Abstract
ORF78 (ac78) of Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is a baculovirus core gene of unknown function. To determine the role of ac78 in the baculovirus life cycle, an AcMNPV mutant with ac78 deleted, Ac78KO, was constructed. Quantitative PCR analysis revealed that ac78 is a late gene in the viral life cycle. After transfection into Spodoptera frugiperda cells, Ac78KO produced a single-cell infection phenotype, indicating that no infectious budded viruses (BVs) were produced. The defect in BV production was also confirmed by both viral titration and Western blotting. However, viral DNA replication was unaffected, and occlusion bodies were formed. An analysis of BVs and occlusion-derived viruses (ODVs) revealed that AC78 is associated with both forms of the virions and is an envelope structural protein. Electron microscopy revealed that AC78 also plays an important role in the embedding of ODV into the occlusion body. The results of this study demonstrate that AC78 is a late virion-associated protein and is essential for the viral life cycle.
INTRODUCTION
The Baculoviridae are a family of arthropod-specific double-stranded DNA (dsDNA) viruses. Viruses from the family have been reported in 600 host species, mainly from insects of the orders Lepidoptera, Diptera, and Hymenoptera (1). Baculoviruses are characterized by rod-shaped, enveloped nucleocapsids with circular, covalently closed, double-stranded DNA genomes of 80 to 180 kbp (2–4). Baculoviruses are subdivided into four genera: alpha-, beta-, gamma-, and deltabaculovirus. Alpha- and betabaculoviruses infect Lepidoptera larvae, whereas gamma- and deltabaculoviruses infect Hymenoptera and Diptera larvae, respectively (5).
Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is the type species of the genus Alphabaculovirus. The infection cycle of AcMNPV includes two distinct viral phenotypes, budded virus (BV) and occlusion-derived virus (ODV). BVs and ODVs have identical nucleocapsid structures and genetic information, but the compositions of their envelopes are different to accommodate their respective functions in the infection cycle (6). The ODVs, which embed in the nucleus in a protein matrix that forms polyhedra or occlusion bodies, are required for the horizontal transmission of the virus. The alkaline environment in the midgut lumen of larvae releases ODV from the occlusion bodies, enabling these viral particles to initiate primary infection of the mature columnar epithelial cells of the midgut (7). The BVs, which are produced as nucleocapsids, egress from the nucleus, migrate through the cytoplasm, bud through a modified plasma membrane of the infected cells (8), and initiate a secondary infection (9).
AcMNPV has a double-stranded DNA genome of approximately 134 kbp that contains 154 predicted open reading frames (ORFs) based on the criterion that the ORF is a single, contiguous, nonoverlapping coding region (2). To date, 58 baculovirus genomes have been completely sequenced, and comparative analyses have reported that 37 core genes are conserved in all genomes and therefore are likely to play essential roles in the baculovirus life cycle (10–12). AcMNPV ORF78 (ac78) is one of the core genes in the family Baculoviridae (10). It has been reported that interruption of Bm64, an ac78 homologue of the Bombyx mori NPV (BmNPV), resulted in a single-cell infection phenotype (13). The genomic sequence of ac78 predicts a gene product of 109 amino acids with a putative molecular mass of 12.5 kDa (2). Orthologs of ac78 have been found in all completely sequenced baculoviruses in previous works (5, 10, 14, 15). In particular, it has been reported that the ac78 ortholog of Culex nigripalpus NPV (CuniNPV), cuni34, encodes an ODV structural protein that may associate with the ODV envelope (16).
In this study, an ac78 knockout virus, Ac78KO, was constructed to investigate the functional role of ac78 in the AcMNPV life cycle. We identified the transcriptional phase of ac78 and the effects of an ac78 deletion on BV production and ODV assembly. In addition, the morphology of BVs and ODVs in Ac78KO-transfected cells was also examined by electron microscopy. Our data indicated that ac78 is essential for BV production, but the deletion of ac78 does not affect viral DNA replication. Electron microscopy observation indicated that ac78 is not required for nucleocapsid formation but is required for ODV morphogenesis and subsequent embedding of virions into polyhedra.
MATERIALS AND METHODS
Bacterial strains and bacmid DNA.
Escherichia coli strains TOP10 and DH10B (Invitrogen) were used throughout the experiments. All restriction endonucleases and modifying enzymes were from Roche Applied Science (Germany). All recombinant bacmids used in this study were propagated in E. coli strain DH10B.
Viruses, insect cells, and transfection.
Spodoptera frugiperda IPLB-Sf21-AE clonal isolate 9 (Sf9) insect cells were cultured at 27°C in TC-100 medium (WelGene, South Korea) supplemented with 10% heat-inactivated (56°C; 30 min) fetal bovine serum (WelGene, South Korea) and subcultured every 3 or 4 days. Wild-type AcMNPV strain C6 and all recombinant AcMNPVs used in this study were propagated in Sf9 cells maintained in TC-100 medium. Transfection was performed using the Cellfectin reagent (Invitrogen) according to the manufacturer's instructions.
Tn7 in vitro transposition.
The Tn7 in vitro transposition procedure was conducted as described previously (17) with slight modification. Briefly, for the transposition reaction, 12 ng of HindIII- and SphI-digested pPCS-S (donor-S) plasmid was combined with 200 ng of Ac-MK bacmid DNA. TnsABC* transposase (New England BioLabs, United Kingdom) was added to the transposition reaction, and the mixture was incubated at 37°C for 10 min. Next, 1 μl of start solution was added to the mixture, which was then incubated at 30°C for at least 2 h. Finally, the transposition reaction was stopped by heating it to 75°C for 10 min. The reacted DNA was transformed into competent E. coli DH10B cells (Invitrogen), and the transformed cells were subsequently plated onto nutrient agar plates containing kanamycin (50 μg/ml) and ampicillin (50 μg/ml). The plates were incubated at 37°C for 2 days. Colonies resistant to both kanamycin and ampicillin were selected, and successful transposition was verified by nucleotide sequence analysis.
Construction of ac78 knockout, repair, and control AcMNPV bacmids.
To construct the ac78 knockout virus, Ac78KO, a baculovirus transfer vector, pBacMKPol, in which the E. coli origin of replication (Mini-F replicon) is coupled with a kanamycin resistance gene (Kan), was inserted into the locus between orf603 and polyhedrin of the AcMNPV genome (Fig. 1A). A novel recombinant bacmid, Ac-MK, was generated via homologous recombination of pBacMKPol and AcMNPV genomic DNA in cotransfected Sf9 cells, and a successful recombinant was selected in E. coli plated on nutrient agar plates containing kanamycin (50 μg/ml). The genomic structure of Ac-MK was verified by endonuclease digestion and nucleotide sequence analysis (bAc-MK; GenBank accession no. KF022001).
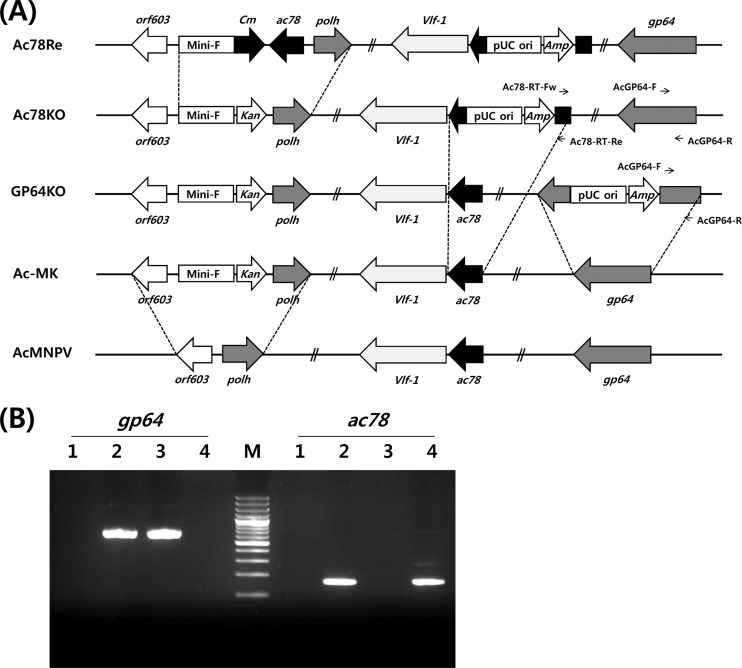
Fig 1.
