Abstract
Although CD8+ cytotoxic T lymphocytes (CTLs) are protective in HIV-1 infection, the factors determining their antiviral efficiency are poorly defined. It is proposed that Gag targeting is superior because of very early Gag epitope presentation, allowing early killing of infected cells before Nef-mediated downregulation of human leukocyte antigen class I (HLA-I). To study Gag epitope presentation kinetics, three epitopes (SL977-85, KF11162-172, and TW10240-249) were genetically translocated from their endogenous location in the Rev-dependent (late) gag gene into the Rev-independent (early) nef gene with concomitant mutation of the corresponding endogenous epitopes to nonrecognized sequences. These viruses were compared to the index virus for CTL-mediated suppression of replication and the susceptibility of this antiviral activity to Nef-mediated HLA-I downregulation. SL9-specific CTLs gained activity after SL9 translocation to Nef, going from Nef sensitive to Nef insensitive, indicating that translocation accelerated infected cell recognition from after to before HLA-I downregulation. KF11-specific CTL antiviral activity was unchanged and insensitive to HLA-I downregulation before and after KF11 translocation, suggesting that already rapid recognition of infected cells was not accelerated. However, TW10-specific CTLs that were insensitive to Nef at the baseline became sensitive with reduced antiviral activity after translocation, indicating that translocation retarded epitope expression. Cytosolic peptide processing assays suggested that TW10 was inefficiently generated after translocation to Nef, compared to SL9 and KF11. As a whole, these data demonstrate that epitope presentation kinetics play an important role in CTL antiviral efficiency, that Gag epitopes are not uniformly presented early, and that the epitope context can play a major role in presentation kinetics.
INTRODUCTION
Human leukocyte antigen class I (HLA-I)-restricted CD8+ cytotoxic T lymphocytes (CTLs) play a protective role in HIV-1 infection, but their control of viral replication in vivo is incomplete and fails to prevent eventual progression to AIDS in the vast majority of infected persons. Multiple factors likely contribute to this failure. One key factor is targeting of CTLs; it has been shown that the magnitude and breadth of Gag-specific CTLs inversely correlate with viremia while Env-targeting CTLs positively correlate with viremia, suggesting that Gag-targeted CTLs are more effective (1–4). The mechanism is unclear, but early Gag epitope presentation kinetics that allow CTL killing of infected cells before Nef-mediated downregulation of HLA-I and virion production have been proposed as an explanation (5).
The influence of epitope presentation kinetics has been demonstrated in two independent studies by translocating epitopes within HIV-1 (6, 7). These studies examined the effect of genetic translocation of epitopes from a late-transcript protein (Pol or Gag) to the early-transcript protein Nef on the antiviral activity of CTLs targeting those epitopes. In both cases, the antiviral activity of CTLs targeting the translocated epitopes was augmented, suggesting that earlier epitope expression allowed earlier infected cell recognition and clearance. A caveat to those studies, however, was that Nef-mediated downregulation of HLA-I, which can reduce the susceptibility of infected cells to CTL recognition (8–10), was not demonstrated (7) or was impaired (6) after epitope translocation into Nef. Thus, it was impossible to separate the direct effect of earlier CTL recognition of infected cells from loss of Nef function, although one study showed that the gain in antiviral activity due to epitope translocation with loss of Nef-mediated HLA-I downregulation exceeded the impact of bypassing downregulation alone (6).
Studies of SIV-specific CTLs have suggested that Gag-specific CTLs can recognize and kill acutely infected cells by recognition of epitopes derived from Gag protein from incoming virions, hours before the translation of any viral proteins (5). However, the antiviral activity of CTLs recognizing several different Gag epitopes has been observed to be susceptible to Nef-mediated HLA-I downregulation (11). Methodological differences related to the infection of target cells likely account for the discrepant results; the killing assays were performed with excess multiplicity of target cell infection, while virus suppression assays for antiviral activity used low multiplicity of target cell infection to allow virus spreading.
In this study, we addressed these issues by creating HIV-1 with genetic translocation of Gag epitopes into the early protein Nef (and modifying the endogenous Gag epitope to ablate CTL recognition) with unimpaired downregulation of HLA-I by Nef after translocation. Index (unmodified NL4-3.1) and epitope-translocated viruses with or without a Nef point mutation to ablate HLA-I downregulation were tested for the effect of epitope presentation kinetics. By controlling the function of Nef, this panel of viruses allows examination of the effects of epitope presentation kinetics by translocation of epitopes from the late protein Gag to the early protein Nef, as well as examination of the impact of Nef-mediated downregulation of HLA-I in the context of epitope expression in Gag versus Nef.
MATERIALS AND METHODS
HIV-1-permissive target cell lines.
The CD4+ HIV-1-permissive cell lines T1 and 1CC-4.14 were used in this study. T1 cells express HLA A*02 (12–14), and the 1CC-4.14 line (11), which was cloned at limiting dilution from a hybridoma generated by fusion of T1 cells and primary CD4+ T lymphocytes, expresses HLA-I from both parental cells, including HLA A*02, A*19, B*51, B*40, C*01, and C*03 from T1 cells and HLA A*01, A*02, B*15, B*57, C*04, and C*06 from primary CD4+ T cells. These cells were maintained in R10 medium, consisting of RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum and 1× penicillin–streptomycin–l-glutamine cocktail (Life Technologies).
HIV-1-specific CTL clones.
CTL clones were obtained by limiting-dilution culture of peripheral blood mononuclear cells (PBMC) from HIV-1-infected persons after epitope-specific stimulation to enrich CTLs of desired specificities, as previously described (15, 16). The clones were maintained in R10 further supplemented with recombinant human interleukin-2 (NIH AIDS Reference and Reagent Repository), with periodic stimulation with anti-CD3 antibody and irradiated allogeneic PBMC. The epitope-specific CTL clones used for this study included two recognizing the Gag77-85 SLYNTVATL (SL9) epitope, four recognizing the Gag162-172 KAFSPEVIPMF (KF11) epitope, and three recognizing the Gag240-249 TSTLQEQIGW (TW10) epitope (Table 1).
