Abstract
Atazanavir (ATV) causes an elevation of unconjugated hyperbilirubinemia (HBR) as a result of UDP glucuronyltransferase (UGT) 1A1 inhibition. Zinc sulfate (ZnSO4) reduces unconjugated hyperbilirubinemia in individuals with Gilbert's syndrome. We assessed the changes in total, conjugated, and unconjugated bilirubin and the effect on ATV pharmacokinetics (PK) after single and 14-day dosing of ZnSO4. HIV patients, stable on ATV/ritonavir (ATV/r)-containing regimens with a total bilirubin level of >25mmol/liter received 125 mg daily of ZnSO4 as Solvazinc tablets for 14 days. ATV/r and bilirubin concentrations were measured pre-ATV/r dose and 2, 4, 6, 8, and 24 h post-ATV/r dose; before ZnSO4 initiation (phase 1), after a single dose (phase 2) and after 14 days (phase 3). Changes in bilirubin and ATV/r concentrations in the absence or presence of ZnSO4 were evaluated by geometric mean ratios (GMRs) and 90% confidence intervals (CIs; we used phase 1 as a reference). Sixteen male patients completed the study maintaining virologic suppression; ZnSO4 was well tolerated. Statistically significant declines in total bilirubin Cmax and AUC0–24 of 16 and 17% were seen in phase2 and 20% in phase 3. Although there were no significant changes in conjugated bilirubin, unconjugated bilirubin Cmax and AUC0–24 of were lower (17 and 19%, phase 2; 20 and 23% during phase 3). The ATV GMRs (90% CI) for Ctrough, Cmax, and AUC0–24 were 0.74 (0.62 to 0.89), 0.82 (0.70 to 0.97), and 0.78 (0.70 to 0.88). Intake of ZnSO4 decreases total and unconjugated bilirubin and causes modest declines in ATV exposure. ZnSO4 supplementation may be useful in management of ATV-related HBR in selected patients.
INTRODUCTION
Atazanavir (ATV) is a commonly prescribed protease inhibitor (PI) approved for use in combination with other antiretroviral (ARV) agents in naive and treatment-experienced adults with HIV infection (1). ATV is a substrate of cytochrome P450 3A4 (CYP3A4) enzyme and coadministration with low doses of the CYP3A4 inhibitor ritonavir (r) increases ATV concentrations and ensures optimal plasma exposures (2). Most individuals on ATV/r-based regimens receive ATV/r at 300/100 mg once daily.
The most frequent symptomatic adverse events associated to ATV/r intake are scleral icterus and jaundice, leading to discontinuation in <1% of recipients in clinical trials, and the most frequent laboratory abnormality is isolated hyperbilirubinemia (HBR) (3, 4). This is consequent to elevations of unconjugated bilirubin due to inhibition of uridine 5′-diphospho-glucuronosyltransferase (UGT) 1A1 enzyme by ATV and leads to a Gilbert's-like syndrome (3, 4). ATV absorption is stomach acid dependent; plasma concentrations vary within and between patients and correlate with increases in unconjugated bilirubin (5). Within the hepatocytes, UGT 1A1 is responsible for the conjugation of bilirubin to form the soluble glucuronide, which is then eliminated (6).
The increase in unconjugated HBR caused by ATV intake appears within the first week of treatment and resolves promptly when the drug is discontinued (4). However, although there are an increasing number of available ARVs, HIV-infected individuals may need to continue ATV/r-based ARV treatment because of the need of a PI/r-based therapy (i.e., second-line therapy in the presence of a resistant virus) (1) because of its favorable toxicity profile compared to other agents of the same class (i.e., limited hyperlipidemia) (7) or in case of local policies favoring less costly ARV combinations (8). Therefore, strategies to limit unconjugated bilirubin increases may benefit HIV-infected patients.
Zinc sulfate (ZnSO4) intake has been investigated in subjects with Gilbert's syndrome, and it has shown to decrease unconjugated bilirubin levels significantly by inhibiting the enterohepatic cycling of unconjugated bilirubin (9). ZnSO4 is a mineral used to treat or prevent zinc deficiency and can be purchased over the counter in most Western countries. Notably, although zinc therapy may shorten symptoms of common cold (10), chronic administration leading to zinc excess should be avoided since this can impair immune function and may contribute to other adverse experiences (11, 12).
The aim of the present study was to investigate the impact of ZnSO4 acute and short-term administration on concentrations of unconjugated bilirubin in HIV-infected individuals with HBR secondary to ATV/r intake. Furthermore, the pharmacokinetics of ATV/r in the presence of ZnSO4 was studied.