Construction of Ac-MK, Ac78KO, Ac78Re, and GP64KO bacmids. (A) Schematic diagram of Ac-MK, Ac78KO, Ac78Re, and GP64KO bacmids. The ac78 gene and gp64 gene were knocked out by insertion of the pUC ori and Amp into amino acid 87 of AC78 and amino acid 337 of GP64, respectively, via Tn7-mediated transposition. The deletion of ac78 was repaired by replacement of Kan with ac78 and Cm via homologous recombination between pUC-19-MCP-ac78 and Ac78KO. The ac78 gene inserted into Ac78Re is driven by its own promoter. (B) RT-PCR analysis of ac78 and gp64 transcription. Total RNA was extracted from transfected Sf9 cells at 72 h p.t. Lanes: M, 100-bp ladder; 1, mock-transfected Sf9 cells; 2, Ac-MK-transfected Sf9 cells; 3, Ac78KO-transfected Sf9 cells; 4, GP64KO-transfected Sf9 cells.
Using Tn7-mediated transposition between Ac-MK and donor-S of the plasmid capture system, we generated the recombinant viruses Ac78KO and GP64KO, in which the ac78 gene or gp64 gene was disrupted with a pUC origin (pUC ori) and an ampicillin resistance gene (Amp), respectively (Fig. 1A). The genomic structure of the bacmids was verified by PCR, using primers specific to the ac78 gene and nucleotide sequence analysis.
To generate the ac78 knockout-repaired bacmid, Ac78Re, the repair transfer vector pUC19-MCP-ac78 was constructed using double-joint PCR (DJ-PCR) coupled with In-Fusion cloning as follows. Double-joint PCR was performed as previously described (18) with modifications. Briefly, in the first round of PCR, the chloramphenicol resistance gene and its promoter were amplified with primers Cm r-Fw (5′-TGAGTCAGCATCACCCGACG-3′) and Cm r-Re (5′-CACCAGCCCCTGTTCTCGAGTCAGC-3′) using the plasmid pDEST-32 as a template. The Mini-F replicon region and the polyhedrin region were amplified with primers MiniF-Fw (5′-GATCTTAAAGGGTTCGAGCCTG-3′) and MiniF-Re (5′-CGTCGGGTGATGCTGACTCAAACGTGCCGGCACGGCCT-3′) and primers Polh-Fw (5′-CTCGAGAACAGGGGCTGGTGCAGCCATTGTAATGAGACGCAC-3′) and Polh-Re (5′-CAATTGCTTACATTGAGCGGTTG-3′), respectively, using the genomic DNA of Ac-MK as a template. For DJ-PCR, the three PCR products, the Mini-F replicon fragment, the chloramphenicol resistance gene, and the polyhedrin gene, were purified using a PCR purification kit (Qiagen, Hamburg, Germany), and then 40 ng of each purified PCR product were combined. In the second round of PCR, the cassette containing a Mini-F replicon, chloramphenicol resistance gene (Cm), and polyhedrin gene was PCR amplified using primers MCP-Fw (5′-GAAGTGCTCCGGGGTGATAG-3′) and MCP-Re (5′-CCATTAGTAGATTTGCCGTCTG-3′) with 5 μl of combined templates. To perform the In-Fusion cloning, the cassette containing the Mini-F replicon 3′-flanking region, chloramphenicol resistance gene, and polyhedrin 5′-flanking region was PCR amplified with primers MCP-In-Fusion-Fw (5′-GACGGCCAGTGAATTGAAGTGCTCCGGGGTGATAG-3′) and MCP-In-Fusion-Re (5′-TACCGAGCTCGAATTCCATTAGTAGATTTGCCGTCTG-3′), using 2 μl of the second-round PCR product as the template. The ac78 gene was PCR amplified with primers Ac78-In-Fusion-Fw (5′-CTTTTGCTGACTCGAGAGTACGACATGTCTTCCAGGT-3′) and Ac78-In-Fusion-Re (5′-AGCCCCTGTTCTCGACACGGGCATCACGAGCAATC-3′), using Ac-MK as the template. All PCR amplifications were performed by using Phusion High-Fidelity DNA Polymerase (Finnzymes, Finland). The In-Fusion cloning procedure was performed using an In-Fusion HD cloning kit (TaKaRa, Japan) according to the manufacturer's instructions. After 100 ng of the purified PCR product was mixed with 50 ng EcoRI-digested pUC19 DNA (TaKaRa, Japan), 2 μl of 5× In-Fusion HD Enzyme Premix was added to the reaction, and the mixture was preincubated at 50°C for 15 min. The resulting DNA was transformed into stellar competent E. coli cells (Invitrogen) according to the manufacturer's instructions, and the transformed cells were subsequently spread onto nutrient agar containing chloramphenicol (50 μg/ml) and ampicillin (50 μg/ml). The plates were incubated overnight at 37°C, and colonies resistant to kanamycin and ampicillin were selected and verified by restriction enzyme digestion and sequence analysis. The resulting plasmid, pUC19-MCP, was then digested with XhoI and mixed with the PCR-amplified ac78 DNA to generate the repair transfer vector pUC19-MCP-ac78 using the In-Fusion HD cloning kit as described above.
The knocked-out ac78 repair bacmid, Ac78Re, was generated via homologous recombination of pUC19-MCP-ac78 and Ac78KO genomic DNA in cotransfected Sf9 cells and selected in E. coli plated on nutrient agar plates containing chloramphenicol (50 μg/ml) and ampicillin (50 μg/ml).
RNA and RT-PCR.
Sf9 cells (1 × 106 cells/35-mm-diameter six-well plate) were transfected with viruses, and total RNA was isolated from transfected Sf9 cells at 72 h posttransfection (p.t.) using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. After the RNA samples were treated with RNase-free DNase I (TaKaRa, Japan), reverse transcription (RT)-PCR was performed using an AccuPower RT/PCR Premix (Bioneer, South Korea) in a 20-μl volume according to the manufacturer's instructions. To amplify the ac78 gene, oligonucleotides Ac78-RT-Fw (5′-GTCGTGTTGTCATAGCCCAC-3′) and Ac78-RT-Re (5′-GAATTTGGACGTGCCCTAC-3′) were used. The gp64 gene was amplified using oligonucleotides AcGP64-F (5′-ATATGTGCTTTTGGCGG-3′) and AcGP64-R (5′-TTTGGCGCGTGGTGAAC-3′).
qPCR.