Table 1.
HIV-1 Gag-specific CTL clones used in this study
| Epitope | Sequence | HLA restriction | Location | CTL clone | SD50 (log pg/ml)a |
|---|---|---|---|---|---|
| SL9 | SLYNTVATL | A*02 | Gag 75–85 (p17) | S00001-SL9-3.23T | 1.80 |
| S00036-SL9-1.9 | 2.40 | ||||
| KF11 | KAFSPEVIPMF | B*57 | Gag 162–172 (p24) | S00014-KF11-10.2 | 3.73 |
| S00014-KF11-1.3 | 3.21 | ||||
| S00014-KF11-3.22 | 3.34 | ||||
| S00014-KF11-10.12 | 2.90 | ||||
| TW10 | TSTLQEQIGW | B*57 | Gag 240–249 (p24) | S00011-TW10-10.38 | 4.49 |
| S00011-TW10-3.24 | 4.99 | ||||
| S00011-TW10-10.47 | 4.66 |
SD50, functional avidity of the CTL measured as the peptide sensitizing dose required to achieve 50% of the maximum killing by chromium release assay.
Genetic translocation of HIV-1 Gag epitopes into Nef and generation of virus stocks.
For epitope translocations, we used half-genome NL4-3 plasmids p83-2.1 and p83-10 (17) with reporter genes in the vpr loci (18, 19) to produce NL4-3.1 reporter virus with various combinations of Gag and Nef mutations (summarized in Table 2). Sequences of three HIV-1 Gag epitopes (SL977-85, KF11162-172, and TW10240-249) with single flanking residues on both ends were genetically inserted into the Nef reading frame (between amino acids 23 and 24) by overlapping PCR with p83-10 as the template. The resulting PCR product was then swapped into plasmid p83-10 with the XbaI and AccIII restriction enzymes, followed by ligation with T4 DNA ligase (New England BioLabs). Additionally, a version of HIV-1 Nef containing the Gag SL9 epitope was created through substitution mutations between residues 40 and 55 of Nef. Epitope mutations known to disrupt CTL recognition were generated in the p83-2.1 Gag reading frame by site-directed mutagenesis (Agilent Technologies), and the M20A mutation, which ablates HLA-I downregulation by Nef (20, 21), also was generated by site-directed mutagenesis in plasmid p83-10. Coelectroporation (Bio-Rad) of combinations of p83-2.1 and p83-10 derivatives into T1 cells allowed the generation of reporter-expressing viruses with desired combinations of variant Gag and variant Nef, also with or without the M20A mutation in Nef. The NL4-3 reporter virus with unmodified (wild-type [WT]) Gag and unmodified Nef (Gag-WT/Nef-WT) served as the control “index virus.”
Table 2.
HIV-1 constructs used in this study
a Name of the virus based on the status of the Gag epitope and modification of Nef, respectively: x, epitope mutated to nonrecognized variant; i, amino acids inserted to create epitope; r, amino acid substitution to create epitope; WT, unaltered WT HIV-1 Gag or Nef protein sequence; M20A, methionine-to-alanine substitution at amino acid position 20 of Nef.
b Kinetics of transcription for the parent protein (Late, Gag; Early, Nef).
c HLA-I downregulation (Downreg.) function of Nef variant.
Assessment of HLA-A*02 expression on HIV-1-infected cells.
To measure the ability of the modified HIV-1 Nef proteins to downregulate HLA-I on infected cells, T1 cells were infected with the indicated recombinant viruses (Table 2) containing the murine CD24 reporter gene in the vpr locus, followed by flow cytometric analysis as previously described (18, 21). Briefly, the cells were costained with anti-murine CD24–fluorescein isothiocyanate and anti-HLA-A*02–phycoerythrin antibodies (BD Biosciences) 5 days after infection. The degree of A*02 downregulation was calculated as the background-subtracted mean fluorescence intensity of A*02 of the test virus compared to that of the Nef-M20A control virus.
Testing of HIV-1 susceptibility to suppression by HIV-1-specific CTL clones.
The susceptibility of each virus to suppression by the CTL clones was quantified by using a virus suppression assay that has been described previously in detail (11, 14–16). In brief, the target cells were infected with the indicated viruses (Table 2) at a multiplicity of approximately 0.01 tissue culture infectious dose per cell and cocultured in 96-well cell culture plates at a CTL effector-to-target cell ratio of 1.25 × 104 with 5 × 104 cells, in triplicate for each condition. A control with no effector cells was included. Supernatant was assessed for p24 antigen concentration (p24 enzyme-linked immunosorbent assay kit; PerkinElmer).
Virus suppression at 7 days after infection was calculated as follows: Suppression = (log10 pg/ml p24 antigen without CTL) − (log10 pg/ml p24 antigen with CTL). Virus suppression efficiency at 7 days after infection was calculated as follows: Suppression Efficiency = Suppression ÷ (log10 pg/ml p24 antigen without CTL). The Nef impact factor was calculated as previously described (11): Nef Impact Factor = Suppression Efficiency with Functional Nef ÷ Suppression Efficiency with M20A Nef.
In vitro peptide degradation assay.