(Some of the results from this study were presented at the Eleventh International Congress on Drug Therapy in HIV Infection, 11 to 15 November 2012, Glasgow, United Kingdom.)
MATERIALS AND METHODS
Subjects.
Adult male and nonpregnant, nonlactating female subjects, with confirmed HIV-1 antibody-positive status, were eligible for enrollment if they provided written informed consent and met the following criteria: (i) if they were between 18 and 65 years old, (ii) if they had a body mass index (BMI) between 18 and 35 kg/m2, and (iii) if they were receiving ongoing treatment with tenofovir, emtricitabine, and ATV/r. Subjects were excluded based on the presence of any active clinically significant disease or AIDS-defining illness; evidence of uncontrolled HIV replication (viral load > 40 copies/ml) or the intake of disallowed concomitant therapies (including the use of zinc supplements for 1 month before screening). Approval for the study was obtained from the Riverside Research Ethics Committee, United Kingdom, and written informed consent was obtained from each subject before study procedures were conducted.
Study design.
This was a 29-day, open-label, three-phase, randomized, crossover, pharmacokinetic study conducted at the Clinical Trial Unit of the St. Stephen's Centre, Chelsea, United Kingdom, and Westminster Hospital, London, United Kingdom. The study was conducted in accordance with the Declaration of Helsinki and with the applicable regulatory requirements (EudraCT 2009-018055-16).
Patients underwent a clinical assessment, and routine laboratory investigations were performed at screening and throughout the study period. The safety and tolerability of the study medications were evaluated throughout the study using the NIAID Division of AIDS table for grading the severity of adult and pediatric adverse events to characterize abnormal findings (published December 2004), vital signs, physical examinations, and clinical laboratory investigations.
After successful screening, eligible subjects were instructed to continue atazanavir/ritonavir (ATV/r) for the whole study and underwent a full pharmacokinetic profile to assess ATV/r and total/conjugated bilirubin concentrations over 24 h in the absence of ZnSO4 intake (day 1). They were then randomized to arm A (ZnSO4 intake from days 2 to 15 and full pharmacokinetic profile on days 2, following a single dose of ZnSO4 and 15, following 14 days of ZnSO4 intake), or arm B (ZnSO4 intake from days 15 to 28 and full pharmacokinetic profile on days 15, following a single dose of ZnSO4 and 28, following 14 days of ZnSO4 intake).
On the pharmacokinetic days, blood samples were drawn pre-ATV/r dose and (after a standard breakfast) 2, 4, 6, 8, and 24 h post dose. On days 2 and 15 for arm A and 15 and 28 for arm B, the ZnSO4 intake was simultaneous to ATV/r intake.
Analytical methods.
Plasma ATV and r concentrations were analyzed by liquid chromatography–mass spectrometry (13). The lower limits of quantification were 10 ng/ml for atazanavir and 5 ng/ml for ritonavir. Intra-assay and interassay coefficients of variation at the low-, medium-, and high-quality controls were <12%. Total and conjugated bilirubin levels were determined by Roche/Hitachi Cobas C systems (Roche Diagnostics GmbH, Mannheim, Germany).
Pharmacokinetic and statistical analysis.
Unconjugated bilirubin values were obtained by subtracting the conjugated value from the total bilirubin value for each sampling time point. The calculated parameters for plasma ATV, r, total bilirubin, conjugated, and unconjugated bilirubin were the concentration measured 24 h after the observed ATV/r dose (C24), the maximum observed concentration (Cmax) according to the dose intake, and the area under the concentration–time curve (AUC) from 0 to 24 h. All of the parameters were calculated by using actual blood sampling times and noncompartmental modeling techniques (WinNonlin Phoenix [version 6.1; Pharsight Corp., Mountain View, CA]). Descriptive statistics, including the geometric mean and 90% confidence intervals (CIs), were calculated for all parameters.
Within-subject changes of ATV concentrations and bilirubin levels (measured in the absence of supplemental zinc intake [the reference value] versus in the presence of ZnSO4) were assessed by calculating the geometric mean ratios (GMRs) and 90% CIs. The CIs were first determined using logarithms of the individual GMR values and then expressed as linear values. The changes in parameters were considered significant when the CIs for the GMR did not exceed 1. The correlation between all measured ATV concentrations and all bilirubin levels was measured following log transformation of the data (since the latter were characterized by a non-normal distribution) by Pearson correlation.