Sf9 cells were infected with viruses at a multiplicity of infection (MOI) of 10. Following gentle rocking for 1 h, the virus-containing culture medium was removed and fresh medium was added after washing two times with incomplete TC-100 medium. Total RNA extracted from infected Sf9 cells at various times postinfection (p.i.) was treated with DNase I prior to cDNA synthesis. Single-stranded cDNA was synthesized from the total RNA using the SuperScript III First-Synthesis System for RT-PCR (Invitrogen, USA) according to the manufacturer's instructions. The quantitative PCR (qPCR) was conducted with a 2× DyNAmo HS SYBR Green qPCR Kit (Finnzymes, Finland) and CFX96 Real-Time System (Bio-Rad). The cycling profile used for qPCR was as follows: a preheating step for enzyme activation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 58°C for 15 s, and 72°C for 30 s. The relative transcription level was calculated using the 2−ΔCt method (19). 28S rRNA was used as a reference gene (20). Oligonucleotides specific to ac78, Ac78-RealTime-Fw (5′-TCGGTGTCAATACTATCCGAA-3′) and Ac78-RealTime-Re (5′-GTGGGCTATGACAACACGAC-3′), were used for qPCR.
Titration of BV.
BV production was determined by both endpoint dilution and qPCR as previously described (21–23) with slight modifications. Sf9 cells (1 × 106 cells/35-mm-diameter six-well plate) were transfected with 1 μg of each bacmid (Ac-MK, Ac78KO, and Ac78Re). At various times p.t., the supernatant containing BV was harvested and cell debris was removed by centrifugation at 8,000 × g for 5 min. The extracellular BV titer in the harvested culture supernatant was determined using the endpoint dilution method in triplicate, as previously reported (22). To determine titers using qPCR, 1 ml of the above-mentioned supernatant containing BV was centrifuged at 80,000 × g for 2 h at 4°C, and the pellets were resuspended with 200 μl of lysis buffer (10 mM Tris-Cl, pH 7.5, 10 mM EDTA, 0.25% SDS, 20 μg/ml RNase A, and 80 μg/ml proteinase K). After overnight incubation at 65°C, the viral DNA was extracted by phenol extraction and alcohol precipitation. To perform qPCR, 2 μl of diluted DNA was used, along with a 2× DyNAmo HS SYBR Green qPCR Kit (Finnzymes, Finland) and the primers IE1-RTF (5′-ACCATCGCCCAGTTCTGCTTATC-3′) and IE1-RTR (5′-GCTTCCGTTTAGTTCCAGTTGCC-3′), which amplify a 100-bp fragment of the ie-1 gene. A stock of wild-type Ac-MK (4.25 × 108 PFU/ml) whose titer was previously determined by endpoint dilution was serially diluted and used to develop a standard curve. The samples were analyzed using a CFX96TM Real-Time System (Bio-Rad) under the following conditions: a preheating step for enzyme activation at 95°C for 15 min, followed by 45 cycles of 95°C for 30 s, 60°C for 20 s, and 72°C for 20 s.
Quantification of viral DNA replication.
To assess viral DNA replication, a qPCR assay was performed as previously described (23) with slight modifications. To prepare viral DNA for analysis, Sf9 cells (1 × 106 cells/35-mm-diameter well of a 6-well plate) were transfected with 1 μg of bacmid DNA (Ac-MK, Ac78KO, repaired bacmid Ac78Re, and GP64KO), and the transfected cells were washed once with 1× phosphate-buffered saline (Sigma) at the designated time p.t. and centrifuged at 8,000 × g for 5 min. The harvested cell pellets were incubated for 30 min at 37°C in 250 μl of lysis buffer (10 mM Tris-Cl, pH 7.5, 10 mM EDTA, 0.25% SDS, 20 μg/ml RNase A) and then incubated overnight at 65°C after the addition of 80 μg/ml proteinase K. Viral DNA was extracted with 250 μl of phenol-chloroform and 250 μl of chloroform, and the aqueous layer containing viral DNA was carefully harvested. Prior to the PCR, 2 μl of total DNA from each time point was digested with 40 U of DpnI restriction enzyme (New England BioLabs) overnight in a 20-μl total reaction volume. To perform qPCR, 2 μl of the digested DNA was used, along with a 2× DyNAmo HS SYBR Green qPCR Kit (Finnzymes, Finland) and the primers gp41-Fw (5′-CGTAGTGGTAGTAATCGCCGC-3′) and gp41-Re (5′-AGTCGAGTCGCGTCGCTTT-3′). The samples were analyzed in a CFX96TM Real-Time System (Bio-Rad) under the following conditions: a preheating step for enzyme activation at 95°C for 15 min, followed by 45 cycles of 95°C for 30 s, 60°C for 20 s, and 72°C for 20 s.
Antibody preparation.
For production of polyclonal antibody against AC78, the PCR-amplified ac78 gene was introduced into the E. coli expression vector pET30a(+) (Novagen, Germany) to obtain pET30a-ac78, which was transformed into E. coli BL21(DE3). AC78 was expressed by IPTG (isopropyl-β-d-thiogalactopyranoside) induction and then purified using Ni-NTA (nitrilotriacetic acid) Superflow Cartridges (Qiagen, Germany) and fast protein liquid chromatography (FPLC) (GE Healthcare). ICR female rabbits were immunized with the purified protein by intravenous injection. The first immunization of 200 μg/rabbit in incomplete Freund's adjuvant (Sigma) was followed at 7-day intervals by a series of 200-μg/rabbit injections in incomplete Freund's adjuvant (Sigma). The rabbits were bled 3 days after the last injection, and antisera were separated after storage of total blood overnight at 4°C.
BV partial purification and concentration.
Sf9 cells (1 × 106 cells/35-mm-diameter well of a 6-well plate) were transfected with 1 μg of bacmid DNA (Ac-MK, Ac78KO, and the repaired bacmid Ac78Re). At 120 h p.t., the supernatant containing BV was harvested and BVs were purified as previously described (24). Briefly, the medium was harvested, and cell debris was removed by centrifugation at 2,000 × g for 20 min. Supernatant (3 ml) was loaded onto a 25% sucrose cushion and centrifuged at 80,000 × g for 90 min at 4°C. The BV pellets were resuspended in 25 μl of 50 mM Tris-Cl (pH 7.5). An equal volume of 2× protein sample buffer (Sigma) was added, and the samples were placed at 100°C for 10 min. Ten microliters of the Ac-MK and Ac78Re samples and the totality of the Ac78KO sample were analyzed by SDS-12% PAGE and Western blotting.
Purification of BV and ODV.
Sf9 cells were infected with Ac-MK at an MOI of 0.1 and harvested at 5 days p.i. by centrifugation at 1,800 × g for 10 min. The resulting supernatant was used for BV purification, and the pellet was used for ODV purification. The purification of BV and ODV and the fractionation of the envelope and nucleocapsid of BV and ODV were performed as previously described (6, 25).
Western blot analysis.
Purified BV and ODV and the fractions of BV and ODV envelope and nucleocapsid were mixed with equal volumes of 2× protein sample buffer (Sigma) and boiled at 100°C for 10 min. These protein samples were resolved in 12% SDS-PAGE, transferred onto a hydrophobic polyvinylidene difluoride (PVDF) membrane (GE Healthcare), and probed with primary antibodies: rabbit polyclonal AC78 antiserum (1:5,000), rabbit polyclonal VP39 antiserum (1:5,000; provided by Kai Yang, Sun Yat-Sen University), or mouse monoclonal GP64 AcV5 antibody (1:5,000; eBioscience). Peroxidase-conjugated goat anti-rabbit antibody (1:50,000; ABM, Canada) or horseradish peroxidase-conjugated sheep anti-mouse antibody (1:10,000; Amersham Biosciences) was used as the secondary antibody. The signals were detected with Western Blotting Luminol Reagent (Santa Cruz Biotechnology).
Electron microscopy.