To assess the processing of Gag epitopes after translocation to the Nef protein, an in vitro peptide degradation assay was performed by the exposure of synthetic peptides containing the Gag epitopes and surrounding Nef sequences to PBMC cytosolic extracts for 60 min, followed by mass spectrometry as described previously (22). In short, 1 nM each synthetic peptide (Bio-Synthesis Inc.) containing a Gag epitope with flanking Nef amino acids (Nef-SL9, MRRAR-SLYNTVATL-YEPA; Nef-KF11, MRRAE-KAFSPEVIPMF-SEPA; Nef-TW10, MRRAT-TSTLQEQIGW-MEPA) was incubated with 20 μg of cytosolic extract. After 1 h, the digested peptide fragments were isolated by trichloroacetic acid purification and identified by mass spectrometry. The mixture was diluted to 400 to 1,600 fmol in 80% water, 15% acetonitrile, and 5% trifluoroethanol; fractionated by reverse-phase high-performance liquid chromatography (Nano-LC; Eksigent); and electrosprayed onto an Orbitrap Discovery mass spectrometer (Thermo) with a flow rate of 400 nl/min as described previously (23). A Nano cHiPLC trap column (200 μm by 0.5 mm ChromXP C18-CL 5 μm 120 Å; Eksigent) was used to remove salts and contaminants from the sample buffers. Peptides were separated in a Nano cHiPLC column (75 μm by 15 cm ChromXP C18-CL 5 μm 300 Å; Eksigent) over a gradient of 2 to 40% acetonitrile with 0.1% formic acid for 20 min. Mass spectra were recorded in the range of 370 to 2,000 Da. Each peptide present in the mixture, the area of the peak it generated, and the proportion of peptides at each time point were calculated with Proteome Discoverer (Thermo). The intensity of a peak generated by a given peptide is proportional to the amount of peptide. Each degradation mixture was run twice on the mass spectrometer.
RESULTS
HIV-1 constructs with Gag epitopes translocated to Nef retain the ability to downregulate HLA-I.
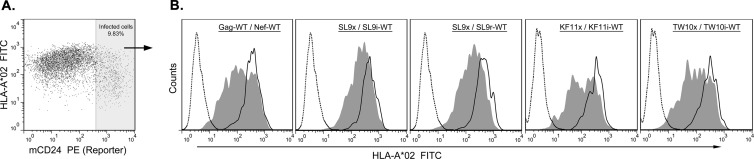
A confounding factor in our previous study to define the role of epitope expression kinetics in CTL antiviral activity was that genetic transfer of a Gag epitope to the C-terminal region of Nef ablated its ability to downregulate HLA-I (6). Three Gag epitopes (SL9, KF11, and TW10) and their flanking amino acids were each translocated genetically into the N-terminal region of Nef (Table 2). Viruses expressing these modified Nef proteins were tested for downregulation of HLA-I on acutely infected cells (Fig. 1). Overall, HLA-I downregulation was similar for these modified Nef proteins, demonstrating that epitope translocation into the N-terminal region of Nef did not impair this function, which is important for CTL evasion.
Fig 1.
Preserved downregulation of HLA-I by modified Nef proteins in HIV-1-infected cells. CD4+ T1 cells were infected with the HIV-1 strains listed in Table 2, with the addition of the murine CD24 reporter gene in the vpr locus. (A) Flow cytometry was performed by gating on the reporter-expressing (infected) cells, with staining for A*02. (B) Results after gating on the infected cells are shown for the indicated viruses, comparing the mutant versions of Nef without (closed lines, filled) and those with (closed lines, unfilled) the M20A mutation, which ablates HLA-I downregulation. Isotype control staining also is shown (dotted lines, unfilled). The percentages of downregulation of HLA-A*02 by WT Nef compared to the corresponding M20A mutant version for Nef-WT, SL9i, SL9r, KF11i, and TW10i were 58% ± 9%, 61% ± 15%, 65% ± 7%, 50% ± 4%, and 62% ± 4%, respectively, based on a minimum of three independent experiments. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
HIV-1 constructs engineered with CTL escape mutations in Gag epitopes maintain replication capacity and acquire resistance to CTLs targeting the epitopes.
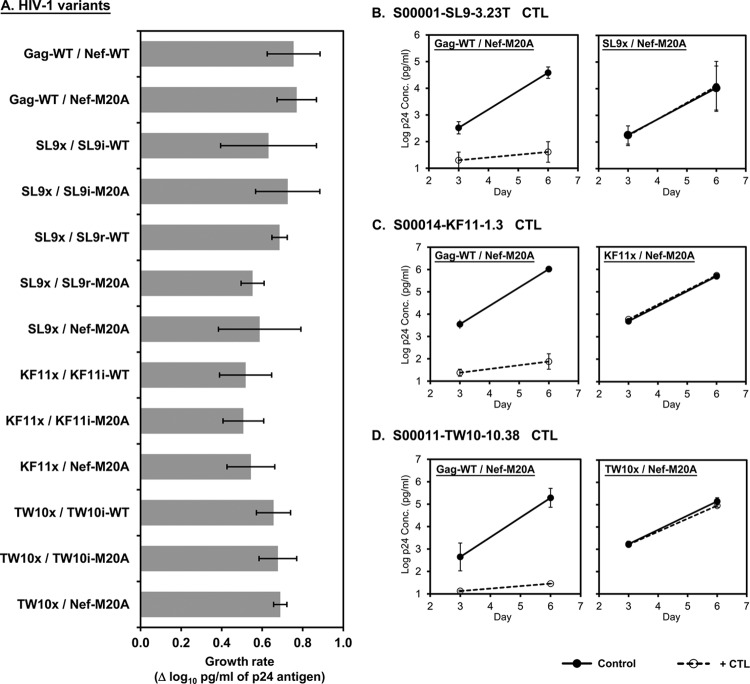
To control for the number of copies of recognized Gag epitopes in the HIV-1 constructs containing translocated Gag epitopes in Nef, genetic mutations were engineered within Gag to ablate CTL recognition of the endogenous cognate epitopes (Table 2). The SL9 epitope was changed to a sequence that we previously found to generate viable HIV-1 that is poorly recognized by SL9-specific CTLs (16). The KF11 and TW10 epitopes were modified to escape mutations that are commonly observed in vivo within persons who have HLA B*57, along with compensatory mutations shown to offset the loss of viral replicative capacity associated with these mutations (24, 25). HIV-1 with these mutations alone or in combination with epitope translocation to Nef showed grossly similar rates of growth (Fig. 2A).
Fig 2.