RESULTS
Demographic and clinical characteristics.
Sixteen male patients completed the study. The median (range) age, weight, BMI, and baseline CD4 cell count were: 46 (25 to 51) years, 80 (63 to 90) kg, 25 (18 to 28) kg/m2, and 599 (317 to 777) cell/mm3. Twelve individuals were Caucasian, two were black, and two defined themselves as “other.” All individuals maintained an undetectable viral load throughout the study period. The study drugs were well tolerated, and no grade 3 or 4 adverse events were reported.
Bilirubin concentrations.
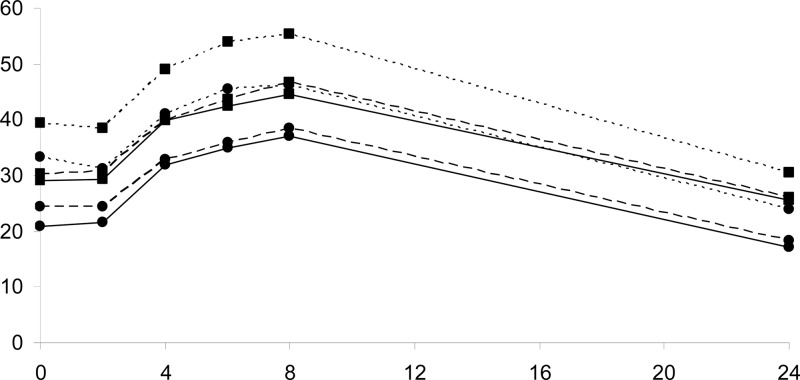
Total bilirubin parameters in the absence and presence of ZnSO4 are illustrated in Table 1. Total bilirubin concentrations are shown in Fig. 1. We observed a decline in total bilirubin Cmax and AUC both after single (GMRs [90% CIs] of 0.84 [0.77 to 0.92] and 0.83 [0.75 to 0.93], respectively) and multiple (GMRs [90% CIs] of 0.80 [0.72 to 0.91] and 0.80 [0.71 to 0.91], respectively) doses of ZnSO4.
Table 1.
Total and unconjugated bilirubin parameters measured during ATV/r intake in the absence or presence of single and multiple (14 days) doses of zinc sulfate (ZnSO4)a
| Parameter | GM (90% CI) |
GMR (90% CI) |
|||
|---|---|---|---|---|---|
| No ZnSO4 | Single dose | Multiple dose | Single dose | Multiple dose | |
| Total bilirubin | |||||
| C24 (μmol/liter) | 31 (28–38) | 26 (24–32) | 26 (23–32) | 0.85 (0.71–1.02) | 0.84 (0.70–1.00) |
| Cmax (μmol/liter) | 56 (51–64) | 47 (43–54) | 45 (41–53) | 0.84 (0.77–0.92) | 0.80 (0.72–0.91) |
| AUC0−24 (μmol · h/liter) | 1,074 (983–1,248) | 896 (817–1,055) | 864 (782–1,032) | 0.83 (0.75–0.93) | 0.80 (0.71–0.91) |
| Unconjugated bilirubin | |||||
| C24 (μmol/liter) | 24 (22–31) | 18 (18–26) | 17 (16–25) | 0.76 (0.55–1.04) | 0.72 (0.54–0.95) |
| Cmax (μmol/liter) | 47 (42–55) | 39 (35–46) | 37 (34–45) | 0.83 (0.75–0.92) | 0.80 (0.70–0.92) |
| AUC0−24 (μmol · h/liter) | 887 (803–1,061) | 721 (655–872) | 684 (615–856) | 0.81 (0.72–0.92) | 0.77 (0.66–0.90) |
C24, bilirubin concentration measured 24 h after atazanavir (without or with ZnSO4) intake; Cmax, maximum bilirubin concentration measured over 24 h after atazanavir (without or with ZnSO4) intake; AUC0−24, AUC exposure to bilirubin measured over 24 h after atazanavir (without or with ZnSO4) intake; GM, geometric mean; GMR, geometric mean ratio; CI, confidence interval.
Fig 1.
Geometric mean total (squares) and unconjugated (circles) bilirubin concentrations measured over 24 h after an atazanavir/ritonavir dose, in the absence of ZnSO4 (dotted lines), after atazanavir/ritonavir plus a single dose of ZnSO4 (dashed lines), and after 14 days of atazanavir/ritonavir plus ZnSO4 coadministration (continuous line).