For transmission electron microscopy (TEM), Sf9 cells (1 × 106 cells/35-mm-diameter well of a 6-well plate) transfected with 1 μg of each bacmid DNA were harvested at the designated time points by centrifugation at 5,000 × g for 5 min and fixed for 4 h at 4°C with 2% paraformaldehyde and 2% glutaraldehyde in 0.05 M sodium cacodylate buffer (pH 7.2). After postfixation with 1% OsO4 in the same buffer, the samples were dehydrated in an ethanol-propylene oxide series and embedded in an Epon-Araldite mixture. Ultrathin sections were obtained with an RMC MT-X ultramicrotome and subsequently stained with a mixture of 2% uranyl acetate and Sato's lead (65). A JEM-1010 transmission electron microscope (JEOL, Japan) was used.
RESULTS
Generation of ac78 knockout, repair, and control bacmids.
To determine whether ac78 is essential for viral replication, we generated an infectious bacmid, Ac78KO, in which the ac78 gene was knocked out. For this, Tn7-mediated transposition between Ac-MK and donor-S of the plasmid capture system was used to generate the recombinant bacmid Ac78KO, in which the ac78 gene was interrupted with a pUC origin (pUC ori) and an ampicillin resistance gene (Amp) 261 bp downstream of the predicted translation start site (Fig. 1A).
RT-PCR analysis was performed to confirm the lack of ac78 expression in Ac78KO-transfected Sf9 cells (Fig. 1B). RT-PCR using a gp64-specific primer set successfully amplified the corresponding gene from the cDNA of Sf9 cells transfected with Ac-MK or Ac78KO. While the single RT-PCR product of expected size was obtained from the Sf9 cells transfected with Ac-MK, no product was amplified from Ac78KO-transfected cells using an ac78-specific primer set. These results demonstrated that the ac78 gene was successfully knocked out in Ac78KO.
To confirm the phenotypes resulting from knockout of ac78, a repaired bacmid, Ac78Re, was used. In this bacmid, ac78, expressed by its own promoter, is inserted upstream of polyhedrin in Ac78KO (Fig. 1A). In addition, as a control, a gp64 gene knockout bacmid, GP64KO, in which the gp64 gene was interrupted with the pUC ori and Amp at 1,013 bp downstream of the predicted translation start site, was also generated from Ac-MK (Fig. 1A). The internal genomic structure of the bacmids Ac-MK, Ac78KO, Ac78Re, and GP64KO was verified by PCR and nucleotide sequence analysis (data not shown).
Transcriptional analysis of ac78.
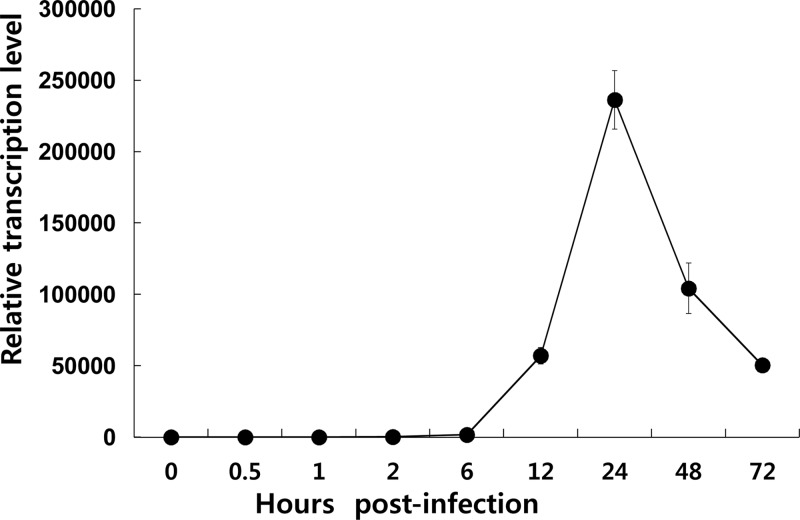
Two consensus sequences of the baculovirus late-gene promoter motif, TAAG, were found at 8 nucleotides (nt) and 89 nt upstream of the translation-initiating codon, ATG, of ac78, indicating that the gene might belong to the late-gene category. To address this, temporal expression of ac78 during viral replication was investigated using qPCR (Fig. 2). In Sf9 cells infected with Ac-MK, transcription of ac78 started at 6 h p.i. Transcription of ac78 continued to increase until 24 h p.i. and then declined slightly up to 72 h p.i. In contrast, no ac78 transcript was detected at any time point in Sf9 cells infected with Ac78KO, which resulted from the knockout of the corresponding gene in Ac78KO.
Fig 2.
Transcription of ac78 in Sf9 cells. Total RNA was extracted from Sf9 cells infected with Ac-MK at 0, 0.5, 1, 2, 6, 12, 24, 48, and 72 h p.i. and subjected to qPCR analysis using an ac78-specific primer set. 28S rRNA was used as a reference gene. The error bars indicate standard deviations.
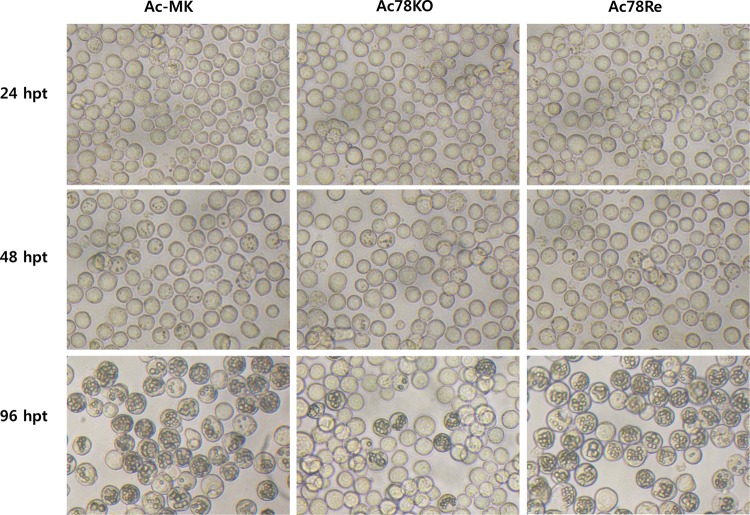
Effect of ac78 deletion on viral replication in transfected Sf9 cells.
To determine the effect of ac78 deletion on viral replication, Sf9 cells were separately transfected with Ac-MK, Ac78KO, or Ac78Re. Light microscopy analysis revealed no difference among these three viruses at 24 h p.t. At 48 h p.t., only a small proportion of the cells contained occlusion bodies (OBs), and the numbers of transfected cells that contained OBs were not significantly different among the three viruses (Fig. 3). By 96 h p.t., significant differences were observed between Ac-MK- and Ac78Re-transfected cells and Ac78KO-transfected cells. A large proportion of the Ac-MK- and Ac78Re-transfected Sf9 cells contained OBs, whereas the number of Ac78KO-transfected cells containing OBs did not increase (Fig. 3).
Fig 3.
Light microscopy of Ac-MK-, Ac78KO-, or Ac78Re-transfected Sf9 cells. Sf9 cells (1 × 106) were transfected with 1 μg of Ac-MK, Ac78KO, or Ac78Re bacmid DNA. At the designated time points, transfected Sf9 cells were observed under a light microscope.
