity and surveillance of Leishmania lines in mouse peritoneal macrophages. Infection of mouse peritoneal macrophages with stationary wild-type (WT), pXG (control), ABCI4, ABCI4K/M, and reverted (ABCI4rev) Leishmania lines using a macrophage/parasite ratio of 1:5 was performed as described in Materials and Methods. The percentage of infected macrophages (A) and the mean number of parasites per macrophage (B) were determined 24 h (black histograms) and 72 h (gray histograms) postinfection. The data are the means ± the SD of three independent experiments. Significant differences versus the control line were determined by using the Student t test (*, P < 0.01 versus respective control).
DISCUSSION
ABC transporters are one of the most intriguing protein families and are of considerable medical significance. However, although eight subfamilies (ABCA to ABCH) (25) have been identified since the first ABC transporter, which is involved in drug resistance, was described in Leishmania (43), only a limited amount of information regarding the functionality of these proteins has been obtained, mainly as regards drug resistance and lipid/heme trafficking (17, 38). In addition, the family of ABC transporters in Leishmania contains an unclassified group of proteins termed as “others” that includes four different transporters with no homology with other eukaryotic ABC proteins, but with orthologues in Trypanosoma brucei and Trypanosoma cruzi (15). We are interested in the functional characterization of this divergent and unclassified group of transporters specific to trypanosomatids and absent in eukaryotic cells. The group “others” includes four different genes, which we have named ABCI1 (LmjF12.1190), ABCI2 (LmjF32.2060), ABCI3 (LmjF33.3040), and ABCI4 (LmjF33.3260), all of which code for half-transporters with a variable topological disposition of the NBD and TMD but with a common requirement to form homo-/heterodimers in order to be functionally active proteins.
We describe here the characterization of ABCI4, a new Leishmania ABC half-transporter with an unusual structure bearing the highly conserved NBD at the COOH terminus and TMD at the NH2 terminus. Coimmunoprecipitation experiments suggest that ABCI4 homodimerizes, although we cannot discard the possibility that it forms heterodimers.
We have determined that ABCI4 has a dual localization in both mitochondria and the PM of Leishmania. Somewhat surprisingly, a similar dual localization has previously been described for human ABCB6, which localizes in both the mitochondria and the PM, thereby contributing to a decreased cellular accumulation of pheophorbide A and hemin (44). Similarly, human ABCG2 has been reported to be distributed in both the PM and in intracellular organelles, including mitochondria, where it regulates ALA-mediated protoporphyrin IX levels via export from mitochondria to the cytosol (45).
We decided to investigate the functionality of ABCI4 by studying the overexpression of this transporter in Leishmania parasites. Interestingly, we found evidence for the antimony transport activity of ABCI4 in Leishmania. Thus, parasites overexpressing ABCI4 were found to be resistant to antimony as well as to other metal ions such as As(III), Cd(II), and Pb(II), suggesting that heavy metals are potential substrates for this transporter.
In addition, ABCI4 overexpression confers resistance to ZPP, a validated fluorescent heme analog transported by ABCG2 (46). Similarly, the resistance to antimonials [Sb(III)] was found to be due to a lower accumulation as a consequence of an increased ABCI4-mediated Sb(III) efflux activity; similar observations were obtained for Cd(II). Since ABCI4 has a significant ability to efflux thiol-conjugated complexes, our results suggest that ABCI4 confers resistance to Sb(III) through an efflux of Sb(III)-thiol complexes. Consequently, the ABCI4 transporter could be considered to be a thiol-X-pump that is able to recognize thiol-conjugated metals. Furthermore, our findings indicate that the ABCI4 localized in the mitochondria is functionally active and may contribute to the traffic of porphyrins as parasites overexpressing ABCI4 are resistant to ZPP, a heme analog, and present a lower accumulation of fluorescent ZnMP due to a significant efflux. In this context, it has previously been described that ABC transporters in mitochondria play important roles in the intracellular traffic of heme and various protoporphyrin intermediates of heme synthesis (47). By analogy, the Leishmania ABCC3 (also known as PGPA or MRPA), which is located in the intracellular vesicular membrane close to the flagellar pocket, has been described to be involved in arsenite and antimonial resistance in Leishmania, although it was initially detected in DNA amplicons (H-circles) of methotrexate-resistant promastigotes (18, 48). The overexpression of MRPA has been described to confer resistance to Sb(III) with an EC50 ∼2-fold increase (19); these results are analogous to the observed in parasites overexpressing ABCI4. In addition, the overexpression of MRPA in combination with other proteins such as γ-glutamylcysteine synthase, increase the resistance index to Sb(III) ∼15-fold (18). These results support that antimony resistance is a multifactorial process that require the combination of different mechanisms to increase the level of resistance. The high levels of ABCC3-mediated arsenite and antimony resistance in Leishmania are associated with the sequestering of metal-thiol conjugates into the intracellular vesicles (18). These conjugates may then move outside the cell by exocytosis, which occurs exclusively through the flagellar pocket, or may be extruded directly outside the cell by a PM thiol-X-efflux pump. Here, the influence of thiol levels on ABCI4-mediated antimonial resistance was confirmed from the decrease in the drug sensitivity profile in Leishmania lines after treatment with BSO and by the ability of this transporter to efflux thiol-conjugated complexes in the presence of Sb(III). We therefore consider that ABCI4 confers resistance to Sb(III) by effluxing an Sb(III)-thiol complex, thereby suggesting that this transporter could be considered to be a putative thiol-X-pump.