Preserved replicative capacity of HIV-1 with mutations in endogenous Gag epitopes to ablate CTL antiviral activity. CD4+ T1 cells were infected with the HIV-1 variants indicated and then assessed for replication and susceptibility to Gag-specific CTLs. (A) The slopes of log10 p24 antigen concentrations (pg/ml) between days 3 and 6 after infection are plotted. (B to D) The growth of each virus with the endogenous WT versus the modified Gag epitope with and without CTLs is plotted. All viruses contained the M20A mutation in Nef.
These viruses with mutations in the endogenous Gag epitopes were tested for susceptibility to suppression of viral replication by CTL clones targeting the epitopes (Fig. 2B to D). To maximize the detection of any residual CTL antiviral activity, these viruses also contained the M20A Nef mutation (which ablates the ability of Nef to downregulate HLA-I on infected cells). In contrast to control virus with unmodified Gag, these Gag epitope mutations rendered HIV-1 resistant to the antiviral activity of corresponding Gag-specific CTLs. As a whole, these data indicated that the Gag epitope escape mutations ablated CTL recognition of the endogenous epitope with minimal impact on viral replicative capacity, allowing functional deletion of the endogenous epitopes after genetic translocation to Nef.
At the baseline, Gag SL9-specific CTLs are sensitive to Nef-mediated HLA-I downregulation but translocation of SL9 into Nef markedly increases viral susceptibility to SL9-specific CTLs and bypasses the impact of Nef-mediated HLA-I downregulation.
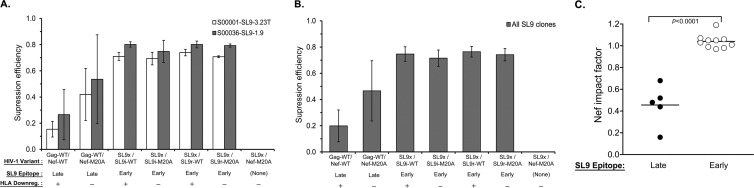
Both versions of HIV-1 with translocation of the SL9 epitope into Nef and functional ablation of the endogenous epitope in Gag were tested for susceptibility to two SL9-specific CTL clones (Fig. 3A and B). The WT index virus (with unmodified Gag and Nef) became more sensitive to CTLs when Nef was altered to contain the M20A mutation, which ablates HLA-I downregulation (Fig. 3A and B, compare Gag-WT/Nef-WT to Gag-WT/Nef-M20A). However, virus with SL9 translocated to Nef was even more sensitive to CTL antiviral activity despite preserved HLA-I downregulation function (Fig. 3A and B, compare Gag-WT/Nef-M20A to SL9x/SL9i-WT and SL9r/SL9r-WT, respectively). Further imposing the M20A mutation to ablate HLA-I downregulation by these viruses did not further increase susceptibility to CTLs (Fig. 3A and B, compare SL9x/SL9i-WT and SL9r/SL9r-WT to SL9x/SL9i-M20A and SL9r/SL9r-M20A), demonstrating that Nef significantly impaired CTL antiviral activity against the index WT virus but conferred no protection for HIV-1 with SL9 expressed in Nef (Fig. 3C). These results suggested that the kinetics of SL9 expression in the WT virus were late enough to allow Nef to reduce SL9 presentation, while SL9 expression after translocation to Nef accelerated epitope presentation to precede the downregulation of HLA-I by Nef.
Fig 3.
Susceptibility of SL9-translocated HIV-1 to SL9-specific CTLs. As described in Materials and Methods, the suppression efficiency of the SL9-specific CTLs for the HIV-1 variants indicated was evaluated by inhibition assays. (A) The mean virus suppression efficiency (based on the log10 p24 concentration [pg/ml] on day 7) for each virus indicated is plotted for two SL9-specific CTL clones. The error bars depict the standard deviations of triplicates within each experiment and across all of the experiments with the same CTL clone. (B) The mean level of suppression of each virus indicated across all of the clones tested is plotted, and the error bars depict the standard deviations across all of the clones tested. (C) The Nef impact factor is plotted for the SL9 epitope expressed endogenously in Gag (Late) versus that translocated in Nef (Early). Each dot represents an independent experiment with an SL9-specific CTL clone. The horizontal line indicates the mean of individual experiments in each group, and the P value is the result of a two-tailed Student t test.
At the baseline, Gag KF11-specific CTLs are minimally sensitive to Nef-mediated HLA-I downregulation and translocation of KF11 into Nef does not enhance the antiviral activity of KF11-specific CTLs or significantly change susceptibility to HLA-I downregulation by Nef.
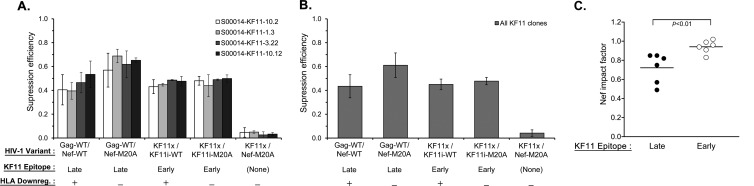
In contrast to SL9-specific CTLs, KF11-specific CTLs were minimally affected by Nef-mediated downregulation of HLA-I at the baseline (Fig. 4A). Translocation of the KF11 epitope into Nef did not enhance the antiviral activity of KF11-specific CTLs or significantly alter their insensitivity to Nef-mediated HLA-I downregulation (Fig. 4B and C). These results suggested that KF11 epitope production from its endogenous position in Gag is rapid and temporally precedes the downregulation of HLA-I by Nef and that translocation into the early protein Nef did not yield any further temporal advantage to CTL antiviral activity.
Fig 4.
Susceptibility of KF11-translocated HIV-1 to KF11-specific CTLs. As described in Materials and Methods, the suppression efficiency of the KF11-specific CTLs for the HIV-1 variants indicated was evaluated by inhibition assays. (A) The mean virus suppression efficiency (based on the log10 p24 concentration [pg/ml] on day 7) for each virus indicated is plotted for four KF11-specific CTL clones. The error bars depict the standard deviations of triplicates within each experiment and across all of the experiments with the same CTL clone. (B) The mean level of suppression of each virus indicated across all of the clones tested is plotted, and the error bars depict the standard deviations across all of the clones tested. (C) The Nef impact factor is plotted for the KF11 epitope expressed endogenously in Gag (Late) versus that translocated in Nef (Early). Each dot represents an independent experiment with a KF11-specific CTL clone. The horizontal line indicates the mean of individual experiments in each group, and the P value is the result a two-tailed Student t test.