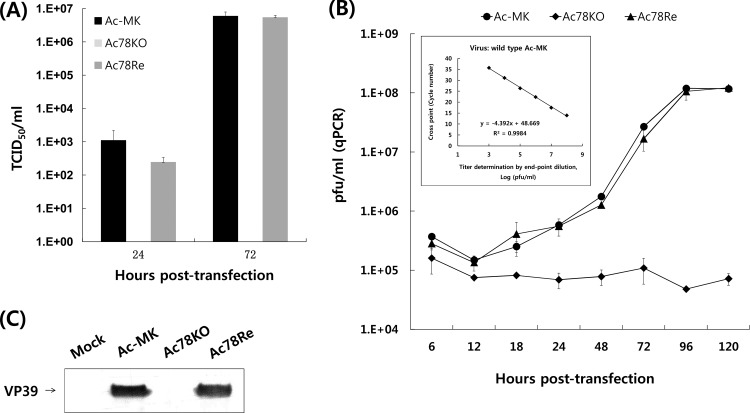
To better define the effect of lacking ac78 on virus replication and to investigate the replication kinetics of the virus constructs, BV levels were analyzed using a 50% tissue culture infective dose (TCID50) and qPCR. Sf9 cells were transfected with Ac-MK, Ac78KO, or Ac78Re bacmid DNA separately, and the BV titers were determined by TCID50 endpoint dilution at selected time points. Whereas Sf9 cells transfected with Ac-MK or Ac78Re displayed a normal increase in BV production that reached equivalent titers, no BV was detectable for Ac78KO-transfected cells at any time point tested, indicating that no infectious virus was produced (Fig. 4A). These results confirmed that ac78 is required for infectious-BV production in Sf9 cells.
Fig 4.
Analysis of BV production. (A) Extracellular BV titers at 24 and 72 h p.t. were determined by TCID50 endpoint dilution from Sf9 cells separately transfected with Ac-MK, Ac78KO, and Ac78Re. Each point represents the average titer derived from three independent TCID50 assays. The error bars represent standard deviations. (B) The virus titer, regardless of virion infectivity, was determined by qPCR analysis of supernatants of Sf9 cells transfected with Ac-MK, Ac78KO, or Ac78Re at the designated time points. A stock of wild-type Ac-MK (4.25 × 108 PFU/ml) whose titer was previously determined by endpoint dilution was serially diluted and used to develop a standard curve (showed in the inset). (C) Western blot analysis of BV isolated from Sf9 cells transfected with Ac-MK, Ac78KO, or Ac78Re at 72 h p.t. The blots were probed with a monoclonal antibody specific for VP39 nucleocapsid protein.
Because the endpoint dilution assay cannot detect the production of noninfectious BVs, the BV titers were also assayed by qPCR, which determines BV titers by detecting viral genomes regardless of infectivity (Fig. 4B). Although there is a background of viral genomes detected at every time point due to the initially transfected bacmid DNA, Sf9 cells transfected with Ac-MK or Ac78Re revealed a steady increase in BV production, whereas no increase in BV production was observed above background level up to 120 h p.t. for Ac78KO-transfected cells.
To additionally confirm the absence of BV production in Ac78KO-transfected cells, Western blotting using the BVs purified from Ac-MK-, Ac78KO-, and Ac78Re-transfected cell supernatants was performed to compare the levels of nucleocapsid protein VP39 (Fig. 4C). Whereas VP39 was detected in the Ac-MK- and Ac78Re-transfected cell supernatants, the nucleocapsid protein was not detected in the Ac78KO-transfected cell supernatants. These results suggested that the deletion of ac78 results in a viral phenotype incapable of producing BV.
Effect of ac78 deletion on viral DNA replication in Sf9 cells.
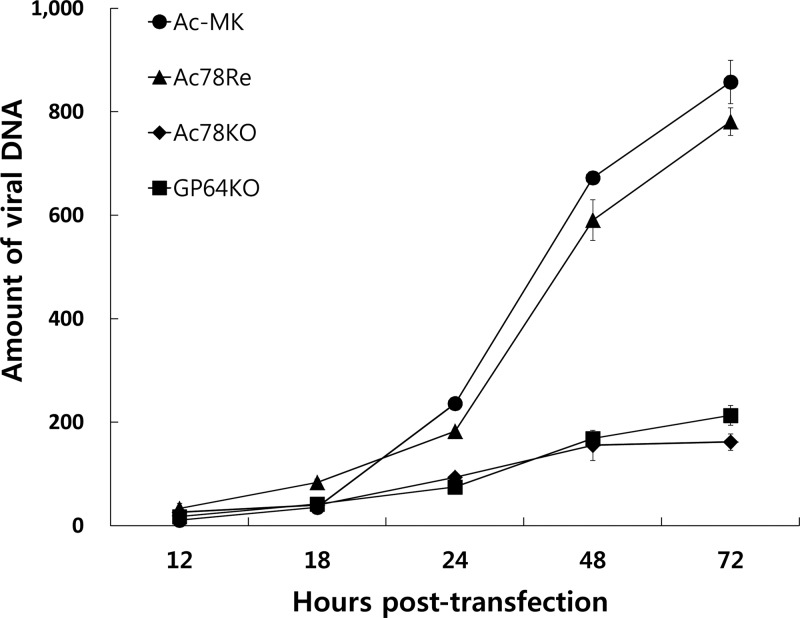
To determine whether ac78 is required for viral DNA replication, a qPCR analysis was performed to investigate the level of viral DNA replication in Ac-MK-, Ac78KO-, and Ac78Re-transfected cells (Fig. 5). In addition, a gp64 gene knockout bacmid, GP64KO, was used as a noninfectious control. Equal amounts of transfected cells were collected at designated time points, and total DNA was extracted from the cell lysates and subjected to qPCR. The results showed that Ac78KO and GP64KO could synthesize levels of nascent DNA similar to those of Ac-MK up to 18 h p.t., suggesting that the onset and level of viral DNA replication in the initially transfected cells are not affected by deletion of ac78. However, though the levels of DNA replication for Ac-MK and Ac78Re increased after 24 h p.t., correlating with the spread of the infection from the production of BV, the DNA replication levels of Ac78KO and GP64KO did not increase up to 72 h p.t., which correlates with the absence of BV production in the transfected cells.
Fig 5.
Time course of viral DNA replication in transfected cells. In total, 1 × 106 Sf9 cells were transfected with 1 μg of Ac-MK, Ac78KO, Ac78Re, and GP64KO bacmids. At the designated time points, total cellular DNA was isolated from Sf9 cells transfected with each bacmid DNA, digested with the restriction enzyme DpnI to eliminate input bacmid, and analyzed by qPCR. The results are from three separate transfections, and the error bars represent the standard deviations.
Substructural localization of AC78 in purified BV and ODV.
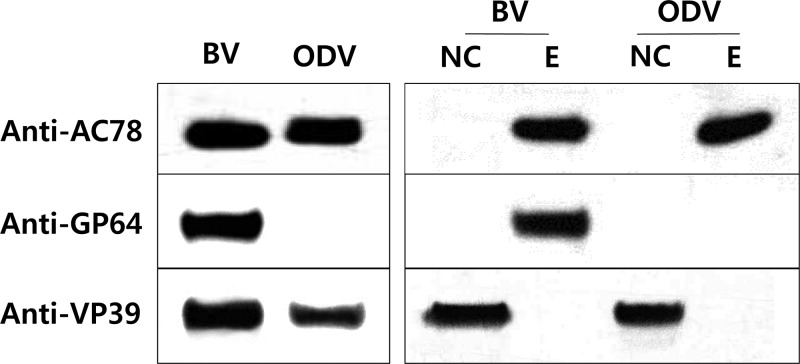
Though two proteomic studies of AcMNPV ODV (26) and BV (27) were reported to identify proteins associated with each virion phenotype, neither study identified AC78 as an ODV or BV structural protein. However, another proteomic analysis demonstrated that the ortholog of AC78 is an ODV structural protein in CuniNPV (16). To confirm whether AC78 is an ODV or BV structural protein, BVs and ODVs were purified from Sf9 cells infected with Ac-MK and analyzed by Western blotting. In addition, the biochemically fractionated nucleocapsid and envelope fractions of the BV and ODV particles were also analyzed by Western blotting. The results of the analyses indicated that AC78 is associated with both BV and ODV and localizes to the envelope fraction (Fig. 6). As a control to confirm the efficiency of the BV fractionation, the nucleocapsid protein VP39 and the BV envelope-specific protein GP64 were analyzed by Western blotting. Both proteins were observed in the expected fractions (Fig. 6).