Several ABC transporters other than ABCC3 also appear to confer metal resistance by sequestration (49). Thus, the yeast ABC transporter HMT1 confers cadmium tolerance by sequestering phytochelatin (a glutathione-like molecule) cadmium complexes in the fission yeast vacuole (50). Similarly, the yeast ABC transporter YCF1 confers cadmium and arsenite resistance by the vacuolar accumulation of metal-glutathione complexes (51), a mechanism that bears a strong resemblance to the MRP1-encoded transporter found in mammalian cells.
The efflux of Sb(III)-thiol complex observed in the Leishmania line overexpressing ABCI4 was supported by the decrease in mitochondrial toxicity of antimony, as determined by the maintenance of ΔΨm and ATP levels, as well as by the lower ROS production at both a cytosolic and a mitochondrial level.
Leishmania parasites are able to survive in macrophages, which provide a hostile environment due to the production and intracellular release of ROS, among other species. Leishmania survives and replicates by inhibiting the oxidative burst or increasing antioxidant protection (52). Interestingly, we have observed that the Leishmania line overexpressing ABCI4 has a better survival inside mouse peritoneal macrophages than the other parasite lines, as determined by the significantly higher replication at 72 h postinfection. Although the mechanism underlying this increased survival capacity is unknown, we suggest that ABCI4 may be able to transport unidentified substrates associated with detoxification mechanisms.
As described previously, Sb(III) resistance is a multifactorial process (22), with differences in the expression level of the known proteins involved in the resistance depending on the resistant line studied, supporting the heterogeneity among drug-resistant parasites (6). Omics techniques, including high-throughput sequencing technologies and mass spectrometry, have been used to screen the whole genome, transcriptome, and metabolome for detection of molecular adaptations to antimony resistance in Leishmania; however, not all changes in expression levels of proteins involved in Sb(III) resistance are detected in all Leishmania clones resistant to Sb(III), explaining the fail of these techniques to detect that ABCI4 is involved in antimony resistance.
In conclusion, overexpression of ABCI4 in the PM may help to protect cells against the toxic effects of antimony and other compounds by efflux as conjugated thiol complexes. In addition, ABCI4 overexpression in mitochondria decreases the toxicity and accumulation of antimony and porphyrins, probably through efflux of these compounds to the cytosol. Future work should lead to the obtention of null mutants for ABCI4 that could potentiality be used to understand the role of ABCI4 in Leishmania, and to determine whether clinical antimony-resistant Leishmania lines overexpress this transporter.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Spanish grants SAF2012-34267 (to F.G.) and SAF2011-28102 (to S.C.), by the Plan Andaluz de Investigación (Cod. BIO130), and by FEDER funds from the EU to F.G. and S.C.
We thank Stephen M. Beverley (Washington University School of Medicine) for providing the vectors pXG-GFP+2′ and pXG-′GFP+ used throughout this research work. We are grateful to José M. Pérez-Victoria for his valuable comments on the manuscript.
Footnotes
Published ahead of print 28 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00211-13.