Although no significant changes in conjugated bilirubin were observed (data not shown), a 28% decline in unconjugated bilirubin C24 was observed after 14 days of ZnSO4 intake. A decline in unconjugated Cmax and AUC both after single (GMRs [90% CIs] of 0.83 [0.75 to 0.92] and 0.81 [0.72 to 0.92], respectively) and multiple (GMRs [90% CIs] of 0.80 [0.70 to 0.92] and 0.77 [0.66 to 0.90], respectively) doses of ZnSO4 (Table 1, Fig. 1) was also measured.
ATV/r pharmacokinetics.
Plasma ATV/r pharmacokinetic parameters in the absence or in the presence of ZnSO4 are reported in Table 2. Steady-state plasma concentrations are shown in Fig. 2.
Table 2.
Pharmacokinetic parameters of ATV and ritonavir measured in the absence or presence of ZnSO4 (zinc sulfate, single and multiple dose)a
| Parameter | GM (90% CI) |
GMR (90% CI) |
|||
|---|---|---|---|---|---|
| No ZnSO4 | Single dose | Multiple dose | Single dose | Multiple dose | |
| ATV | |||||
| C24 (ng/ml) | 538 (485–842) | 454 (419–598) | 400 (377–532) | 0.84 (0.67–1.06) | 0.74 (0.62–0.89) |
| Cmax (ng/ml) | 3,061 (2,722–4,838) | 2,842 (2,592–3,576) | 2,520 (2,349–3,286) | 0.93 (0.80–1.08) | 0.82 (0.70–0.97) |
| AUC0−24 (ng · h/ml) | 35,340 (31,603–46,551) | 31,014 (28,475–37,687) | 27,726 (25,770–34,331) | 0.88 (0.77–1.01) | 0.78 (0.70–0.88) |
| RTV | |||||
| C24 (ng/ml) | 46 (41–80) | 43 (38–67) | 39 (35–64) | 0.93 (0.78–1.12) | 0.85 (0.71–1.03) |
| Cmax (ng/ml) | 1,104 (1,009–1,518) | 891 (816–1,199) | 888 (789–1,274) | 0.81 (0.74–0.88) | 0.80 (0.71–0.91) |
| AUC0−24 (ng · h/ml) | 8,702 (7,980–12,178) | 7,341 (6,777–9720) | 7,312 (6,526–9,847) | 0.84 (0.77–0.92) | 0.84 (0.75–0.94) |
RTV, ritonavir; C24, atazanavir/ritonavir concentration measured 24 h after drug intake; Cmax, maximum concentration measured over 24 h after atazanavir/ritonavir intake; AUC, area under the curve; GM, geometric mean; GMR, geometric mean ratio; CI, confidence interval.
Fig 2.
Geometric mean steady-state atazanavir concentrations over the 24-h dosing interval in the absence (diamonds), in the presence of single dose of ZnSO4 (squares) and in the presence of multiple dose of ZnSO4 (triangles). The inset shows the graph in logarithmic scale.
Although a single dose of ZnSO4 did not seem to have any significant impact on ATV plasma concentrations, 14 days of ZnSO4 intake was associated with a significant decrease in ATV C24 (GMR [90% CI] of 0.74 [0.62 to 0.89]), Cmax (0.82 [0.70 to 0.97]), and AUC (0.78 [0.70 to 0.88]). All individuals with the exception of one (whose ATV concentrations were lower than expected throughout the study) maintained ATV C24 concentrations above the suggested minimum effective concentration (MEC) of 150 ng/ml (14). Interestingly, ritonavir Cmax and AUC0−24 were significantly decreased by single and multiple ZnSO4 dose intake: 19 to 20% and 16%, respectively (Table 2). Finally, a significant correlation between ATV concentrations and total bilirubin levels was observed without zinc (r = 0.298; P = 0.005) and in the presence of zinc (r = 0.332; P < 0.001).
DISCUSSION
The administration of both single and multiple doses of ZnSO4 to HIV-infected individuals stable on ATV/r-based ARV therapy leads to a decrease in both total and unconjugated bilirubin consistent with data in Gilbert's syndrome. A modest decrease in ATV and ritonavir concentrations was also observed, suggesting that ZnSO4 may affect ATV/r absorption.