Fig 6.
Western blot analysis of AC78 in purified and fractionated virions. BV and ODV were purified using a sucrose gradient and analyzed by SDS-PAGE and Western blotting. The blots were probed individually with an anti-Ac78 antibody to detect AC78, anti-AcV5 monoclonal antibody to detect the BV envelope protein GP64, and anti-VP39 to detect the nucleocapsid protein VP39. NC, nucleocapsid fraction; E, envelope fraction. GP64 and VP39 were analyzed to confirm correct fractionation of the BV particle in envelope and nucleocapsid fractions, respectively.
Electron microscopy of Ac-MK-, Ac78KO-, or Ac78Re-transfected cells.
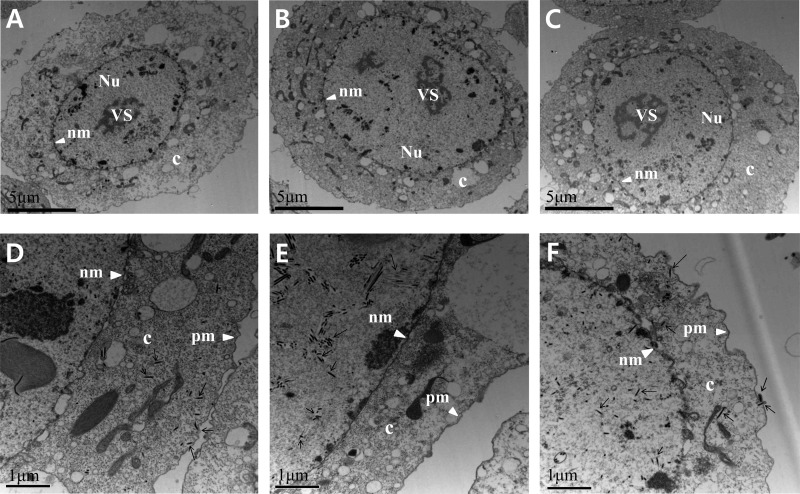
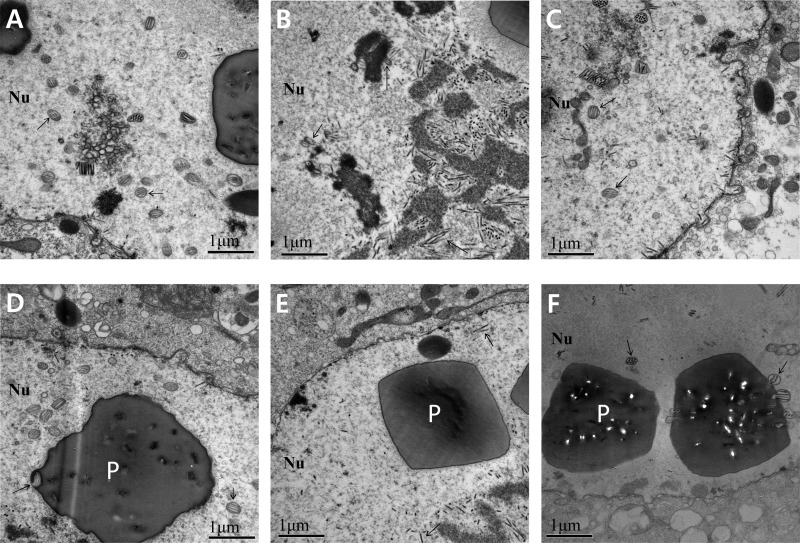
As evidenced by the presence of OBs in the nuclei of transfected Sf9 cells, the viral infection of Ac78KO can progress to very late phases. Whereas the deletion of ac78 did not affect viral DNA replication, TCID50 endpoint dilution and qPCR results indicated that ac78 is essential for BV production. Western blot analysis indicated that AC78 is associated with the envelopes of both BV and ODV. To further analyze whether the deletion of ac78 has any effect on viral morphogenesis, electron microscopic analysis was performed (Fig. 7 and 8). At 24 h p.t., cells transfected with Ac-MK, Ac78KO, or Ac78Re exhibited the typical baculovirus infection symptoms, including enlarged nuclei and a typically reorganized electron-dense virogenic stroma and rod-shaped nucleocapsids associating with the electron-dense edges of the virogenic stroma. The virogenic stroma is the active site for viral DNA replication and nucleocapsid assembly. In Ac-MK- or Ac78Re-transfected cells, most nucleocapsids were mainly concentrated in the nucleus, but a significant number were observed in the cytoplasm and budding at the plasma membrane (Fig. 7D and F). In contrast, in Ac78KO-transfected cells observed up to 72 h p.t., the nucleocapsids were consistently observed only in the nucleus (Fig. 7E). At 48 and 72 h p.t., the nucleocapsids associated with edges of the virogenic stroma, forming bundles, aligning with de novo-developed nuclear envelopes, and acquiring envelopes, were observed in both Ac-MK- and Ac78Re-transfected cells. These enveloped virions contain multiple nucleocapsids prior to occlusion in the protein crystalline matrix of the developing OBs (Fig. 8A and C), and OBs containing numerous enveloped virions were observed in the ring zone (Fig. 8D and F). In Ac78KO-transfected cells, while bundles of nucleocapsids were also observed, only a few of them were enveloped, and the enveloped virions contained fewer nucleocapsids than Ac-MK or Ac78Re (Fig. 8B). In addition, ODVs were not embedded in the OBs, although their shapes and sizes were similar to those of Ac-MK and Ac78Re (Fig. 8E). These observations indicated that deletion of ac78 affects not only BV production, but also ODV morphogenesis and subsequent embedding of ODVs into OBs.
Fig 7.
Transmission electron microscopy analysis of Sf9 cells transfected with Ac-MK (A and D), Ac78KO (B and E), or Ac78Re (C and F) at 24 h p.t. (A, B, and C) Enlarged nucleus (Nu) and virogenic stroma (VS) in Ac-MK-, Ac78KO-, or Ac78Re-transfected cells. c, cytoplasm; nm, nuclear membrane. (D and F) Higher-magnification micrographs of Ac-MK- or Ac78Re-transfected cells displaying normal nucleocapsids residing in the cytoplasm (c) and budding from the plasma membrane (pm). (E) In Ac78KO-transfected cells, nucleocapsids and masses of electron-lucent tubular structures (arrows) were observed in the nucleus, but no nucleocapsids were observed in the cytoplasm.
Fig 8.
Nucleocapsid envelopment and OB morphogenesis. In Ac-MK- or Ac78Re-transfected cells, normally enveloped virions containing multiple nucleocapsids (A and C, arrows) were embedded within the polyhedra (P) (D and F, arrows). In Ac78KO-transfected cells, the nucleocapsids and masses of electron-lucent tubular structures appeared at the electron-dense edges of the stroma and enveloped virions contained few nucleocapsids (arrows) (B), and no normal virions were embedded in the polyhedra (E).