REFERENCES
- 1. Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sundar S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 6:849–854 [DOI] [PubMed] [Google Scholar]
- 3. Olliaro PL. 2010. Drug combinations for visceral leishmaniasis. Curr. Opin. Infect. Dis. 23:595–602 [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Hernandez R, Manzano JI, Castanys S, Gamarro F. 2012. Leishmania donovani develops resistance to drug combinations. PLoS Negl. Trop. Dis. 6:e1974. 10.1371/journal.pntd.0001974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Croft SL, Sundar S, Fairlamb AH. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Decuypere S, Vanaerschot M, Brunker K, Imamura H, Muller S, Khanal B, Rijal S, Dujardin JC, Coombs GH. 2012. Molecular mechanisms of drug resistance in natural Leishmania populations vary with genetic background. PLoS Negl. Trop. Dis. 6:e1514. 10.1371/journal.pntd.0001514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brochu C, Wang J, Roy G, Messier N, Wang XY, Saravia NG, Ouellette M. 2003. Antimony uptake systems in the protozoan parasite Leishmania and accumulation differences in antimony-resistant parasites. Antimicrob. Agents Chemother. 47:3073–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dey S, Papadopoulou B, Haimeur A, Roy G, Grondin K, Dou D, Rosen BP, Ouellette M. 1994. High level arsenite resistance in Leishmania tarentolae is mediated by an active extrusion system. Mol. Biochem. Parasitol. 67:49–57 [DOI] [PubMed] [Google Scholar]
- 9. Higgins CF. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67–113 [DOI] [PubMed] [Google Scholar]
- 10. Ouellette M, Drummelsmith J, Papadopoulou B. 2004. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist. Update 7:257–266 [DOI] [PubMed] [Google Scholar]
- 11. Perez-Victoria JM, Perez-Victoria FJ, Parodi-Talice A, Jimenez IA, Ravelo AG, Castanys S, Gamarro F. 2001. Alkyl-lysophospholipid resistance in multidrug-resistant Leishmania tropica and chemosensitization by a novel P-glycoprotein-like transporter modulator. Antimicrob. Agents Chemother. 45:2468–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coelho AC, Beverley SM, Cotrim PC. 2003. Functional genetic identification of PRP1, an ABC transporter superfamily member conferring pentamidine resistance in Leishmania major. Mol. Biochem. Parasitol. 130:83–90 [DOI] [PubMed] [Google Scholar]
- 13. Castanys-Munoz E, Perez-Victoria JM, Gamarro F, Castanys S. 2008. Characterization of an ABCG-like transporter from the protozoan parasite Leishmania with a role in drug resistance and transbilayer lipid movement. Antimicrob. Agents Chemother. 52:3573–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bates SE, Robey R, Miyake K, Rao K, Ross DD, Litman T. 2001. The role of half-transporters in multidrug resistance. J. Bioenerg. Biomembr. 33:503–511 [DOI] [PubMed] [Google Scholar]
- 15. Leprohon P, Legare D, Girard I, Papadopoulou B, Ouellette M. 2006. Modulation of Leishmania ABC protein gene expression through life stages and among drug-resistant parasites. Eukaryot. Cell 5:1713–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perez-Victoria JM, Parodi-Talice A, Torres C, Gamarro F, Castanys S. 2001. ABC transporters in the protozoan parasite Leishmania. Int. Microbiol. 4:159–166 [DOI] [PubMed] [Google Scholar]
- 17. Coelho AC, Cotrim PC. 2013. The role of ABC transporters in drug-resistant Leishmania, p 237–258 In Ponte-Sucre A, Padrón-Nieves EDM. (ed), Drug resistance in leishmania parasites. Springer-Verlag, Vienna, Austria [Google Scholar]
- 18. Légaré D, Richard D, Mukhopadhyay R, Stierhof YD, Rosen BP, Haimeur A, Papadopoulou B, Ouellette M. 2001. The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase. J. Biol. Chem. 276:26301–26307 [DOI] [PubMed] [Google Scholar]