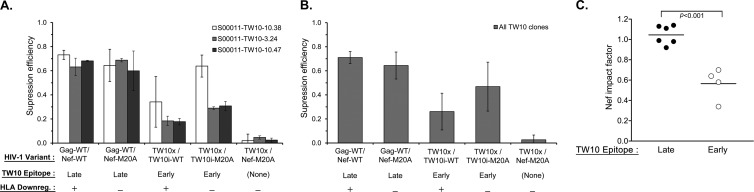
Translocation of the Gag TW10 epitope into Nef reduces the antiviral efficacy of TW10-specific CTLs and increases their sensitivity to HLA-I downregulation by Nef.
CTLs targeting the Gag TW10 epitope were tested for antiviral activity against index and modified viruses. With endogenous TW10 in Gag, CTL antiviral activity was relatively unaffected by Nef-mediated HLA-I downregulation (Fig. 5A). In contrast to the SL9 and KF11 epitopes, translocation of TW10 to Nef both reduced the antiviral activity of the CTLs and rendered them susceptible to the impact of Nef-mediated HLA-I downregulation (Fig. 5B and C). This suggested that epitope translocation reduced the efficiency and/or slowed the kinetics of TW10 expression to prevent CTL recognition of infected cells before Nef-mediated HLA-I downregulation.
Fig 5.
Susceptibility of TW10-translocated HIV-1 to TW10-specific CTLs. As described in Materials and Methods, the suppression efficiency of the TW10-specific CTLs for the HIV-1 variants indicated was evaluated by inhibition assays. (A) The mean amount of virus suppression efficiency (based on the log10 p24 concentration [pg/ml] on day 7) for each virus indicated is plotted for three TW10-specific CTL clones. The error bars depict the standard deviations of the triplicates within each experiment and across all of the experiments with the same CTL clone. (B) The mean level of suppression of each virus indicated across all of the clones tested is plotted, and the error bars depict the standard deviations across all of the clones tested. (C) The Nef impact factor is plotted for the TW10 epitope expressed endogenously in Gag (Late) versus that translocated in Nef (Early). Each dot represents an independent experiment with a TW10-specific CTL clone. The horizontal line indicates the mean of individual experiments in each group, and the P value is the result of a two-tailed Student t test.
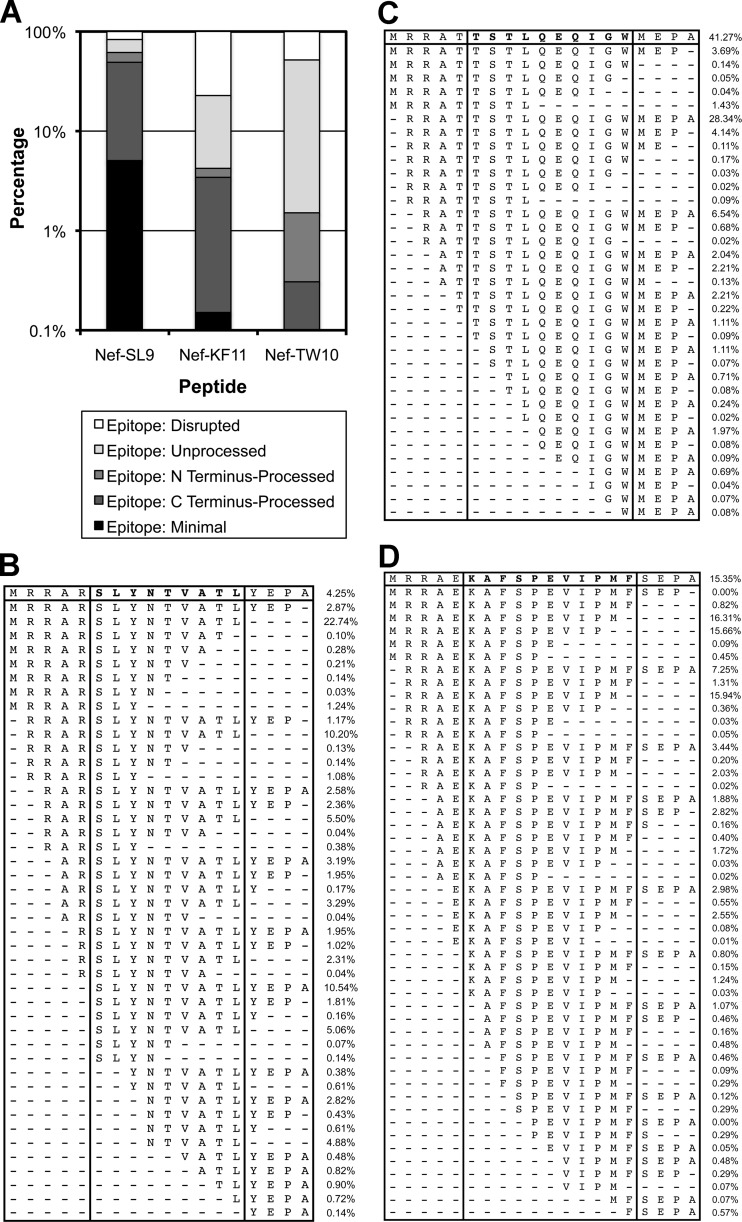
The translocated TW10 Gag epitope in Nef is inefficiently processed compared to the translocated SL9 and KF11 Gag epitopes.