DISCUSSION
AcMNPV ac78 has been observed in all the baculovirus genomes sequenced thus far and is one of 37 core genes (10), which suggests it performs a common key function in the baculovirus life cycle. However, the function of the gene is still unknown. In this study, the role of ac78 was investigated. Our results suggested that ac78 is essential for BV production and the formation of normal OBs.
To investigate the functional role of ac78 in the baculovirus life cycle, we generated an ac78 knockout recombinant bacmid, Ac78KO, via Tn7-mediated transposition. In the genome of Ac78KO, the ac78 gene was interrupted with a pUC ori-Amp cassette at 261 bp downstream of its predicted translation start site. At the insertion site, a copy of a 5-nt sequence, ATATT, was generated at both ends of the Tn7 sequence, which avoided disruption of the TAAG motif for the downstream vlf-1 gene. Since a previous study reported that insertion anywhere within or downstream of the promoter motif disrupted expression of vlf-1 (28), which was previously shown to affect BV production and nucleocapsid assembly (29), we analyzed the expression of vlf-1 using qPCR. The result revealed that vlf-1 expression was not disrupted in Ac78KO (data not shown). Therefore, the defect in BV production in the Ac78KO-transfected Sf9 cells did not result from the disruption of vlf-1 expression, but from the disruption of the ac78 gene.
To determine if ac78 is required for viral DNA replication within transfected cells via qPCR, a gp64 knockout bacmid, GP64KO, was generated to serve as a control virus. Although deletion of gp64 lacks the ability to initiate cell-to-cell infection because the corresponding protein, GP64, is required for nucleocapsids to egress from infected cells, all other replication processes are unaffected (24, 30). Ac78KO showed a replication level similar to that of GP64KO, indicating that ac78 is not essential for viral DNA replication.
Western blot analysis indicated that AC78 is an envelope component of both BV and ODV. To date, only a small number of proteins have been reported to be associated with both the BV and ODV envelopes, including AC16 (BV/ODV-E26) (31), AC23 (F-like) (26, 27), AC64 (GP37) (32), AC94 (ODV-E25) (27, 33), AC96 (34), and AC143 (ODV-E18) (27). AC16 (BV/ODV-E26) is a peripheral membrane protein that may interact with other proteins to play an important role in trafficking ODV envelope proteins (31, 35). AC23 (F-like) is important for BV infectivity (36), and AC64 (GP37) is a chitin-binding protein (32). Both of these proteins are not essential for viral replication (37, 38). In addition, AC94 (ODV-E25) and AC96 are not essential for viral DNA replication or BV production (34, 39). AC94 (ODV-E25) is an integral ODV envelope protein with an envelope-anchored N terminus (33, 40) that is required for the formation of intranuclear microvesicles and ODV (39). AC96 is a per os infectivity factor (PIF) that is not related to BV production or viral DNA replication (34). AC143 (ODV-E18) is an integral ODV envelope protein, and all homologues have N-terminal transmembrane motif regions (41), which are essential for BV production (42).
In addition, there are two envelope proteins present only in BV, AC35 (V-ubiquitin) and AC128 (GP64). AC35 is not essential for viral replication, but viruses with frameshift mutations of v-ubiquitin have reduced BV production (43). GP64 is the major envelope protein of BV responsible for membrane fusion and is important for efficient BV budding (24, 44).
ODV envelope proteins are much more complex in composition than those of BV envelopes. ODV envelope proteins are composed of AC46 (ODV-E66), AC80 (GP41) (45), AC148 (ODV-E56), and a group of PIFs, including P74 (PIF-0) (46–48), PIF-1 (AC119) (49), PIF-2 (AC22) (50), PIF-3 (AC115) (51), and PIF-4 (AC96) (34). AC46 (ODV-E66) is an integral ODV envelope protein with an envelope-anchored N terminus (40) that is not required for virion morphogenesis or occlusion assembly but is required for oral infectivity (52). AC80 (GP41) is a tegument protein modified with O-linked N-acetylglucosamine located between the virion envelope and capsid (53, 54) that is required for the egress of nucleocapsids from the nucleus, forming BV (55). AC148 (ODV-E56) has a transmembrane motif on its C terminus and is not required for BV production, ODV assembly, or occlusion (56, 57).
Using electron microscopy, abundant nucleocapsids with a normal appearance were observed in the intrastromal space of the virogenic stroma of Ac78KO-transfected cells and were morphologically indistinguishable from those in cells transfected with either Ac-MK or Ac78Re bacmid, indicating that nucleocapsid assembly was not affected by the deletion of ac78. The normal nucleocapsids also indicated that new viral DNA was incorporated into the capsids. This is consistent with the qPCR analysis, which indicated that ac78 is not involved in viral DNA synthesis. However, no nucleocapsid budding through the nuclear membrane was observed in the cytoplasm of transfected cells. These results revealed that the inefficient BV production of Ac78KO-transfected cells was due to inefficient egress of nucleocapsids from the nucleus to the cytoplasm. In Ac78KO-transfected cells, the nucleocapsids could normally bundle together in the nuclear ring zone; however, most of the nucleocapsids formed nonenveloped bundles, and abnormal OBs devoid of virions developed. Several morphogenetic processes, such as nucleocapsid bundling, envelopment of ODVs, and embedding of ODVs into OB, have been reported to occur in the ring zone (7). Therefore, our results suggest that AC78 executes its function after nucleocapsid assembly, during nucleocapsid envelopment and ODV embedding. Previous studies have reported that OBs without embedded virions can be formed in cells transfected with knockout viruses, ac98 (38K) (58), ac142 (59), ac103 (p48) (60), ac53 (61), ac76 (62), ac94 (39), and ac109 (63, 64). Deletion of ac98 (38K) or ac53 leads to defects in nucleocapsid assembly, whereas knockout of ac142, ac103 (p48), ac76, or ac109 interferes with nucleocapsid envelopment, which is similar to what we observed for ac78 knockout in this study.
In conclusion, this study suggests that ac78 is an essential gene for BV production and normal occlusion body formation. Deletion of ac78 does not affect viral DNA replication, and AC78 was shown to be an envelope component of both BV and ODV. Although the exact role of ac78 in the processes of nucleocapsid egress from the nucleus, as well as occlusion synthesis, is still unclear, our study will contribute to a better understanding of the baculovirus infection processes.
ACKNOWLEDGMENTS
This work was supported by a grant from the Next-Generation BioGreen 21 Program (no. PJ009031), Rural Development Administration, Republic of Korea. Xue Ying Tao, Joo Hyun Lee, Qin Liu, Song Eun Kim, and Saes Byeol An were supported by the second stage of the Brain Korea 21 project.