- 19. El Fadili K, Messier N, Leprohon P, Roy G, Guimond C, Trudel N, Saravia NG, Papadopoulou B, Legare D, Ouellette M. 2005. Role of the ABC transporter MRPA (PGPA) in antimony resistance in Leishmania infantum axenic and intracellular amastigotes. Antimicrob. Agents Chemother. 49:1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dey S, Ouellette M, Lightbody J, Papadopoulou B, Rosen BP. 1996. An ATP-dependent As(III)-glutathione transport system in membrane vesicles of Leishmania tarentolae. Proc. Natl. Acad. Sci. U. S. A. 93:2192–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haimeur A, Guimond C, Pilote S, Mukhopadhyay R, Rosen BP, Poulin R, Ouellette M. 1999. Elevated levels of polyamines and trypanothione resulting from overexpression of the ornithine decarboxylase gene in arsenite-resistant Leishmania. Mol. Microbiol. 34:726–735 [DOI] [PubMed] [Google Scholar]
- 22. Leprohon P, Legare D, Ouellette M. 2009. Intracellular localization of the ABCC proteins of Leishmania and their role in resistance to antimonials. Antimicrob. Agents Chemother. 53:2646–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mukhopadhyay R, Dey S, Xu N, Gage D, Lightbody J, Ouellette M, Rosen BP. 1996. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc. Natl. Acad. Sci. U. S. A. 93:10383–10387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mukherjee A, Padmanabhan PK, Singh S, Roy G, Girard I, Chatterjee M, Ouellette M, Madhubala R. 2007. Role of ABC transporter MRPA, gamma-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J. Antimicrob. Chemother. 59:204–211 [DOI] [PubMed] [Google Scholar]
- 25. Mandal G, Wyllie S, Singh N, Sundar S, Fairlamb AH, Chatterjee M. 2007. Increased levels of thiols protect antimony unresponsive Leishmania donovani field isolates against reactive oxygen species generated by trivalent antimony. Parasitology 134:1679–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coelho AC, Messier N, Ouellette M, Cotrim PC. 2007. Role of the ABC transporter PRP1 (ABCC7) in pentamidine resistance in Leishmania amastigotes. Antimicrob. Agents Chemother. 51:3030–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coelho AC, Gentil LG, JF da Silveira Cotrim PC. 2008. Characterization of Leishmania (Leishmania) amazonensis promastigotes resistant to pentamidine. Exp. Parasitol. 120:98–102 [DOI] [PubMed] [Google Scholar]
- 28. Manzano JI, Carvalho L, Perez-Victoria JM, Castanys S, Gamarro F. 2011. Increased glycolytic ATP synthesis is associated with tafenoquine resistance in Leishmania major. Antimicrob. Agents Chemother. 55:1045–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ha DS, Schwarz JK, Turco SJ, Beverley SM. 1996. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol. Biochem. Parasitol. 77:57–64 [DOI] [PubMed] [Google Scholar]
- 30. Perez-Victoria FJ, Gamarro F, Ouellette M, Castanys S. 2003. Functional cloning of the miltefosine transporter: a novel P-type phospholipid translocase from Leishmania involved in drug resistance. J. Biol. Chem. 278:49965–49971 [DOI] [PubMed] [Google Scholar]
- 31. Kennedy ML, Cortes-Selva F, Perez-Victoria JM, Jimenez IA, Gonzalez AG, Munoz OM, Gamarro F, Castanys S, Ravelo AG. 2001. Chemosensitization of a multidrug-resistant Leishmania tropica line by new sesquiterpenes from Maytenus magellanica and Maytenus chubutensis. J. Med. Chem. 44:4668–4676 [DOI] [PubMed] [Google Scholar]
- 32. Sanchez-Canete MP, Carvalho L, Perez-Victoria FJ, Gamarro F, Castanys S. 2009. Low plasma membrane expression of the miltefosine transport complex renders Leishmania braziliensis refractory to the drug. Antimicrob. Agents Chemother. 53:1305–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahto SK, Yoon TH, Rhee SW. 2010. A new perspective on in vitro assessment method for evaluating quantum dot toxicity by using microfluidics technology. Biomicrofluidics 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE. 2004. Pheophorbide, a is a specific probe for ABCG2 function and inhibition. Cancer Res. 64:1242–1246 [DOI] [PubMed] [Google Scholar]
- 35. Sarkar A, Mandal G, Singh N, Sundar S, Chatterjee M. 2009. Flow cytometric determination of intracellular non-protein thiols in Leishmania promastigotes using 5-chloromethyl fluorescein diacetate. Exp. Parasitol. 122:299–305 [DOI] [PubMed] [Google Scholar]