Because of the need for lifelong ARV intake, strategies on how to manage ARV therapy-related adverse effects are often investigated in order to improve the quality of life for HIV-infected individuals. Unconjugated HBR is the most commonly described laboratory abnormality associated with ATV and ATV/r intake, occasionally leading to icterus, jaundice, and treatment discontinuation (4). In our study, 14-day intake of ZnSO4 has shown to decrease unconjugated bilirubin exposure measured over 24 h by 23%. This is probably a consequence of ZnSO4 chelating unconjugated bilirubin after biliary elimination (15).
A previous study showed that acute and chronic administration of oral ZnSO4 is able to decrease unconjugated bilirubin concentrations in subjects with Gilbert's syndrome. The authors of that suggested a role for zinc in inhibiting the enterohepatic circulation of unconjugated bilirubin and therefore discussed the potential usefulness of ZnSO4 in the treatment of different conditions associated with hyperbilirubinemia (9).
Our data, showing an average 20% reduction in bilirubin due to ZnSO4, would support this theory. However, the chronic use of ZnSO4 in patients without zinc depletion requires further evaluation to ensure the lack of unwanted side effects (11, 12). Furthermore, we observed an effect of zinc intake on the pharmacokinetics of ATV/r. This is unlikely to be due to a metabolic drug interaction mechanism. Interestingly, ritonavir Cmax was decreased by ca. 20% by ZnSO4, suggesting a role of the latter in affecting ritonavir absorption. Zinc was already shown to affect ciprofloxacin exposure and cause a similar decrease (20%) in its absorption (16). Importantly, the chelating effect of zinc that leads to the lower ciprofloxacin exposures is minimized by separating the doses of zinc and ciprofloxacin by at least 2 h. However, in our study ZnSO4 and ATV/r were administered simultaneously. This suggests that the chelating effect of zinc may be extended to lipophilic drugs in the gastrointestinal tract, causing the formation of complexes between the salt substance and the therapeutic drug (in this case ATV or r), and limiting the absorption of the latter. Further study is needed to understand if the benefit of zinc on bilirubin levels can be observed without a parallel impact on ATV concentrations.
ATV absorption seemed to be less affected after a single dose administration of ZnSO4, but Cmax was decreased by 18% after 14 days of ZnSO4 intake. ATV total exposure and Ctrough were also decreased by 18 and 26%, respectively. Whether the effect on ATV PK was due to ZnSO4 intake per se or secondary to the decrease in r absorption is unclear. When looking at the correlation between ATV concentrations and bilirubin, this seems stronger in the presence of ZnSO4, suggesting that ZnSO4 may drive a parallel reduction in both ATV and bilirubin.
Importantly, all individuals, with the exception of one patient, maintained concentrations above the suggested MEC of ATV of 150 ng/ml (14) throughout the study. This patient had lower than expected ATV concentrations both in the presence and in the absence of ZnSO4. Whether the limited decrease in ATV exposure is significant in HIV-infected individuals long term, especially in those with an extensive history of ARV intake and limited future treatment options due to infection by resistant viruses, remains unclear.
Furthermore, one of the limitations of our study was that we investigated the effect of ZnSO4 intake over a short period of 14 days only. Therefore, data on the impact of chronic ZnSO4 intake are warranted. We have not measured the plasma exposure of the other components of the ARV regimen (the NRTIs tenofovir and emtricitabine), since it is unlikely that zinc intake affects their efficacy, because the efficacy of these hydrophilic prodrugs is determined by their intracellular anabolite concentrations (14). The long-term use of metal ions known to bind to the bile and chelate drugs in the gastrointestinal tract to form complexes that are poorly absorbed should be undertaken with caution, and its benefits carefully weighed against its risks.
In conclusion, this is the first study to investigate a novel potential means of managing ATV-associated HBR in HIV-infected individuals. We observed a meaningful decrease in unconjugated and total bilirubin following ZnSO4 intake and a limited decrease in ATV/r plasma exposure. ZnSO4 supplementation may represent a useful tool in the short-term management of ATV-related HBR in selected patients.
ACKNOWLEDGMENTS
We thank the St. Stephen's AIDS Trust Research Team for their hard work and the volunteers who took part in the study.
A.J., G.M., D.B., B.G., and M.B. received travel and research grants from and have been advisers for Tibotec, Roche, Pfizer, GlaxoSmithKline, Bristol-Myers Squibb, Merck Sharp & Dohme, Abbott, and Boehringer Ingelheim.
This study was supported in part by a research grant from Bristol-Myers-Squibb. Funding support was also provided by the St. Stephen's AIDS Trust.