To examine the efficiency of Gag epitope processing after translocation into Nef, peptides corresponding to these Gag epitopes in the context of the flanking Nef residues were exposed to PBMC cytosolic extracts (22). The resulting peptide fragmentation revealed different efficiencies for the three epitopes (Fig. 6). The SL9 and KF11 epitopes were generated as optimally processed minimal epitope sequences (5.3 and 0.2% of the total peptides, respectively), while the minimal TW10 epitope was not produced. Considering peptide fragments that were properly cleaved at either the N or the C terminus, the SL9 epitope was the most efficiently processed (64.4% of the total), followed by the KF11 epitope (5.0% of the total), and the TW10 epitope was the least efficiently processed (2.6% of the total). These findings indicated that processing of the translocated TW10 epitope was particularly inefficient, suggesting an explanation for the poor antiviral function of TW10-specific CTLs against HIV-1 with Nef-TW10.
Fig 6.
Processing efficiency of Gag epitopes translocated to Nef. Synthetic peptides corresponding to the Gag epitopes and their flanking sequences after translocation to Nef were incubated with PBMC cytosolic extracts, and the resulting degradation peptides were analyzed by mass spectrometry. (A) The percentages of peptide cleavage products representing the minimal epitopes, partially processed epitopes (cleaved at the N or C terminus), unprocessed epitopes (not cleaved at either terminus), and disrupted epitopes are indicated on a log10 scale. (B) Full list of the cleavage products detected. The top row indicates the input peptide.
DISCUSSION
The HIV-1 life cycle is relatively rapid after the infection of a target CD4+ T lymphocyte, with an eclipse period of approximately 1 to 2 days before the release of new virions (13, 26, 27). This period includes the time required for viral entry, uncoating, reverse transcription, integration, transcription, and nuclear export of viral RNA, which are steps required before viral protein translation and processing into epitopes via the HLA-I pathway. The interval between the start of translation and the release of viral progeny is the window in which CTLs have the opportunity to recognize infected cells and arrest viral replication. Although it has been demonstrated that some HIV-1-specific CTLs can lyse infected cells within this interval (13, 28), clearly CTLs do not achieve this consistently in vivo. Other than viral sequence variation leading to escape mutation (17), at least two factors likely contribute to the inability of CTLs to clear infected cells during eclipse: varying kinetics of epitope expression (6, 7) (due to factors such as differential timing of viral protein production [29]) and Nef-mediated downregulation of HLA-I (8–11). Thus, the timing of epitope presentation in relation to the viral life cycle and downregulation of HLA-I by Nef is a key determinant of the ultimate antiviral activity of CTLs. Earlier studies partially addressed this issue by manipulating epitope kinetics but were unable to separate the contribution of earlier infected cell killing relative to viral replication itself from that of HLA-I downregulation by Nef.
We studied the impact of translocating three epitopes from Gag to Nef, thereby moving these epitopes from a late to an early protein. The genetic modification of Nef with translocated Gag epitopes did not affect the ability of Nef to downregulate HLA-I on infected cells, allowing us to separate the manipulation of this activity. First examining the WT virus with the epitopes in their endogenous position within Gag, it was apparent that despite the common protein source of their epitopes, the Gag-specific CTLs varied in their susceptibility to the downregulation of HLA-I by Nef. This suggested that the kinetics of epitope expression/recognition might vary. SL9-specific CTLs were susceptible to Nef, while KF11- and TW10-specific CTLs were relatively unaffected, despite the higher functional avidity of the SL9-specific CTLs (Table 1). This suggested that the latter two epitopes might be presented before the downregulation of HLA-I.
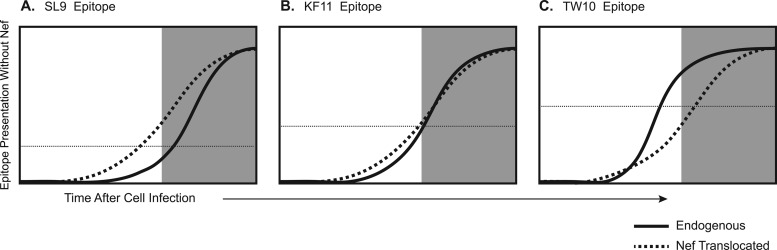
The functional result of translocation of these epitopes to the early protein Nef gave further insight into the kinetics of epitope presentation. Translocation of the SL9 epitope both enhanced the antiviral activity of SL9-specific CTLs and rendered this activity resistant to Nef-mediated HLA-I downregulation. This suggested that epitope presentation was accelerated and increased CTL antiviral efficiency by shifting killing from after to before virion release, as well as from after to before functionally significant HLA-I downregulation by Nef. Translocation of the KF11 epitope, however, had no impact on the antiviral activity of KF11-specific CTLs or their resistance to the effect of Nef. This indicated that earlier expression of this epitope in Nef did not yield an additional benefit, suggesting that the KF11 epitope is equally presented early before virion release and downregulation of HLA-I when expressed in Gag. Finally, although the antiviral activities of TW10-specific CTLs were much higher against the WT index virus and also insensitive to HLA-I downregulation by Nef, translocation of the TW10 epitopes to Nef diminished antiviral activity and rendered these CTLs susceptible to Nef. Examination of epitope processing with an in vitro peptide degradation assay indicated that TW10 processing after translocation to Nef was inefficient, suggesting that impairment of epitope processing can significantly alter the antiviral activity of CTLs directly and via susceptibility to Nef, in agreement with prior studies demonstrating the impact of mutations in epitope-flanking amino acids that result in the escape of HIV-1 from CTLs (30, 31). Figure 7 provides a schematic summary of the results.
Fig 7.
Proposed kinetic relationships of epitope presentation, Nef-mediated HLA-I downregulation, and CTL recognition of HIV-1-infected cells. On the basis of our data and the functional avidity of the CTL clones used in this study, the presumed epitope presentation kinetics are depicted. The x axis represents time after a cell is infected, and the y axis represents the amount of epitope presentation in the absence of Nef. The shaded area represents the time that Nef-mediated downregulation of HLA-I would be in effect.