- 36. Goemann IM, Gereben B, Harney JW, Zhu B, Maia AL, Larsen PR. 2010. Substitution of serine for proline in the active center of type 2 iodothyronine deiodinase substantially alters its in vitro biochemical properties with dithiothreitol but not its function in intact cells. Endocrinology 151:821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moreira W, Leprohon P, Ouellette M. 2011. Tolerance to drug-induced cell death favours the acquisition of multidrug resistance in Leishmania. Cell Death Dis. 2:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Campos-Salinas J, Cabello-Donayre M, Garcia-Hernandez R, Perez-Victoria I, Castanys S, Gamarro F, Perez-Victoria JM. 2011. A new ATP-binding cassette protein is involved in intracellular haem trafficking in Leishmania. Mol. Microbiol. 79:1430–1444 [DOI] [PubMed] [Google Scholar]
- 39. Ozvegy C, Varadi A, Sarkadi B. 2002. Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter: modulation of substrate specificity by a point mutation. J. Biol. Chem. 277:47980–47990 [DOI] [PubMed] [Google Scholar]
- 40. Henriksen U, Gether U, Litman T. 2005. Effect of Walker A mutation (K86M) on oligomerization and surface targeting of the multidrug resistance transporter ABCG2. J. Cell Sci. 118:1417–1426 [DOI] [PubMed] [Google Scholar]
- 41. Mehta A, Shaha C. 2006. Mechanism of metalloid-induced death in Leishmania spp.: role of iron, reactive oxygen species, Ca2+, and glutathione. Free Radic. Biol. Med. 40:1857–1868 [DOI] [PubMed] [Google Scholar]
- 42. Sudhandiran G, Shaha C. 2003. Antimonial-induced increase in intracellular Ca2+ through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes. J. Biol. Chem. 278:25120–25132 [DOI] [PubMed] [Google Scholar]
- 43. Ouellette M, Fase-Fowler F, Borst P. 1990. The amplified H circle of methotrexate-resistant Leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 9:1027–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paterson JK, Shukla S, Black CM, Tachiwada T, Garfield S, Wincovitch S, Ernst DN, Agadir A, Li X, Ambudkar SV, Szakacs G, Akiyama S, Gottesman MM. 2007. Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry 46:9443–9452 [DOI] [PubMed] [Google Scholar]
- 45. Kobuchi H, Moriya K, Ogino T, Fujita H, Inoue K, Shuin T, Yasuda T, Utsumi K, Utsumi T. 2012. Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS One 7:e50082. 10.1371/journal.pone.0050082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Desuzinges-Mandon E, Arnaud O, Martinez L, Huche F, Di Pietro A, Falson P. 2010. ABCG2 transports and transfers heme to albumin through its large extracellular loop. J. Biol. Chem. 285:33123–33133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zutz A, Gompf S, Schagger H, Tampe R. 2009. Mitochondrial ABC proteins in health and disease. Biochim. Biophys. Acta 1787:681–690 [DOI] [PubMed] [Google Scholar]
- 48. Callahan HL, Beverley SM. 1991. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J. Biol. Chem. 266:18427–18430 [PubMed] [Google Scholar]
- 49. Ishikawa T, Li ZS, Lu YP, Rea PA. 1997. The GS-X pump in plant, yeast, and animal cells: structure, function, and gene expression. Biosci. Rep. 17:189–207 [DOI] [PubMed] [Google Scholar]
- 50. Ortiz DF, Ruscitti T, McCue KF, Ow DW. 1995. Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J. Biol. Chem. 270:4721–4728 [DOI] [PubMed] [Google Scholar]
- 51. Li ZS, Szczypka M, Lu YP, Thiele DJ, Rea PA. 1996. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J. Biol. Chem. 271:6509–6517 [DOI] [PubMed] [Google Scholar]
- 52. Van Assche T, Deschacht M, RA da Luz Maes L, Cos P. 2011. Leishmania-macrophage interactions: insights into the redox biology. Free Radic. Biol. Med. 51:337–351 [DOI] [PubMed] [Google Scholar]
- 53. Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Klein M, Kolukisaoglu U, Lee Y, Martinoia E, Murphy A, Rea PA, Samuels L, Schulz B, Spalding EJ, Yazaki K, Theodoulou FL. 2008. Plant ABC proteins—a unified nomenclature and updated inventory. Trends Plant Sci. 13:151–159 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.