Footnotes
Published ahead of print 20 May 2013
REFERENCES
- 1. British HIV Association 2012. BHIVA guidelines for the treatment of HIV-1 positive adults with antiretroviral therapy 2012. BHIVA, London, United Kingdom [Google Scholar]
- 2. Ford JJ, Boffito MM, Maitland DD, Hill AA, Back DD, Khoo SS, Nelson MM, Moyle GG, Gazzard BB, Pozniak AA. 2006. Influence of atazanavir 200 mg on the intracellular and plasma pharmacokinetics of saquinavir and ritonavir 1,600/100 mg administered once daily in HIV-infected patients. J. Antimicrob. Chemother. 58:1009–1016 [DOI] [PubMed] [Google Scholar]
- 3. Goldsmith DRD, Perry CMC. 2003. Atazanavir. Drugs 63:1679–1693 [DOI] [PubMed] [Google Scholar]
- 4. Croom KF, Dhillon S, Keam SJ. 2009. Atazanavir: a review of its use in the management of HIV-1 infection. Drugs 69:1107–1140 [DOI] [PubMed] [Google Scholar]
- 5. McDonald CC, Uy JJ, Hu WW, Wirtz VV, Juethner SS, Butcher DD, McGrath DD, Farajallah AA, Moyle GG. 2012. Clinical significance of hyperbilirubinemia among HIV-1-infected patients treated with atazanavir/ritonavir through 96 weeks in the CASTLE study. AIDS Patient Care STDS 26:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lankisch TOT, Moebius UU, Wehmeier MM, Behrens GG, Manns MPM, Schmidt RER, Strassburg CPC. 2006. Gilbert's disease and atazanavir: from phenotype to UDP-glucuronosyltransferase haplotype. Hepatology 44:1324–1332 [DOI] [PubMed] [Google Scholar]
- 7. Molina J-M, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, Moyle G, Mancini M, Percival L, Yang R, Wirtz V, Lataillade M, Absalon J, McGrath D, CASTLE Study team 2010. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J. Acquir. Immune Defic. Syndr. 53:323–332 [DOI] [PubMed] [Google Scholar]
- 8. London HIV Consortium 2011. Improving the cost of ARVs in London: summary of ARV prescribing messages for London. London HIV Consortium, London, United Kingdom: www.londonspecializedcommissioning.nhs.uk [Google Scholar]
- 9. Méndez-Sánchez N, Martínez M, González V, Roldán-Valadez E, Flores MA, Uribe M. 2002. Zinc sulfate inhibits the enterohepatic cycling of unconjugated bilirubin in subjects with Gilbert's syndrome. Ann. Hepatol. 1:40–43 [PubMed] [Google Scholar]
- 10. Science M, Johnstone J, Roth DE, Guyatt G, Loeb M. 2012. Zinc for the treatment of the common cold: a systematic review and meta-analysis of randomized controlled trials. CMAJ 184:E551–E561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. L'Abbe MR, Fischer PWF. 1984. The effects of high dietary zinc and copper deficiency on the activity of copper-requiring metalloenzymes in the growing rat. J. Nutr. 114:813–822 [DOI] [PubMed] [Google Scholar]
- 12. Willis MSM, Monaghan SAS, Miller MLM, McKenna RWR, Perkins WDW, Levinson BSB, Bhushan VV, Kroft SHS. 2005. Zinc-induced copper deficiency: a report of three cases initially recognized on bone marrow examination. Am. J. Clin. Pathol. 123:125–131 [DOI] [PubMed] [Google Scholar]
- 13. Else LL, Watson VV, Tjia JJ, Hughes AA, Siccardi MM, Khoo SS, Back DD. 2010. Validation of a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. CORD Conf. Proc. 878:1455–1465 [DOI] [PubMed] [Google Scholar]
- 14. Porte CJ, Back D, Blaschke T, Boucher CA, Fletcher CV, Flexner C, Gerber J, Kashuba AD, Schapiro JM, Burger DM. 2006. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev. Antivir. 4:14 [Google Scholar]
- 15. Ostrow JDJ, Celic LL. 1984. Bilirubin chemistry, ionization and solubilization by bile salts. Hepatology 4:38S–45S [DOI] [PubMed] [Google Scholar]
- 16. Polk RER, Healy DPD, Sahai JJ, Drwal LL, Racht EE. 1989. Effect of ferrous sulfate and multivitamins with zinc on absorption of ciprofloxacin in normal volunteers. Antimicrob. Agents Chemother. 33:1841–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]