These data indicate heterogeneity in the presentation and recognition of Gag epitopes from HIV-1 and suggest that early epitope presentation leading to early killing of infected cells is not necessarily a property of Gag-specific CTLs in general. This agrees with recent work demonstrating that Gag-specific CTLs vary in their timing of recognizing infected cells (28). Although it has been observed that the magnitude and/or breadth of Gag targeting by CTLs correlates with lower viremia (1–4), these statistical associations have been demonstrable only with relatively large cohorts, suggesting a relatively weak and/or inconsistent advantage of Gag targeting. An explanation for this could be that the earliness of Gag epitope presentation is not uniform, as suggested by our data, or that other non-Gag epitopes may also vary in their antiviral impact.
A recent report by Rajapaksa et al. (32) suggested that HLA class I B types are less susceptible to Nef-mediated downregulation than A types are. It is possible that a greater effect of Nef on A*02 versus B*57 could be a contributing factor in the impact of SL9 epitope translocation to Nef, which resulted in a marked loss of Nef susceptibility compared to the other epitopes. Another recent report from our group, however, failed to demonstrate a significant difference in the timing or magnitude of A*02 versus B*57 downregulation by Nef and suggested that epitope targeting is the major determinant of CTL susceptibility to Nef (likely because of efficiency of processing and presentation), independent of HLA restriction or protein targeting (28), although this did not exclude a contribution by HLA restriction.
Finally, interpretation of our findings is dependent on the context of target cell infection. It has been demonstrated that the early-killing phenomenon first described by Sacha et al. (5) varies, depending on the virus inoculum, and that CTLs targeting different epitopes require different amounts of input virus to achieve early killing (28). Our earlier data obtained with a virus suppression assay have suggested that most Gag-specific CTLs are susceptible to Nef (10, 11, 21). In these assays, a low initial inoculum of virus is allowed to spread, and thus the dose of virus for each infected cell is unclear in comparison to controlled single-round infections such as those used by Sacha et al. (5). However, our data are consistent with early killing of infected cells by KF11- and TW10-specific CTLs under these conditions.
Additionally, our data indicate that epitopes from the early protein Nef are not necessarily presented early in the viral life cycle. We found that translocation of the TW10 Gag epitope (for which early presentation is very efficient [28]) to Nef reduced CTL recognition of epitope expression to lag behind Nef-mediated HLA-I downregulation (Fig. 6C). This agrees with our prior finding that Nef-specific CTLs can be susceptible to HLA-I downregulation by Nef in virus suppression assays (11). Thus, while the kinetics of expression of the parent protein may influence the timing of epitope presentation, other varying factors, such as efficiency of processing, transport, and HLA-I binding, are important in the ultimate timing of presentation and recognition by CTLs.
In conclusion, our data indicate that Gag-derived CTL epitopes vary in their kinetics of presentation and triggering of CTL recognition of acutely infected cells. Of the three Gag epitopes studied, two appeared to yield CTL recognition before Nef-mediated HLA-I downregulation and one yielded relatively late CTL recognition that followed Nef-mediated HLA-I downregulation. Thus, while early presentation of Gag epitopes could be a contributing factor in the protective effect of Gag targeting by CTLs, it is not universal. The protein source of epitopes is only one factor of many that determine the ultimate timing of CTL recognition of infected cells.
ACKNOWLEDGMENTS
This work was funded by PHS grants AI043203 and AI051970 and a generous gift from the AIDS Healthcare Foundation.
Footnotes
Published ahead of print 5 June 2013
REFERENCES
- 1. Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53 [DOI] [PubMed] [Google Scholar]
- 2. Masemola A, Mashishi T, Khoury G, Mohube P, Mokgotho P, Vardas E, Colvin M, Zijenah L, Katzenstein D, Musonda R, Allen S, Kumwenda N, Taha T, Gray G, McIntyre J, Karim SA, Sheppard HW, Gray CM. 2004. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. J. Virol. 78:3233–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rivière Y, McChesney MB, Porrot F, Tanneau-Salvadori F, Sansonetti P, Lopez O, Pialoux G, Feuillie V, Mollereau M, Chamaret S, et al. 1995. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res. Hum. Retroviruses 11:903–907 [DOI] [PubMed] [Google Scholar]
- 4. Rolland M, Heckerman D, Deng W, Rousseau CM, Coovadia H, Bishop K, Goulder PJ, Walker BD, Brander C, Mullins JI. 2008. Broad and Gag-biased HIV-1 epitope repertoires are associated with lower viral loads. PLoS One 3:e1424. 10.1371/journal.pone.0001424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sacha JB, Chung C, Rakasz EG, Spencer SP, Jonas AK, Bean AT, Lee W, Burwitz BJ, Stephany JJ, Loffredo JT, Allison DB, Adnan S, Hoji A, Wilson NA, Friedrich TC, Lifson JD, Yang OO, Watkins DI. 2007. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol. 178:2746–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ali A, Lubong R, Ng H, Brooks DG, Zack JA, Yang OO. 2004. Impacts of epitope expression kinetics and class I downregulation on the antiviral activity of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. J. Virol. 78:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Baalen CA, Guillon C, van Baalen M, Verschuren EJ, Boers PH, Osterhaus AD, Gruters RA. 2002. Impact of antigen expression kinetics on the effectiveness of HIV-specific cytotoxic T lymphocytes. Eur. J. Immunol. 32:2644–2652 [DOI] [PubMed] [Google Scholar]
- 8. Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397–401 [DOI] [PubMed] [Google Scholar]
- 9. Tomiyama H, Akari H, Adachi A, Takiguchi M. 2002. Different effects of Nef-mediated HLA class I down-regulation on human immunodeficiency virus type 1-specific CD8(+) T-cell cytolytic activity and cytokine production. J. Virol. 76:7535–7543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang OO, Nguyen PT, Kalams SA, Dorfman T, Gottlinger HG, Stewart S, Chen IS, Threlkeld S, Walker BD. 2002. Nef-mediated resistance of human immunodeficiency virus type 1 to antiviral cytotoxic T lymphocytes. J. Virol. 76:1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adnan S, Balamurugan A, Trocha A, Bennett MS, Ng HL, Ali A, Brander C, Yang OO. 2006. Nef interference with HIV-1-specific CTL antiviral activity is epitope specific. Blood 108:3414–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salter RD, Howell DN, Cresswell P. 1985. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics 21:235–246 [DOI] [PubMed] [Google Scholar]
- 13. Yang OO, Kalams SA, Rosenzweig M, Trocha A, Jones N, Koziel M, Walker BD, Johnson RP. 1996. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J. Virol. 70:5799–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang OO, Kalams SA, Trocha A, Cao H, Luster A, Johnson RP, Walker BD. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bennett MS, Ng HL, Ali A, Yang OO. 2008. Cross-clade detection of HIV-1-specific cytotoxic T lymphocytes does not reflect cross-clade antiviral activity. J. Infect. Dis. 197:390–397 [DOI] [PubMed] [Google Scholar]
- 16. Bennett MS, Ng HL, Dagarag M, Ali A, Yang OO. 2007. Epitope-dependent avidity thresholds for cytotoxic T-lymphocyte clearance of virus-infected cells. J. Virol. 81:4973–4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang OO, Sarkis PT, Ali A, Harlow JD, Brander C, Kalams SA, Walker BD. 2003. Determinants of HIV-1 mutational escape from cytotoxic T lymphocytes. J. Exp. Med. 197:1365–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ali A, Jamieson BD, Yang OO. 2003. Half-genome human immunodeficiency virus type 1 constructs for rapid production of reporter viruses. J. Virol. Methods 110:137–142 [DOI] [PubMed] [Google Scholar]
- 19. Ali A, Yang OO. 2006. A novel small reporter gene and HIV-1 fitness assay. J. Virol. Methods 133:41–47 [DOI] [PubMed] [Google Scholar]
- 20. Akari H, Arold S, Fukumori T, Okazaki T, Strebel K, Adachi A. 2000. Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation. J. Virol. 74:2907–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ali A, Ng HL, Dagarag MD, Yang OO. 2005. Evasion of cytotoxic T lymphocytes is a functional constraint maintaining HIV-1 Nef expression. Eur. J. Immunol. 35:3221–3228 [DOI] [PubMed] [Google Scholar]
- 22. Le Gall S, Stamegna P, Walker BD. 2007. Portable flanking sequences modulate CTL epitope processing. J. Clin. Invest. 117:3563–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang SC, Martin E, Shimada M, Godfrey SB, Fricke J, Locastro S, Lai NY, Liebesny P, Carlson JM, Brumme CJ, Ogbechie OA, Chen H, Walker BD, Brumme ZL, Kavanagh DG, Le Gall S. 2012. Aminopeptidase substrate preference affects HIV epitope presentation and predicts immune escape patterns in HIV-infected individuals. J. Immunol. 188:5924–5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crawford H, Prado JG, Leslie A, Hue S, Honeyborne I, Reddy S, van der Stok M, Mncube Z, Brander C, Rousseau C, Mullins JI, Kaslow R, Goepfert P, Allen S, Hunter E, Mulenga J, Kiepiela P, Walker BD, Goulder PJ. 2007. Compensatory mutation partially restores fitness and delays reversion of escape mutation within the immunodominant HLA-B*5703-restricted Gag epitope in chronic human immunodeficiency virus type 1 infection. J. Virol. 81:8346–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schneidewind A, Tang Y, Brockman MA, Ryland EG, Dunkley-Thompson J, Steel-Duncan JC, St John MA, Conrad JA, Kalams SA, Noel F, Allen TM, Christie CD, Feeney ME. 2009. Maternal transmission of human immunodeficiency virus escape mutations subverts HLA-B57 immunodominance but facilitates viral control in the haploidentical infant. J. Virol. 83:8616–8627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271:1582–1586 [DOI] [PubMed] [Google Scholar]
- 27. Srivastava KK, Fernandez-Larsson R, Zinkus DM, Robinson HL. 1991. Human immunodeficiency virus type 1 NL4-3 replication in four T-cell lines: rate and efficiency of entry, a major determinant of permissiveness. J. Virol. 65:3900–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen DY, Balamurugan A, Ng HL, Cumberland WG, Yang OO. 2012. Epitope targeting and viral inoculum are determinants of Nef-mediated immune evasion of HIV-1 from cytotoxic T lymphocytes. Blood 120:100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ranki A, Lagerstedt A, Ovod V, Aavik E, Krohn KJ. 1994. Expression kinetics and subcellular localization of HIV-1 regulatory proteins Nef, Tat and Rev. in acutely and chronically infected lymphoid cell lines. Arch. Virol. 139:365–378 [DOI] [PubMed] [Google Scholar]
- 30. Milicic A, Price DA, Zimbwa P, Booth BL, Brown HL, Easterbrook PJ, Olsen K, Robinson N, Gileadi U, Sewell AK, Cerundolo V, Phillips RE. 2005. CD8+ T cell epitope-flanking mutations disrupt proteasomal processing of HIV-1 Nef. J. Immunol. 175:4618–4626 [DOI] [PubMed] [Google Scholar]
- 31. Yokomaku Y, Miura H, Tomiyama H, Kawana-Tachikawa A, Takiguchi M, Kojima A, Nagai Y, Iwamoto A, Matsuda Z, Ariyoshi K. 2004. Impaired processing and presentation of cytotoxic-T-lymphocyte (CTL) epitopes are major escape mechanisms from CTL immune pressure in human immunodeficiency virus type 1 infection. J. Virol. 78:1324–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rajapaksa US, Li D, Peng YC, McMichael AJ, Dong T, Xu XN. 2012. HLA-B may be more protective against HIV-1 than HLA-A because it resists negative regulatory factor (Nef) mediated down-regulation. Proc. Natl. Acad. Sci. U. S. A. 109:13353–13358 [DOI] [PMC free article] [PubMed] [Google Scholar]