Footnotes
Published ahead of print 22 May 2013
REFERENCES
- 1. Herniou EA, Jehle JA. 2007. Baculovirus phylogeny and evolution. Curr. Drug Targets 8:1043–1050 [DOI] [PubMed] [Google Scholar]
- 2. Ayres MD, Howard SC, Kuzio J, Lopez-Ferber M, Possee RD. 1994. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 202:586–605 [DOI] [PubMed] [Google Scholar]
- 3. Herniou EA, Olszewski JA, Cory JS, O'Reilly DR. 2003. The genome sequence and evolution of baculoviruses. Annu. Rev. Entomol. 48:211–234 [DOI] [PubMed] [Google Scholar]
- 4. Jakubowska AK, Peters SA, Ziemnicka J, Vlak JM, van Oers MM. 2006. Genome sequence of an enhancin gene-rich nucleopolyhedrovirus (NPV) from Agrotis segetum: collinearity with Spodoptera exigua multiple NPV. J. Gen. Virol. 87:537–551 [DOI] [PubMed] [Google Scholar]
- 5. Jehle JA, Blissard GW, Bonning BC, Cory JS, Herniou EA, Rohrmann GF, Theilmann DA, Thiem SM, Vlak JM. 2006. On the classification and nomenclature of baculoviruses: a proposal for revision. Arch. Virol. 151:1257–1266 [DOI] [PubMed] [Google Scholar]
- 6. Braunagel SC, Summers MD. 1994. Autographa californica nuclear polyhedrosis virus, PDV, and ECV viral envelopes and nucleocapsids: structural proteins, antigens, lipid and fatty acid profiles. Virology 202:315–328 [DOI] [PubMed] [Google Scholar]
- 7. Williams GV, Faulkner P. 1997. Cytological changes and viral morphogenesis during baculovirus infection, p 61–108 Miller LK. (ed), The baculovirus. Plenum Publishing Corporation, New York, NY [Google Scholar]
- 8. Monsma SA, Oomens AG, Blissard GW. 1996. The GP64 envelope fusion protein is an essential baculovirus protein required for cell-to-cell transmission of infection. J. Virol. 70:4607–4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Federici BA. 1997. Baculovirus pathogenesis, p 33–56 Miller LK. (ed), The baculoviruses. Plenum Publishing Corporation, New York, NY [Google Scholar]
- 10. Garavaglia MJ, Miele SA, Iserte JA, Belaich MN, Ghiringhelli PD. 2012. The ac53, ac78, ac101, and ac103 genes are newly discovered core genes in the family Baculoviridae. J. Virol. 86:12069–12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rohrmann GF. 2011. Baculovirus molecular biology, 2nd ed NCBI, Bethesda, MD [Google Scholar]
- 12. Yuan MJ, Huang ZQ, Wei DH, Hu ZY, Yang K, Pang Y. 2011. Identification of Autographa californica nucleopolyhedrovirus ac93 as a core gene and its requirement for intranuclear microvesicle formation and nuclear egress of nucleocapsids. J. Virol. 85:11664–11674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ono C, Kamagata T, Taka H, Sahara K, Asano S, Bando H. 2012. Phenotypic grouping of 141 BmNPVs lacking viral gene sequences. Virus Res. 165:197–206 [DOI] [PubMed] [Google Scholar]
- 14. Cohen DPA, Davies MMBG, Vlak JM, van Oers MM. 2009. Encyclopedia of Autographa californica nucleopolyhedrovirus genes. Virol. Sinica 24:6 [Google Scholar]
- 15. Miele SAB, Belaich GMMN, Ghiringhelli PD. 2011. Baculovirus: molecular insights on their diversity and conservation. Int. J. Evol. Biol. 2011:379424. 10.4061/2011/379424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perera O, Green TB, Stevens SM, White S, Becnel JJ. 2007. Proteins associated with Culex nigripalpus nucleopolyhedrovirus occluded virions. J. Virol. 81:4585–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi JY, Roh JY, Kang JN, Shim HJ, Woo SD, Jin BR, Li MS, Je YH. 2005. Genomic segments cloning and analysis of Cotesia plutellae polydnavirus using plasmid capture system. Biochem. Biophys. Res. Commun. 332:487–493 [DOI] [PubMed] [Google Scholar]
- 18. Kim MS, Kim SY, Yoon JK, Lee YW, Bahn YS. 2009. An efficient gene-disruption method in Cryptococcus neoformans by double-joint PCR with NAT-split markers. Biochem. Biophys. Res. Commun. 390:983–988 [DOI] [PubMed] [Google Scholar]
- 19. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xue JL, Salem TZ, Turney CM, Cheng XW. 2010. Strategy of the use of 28S rRNA as a housekeeping gene in real-time quantitative PCR analysis of gene transcription in insect cells infected by viruses. J. Virol. Methods 163:210–215 [DOI] [PubMed] [Google Scholar]
- 21. Lo HR, Chao YC. 2004. Rapid titer determination of baculovirus by quantitative real-time polymerase chain reaction. Biotechnol. Prog. 20:354–360 [DOI] [PubMed] [Google Scholar]
- 22. O'Reilly DR, Miller LK, Luckow VA. 1992. Baculovirus expression vector: a laboratory manual. W.H. Freeman & Company, New York, NY [Google Scholar]
- 23. Vanarsdall AL, Okano K, Rohrmann GF. 2005. Characterization of the replication of a baculovirus mutant lacking the DNA polymerase gene. Virology 331:175–180 [DOI] [PubMed] [Google Scholar]
- 24. Oomens AG, Blissard GW. 1999. Requirement for GP64 to drive efficient budding of Autographa californica multicapsid nucleopolyhedrovirus. Virology 254:297–314 [DOI] [PubMed] [Google Scholar]
- 25. Wu W, Liang H, Kan J, Liu C, Yuan M, Liang C, Yang K, Pang Y. 2008. Autographa californica multiple nucleopolyhedrovirus 38K is a novel nucleocapsid protein that interacts with VP1054, VP39, VP80, and itself. J. Virol. 82:12356–12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Braunagel SC, Russell WK, Rosas-Acosta G, Russell DH, Summers MD. 2003. Determination of the protein composition of the occlusion-derived virus of Autographa californica nucleopolyhedrovirus. Proc. Natl. Acad. Sci. U. S. A. 100:9797–9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang R, Deng F, Hou D, Zhao Y, Guo L, Wang H, Hu Z. 2010. Proteomics of the Autographa californica nucleopolyhedrovirus budded virions. J. Virol. 84:7233–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang S, Miller LK. 1998. Expression and mutational analysis of the baculovirus very late factor 1 (vlf-1) gene. Virology 245:99–109 [DOI] [PubMed] [Google Scholar]
- 29. Vanarsdall AL, Okano K, Rohrmann GF. 2004. Characterization of a baculovirus with a deletion of vlf-1. Virology 326:191–201 [DOI] [PubMed] [Google Scholar]
- 30. Vanarsdall AL, Okano K, Rohrmann GF. 2006. Characterization of the role of very late expression factor 1 in baculovirus capsid structure and DNA processing. J. Virol. 80:1724–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beniya H, Braunagel SC, Summers MD. 1998. Autographa californica nuclear polyhedrosis virus: subcellular localization and protein trafficking of BV/ODV-E26 to intranuclear membranes and viral envelopes. Virology 240:64–75 [DOI] [PubMed] [Google Scholar]
- 32. Li Z, Li C, Yang K, Wang L, Yin C, Gong Y, Pang Y. 2003. Characterization of a chitin-binding protein GP37 of Spodoptera litura multicapsid nucleopolyhedrovirus. Virus Res. 96:113–122 [DOI] [PubMed] [Google Scholar]
- 33. Russell RLQ, Rohrmann GF. 1993. A 25-kDa protein is associated with the envelopes of occluded baculovirus virions. Virology 195:532–540 [DOI] [PubMed] [Google Scholar]
- 34. Fang M, Nie Y, Harris S, Erlandson MA, Theilmann DA. 2009. Autographa californica multiple nucleopolyhedrovirus core gene ac96 encodes a per Os infectivity factor (PIF-4). J. Virol. 83:12569–12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burks JK, Summers MD, Braunagel SC. 2007. BV/ODV-E26: a palmitoylated, multifunctional structural protein of Autographa californica nucleopolyhedrovirus. Virology 361:194–203 [DOI] [PubMed] [Google Scholar]
- 36. Wang M, Tan Y, Yin F, Deng F, Vlak JM, Hu Z, Wang H. 2008. The F-like protein Ac23 enhances the infectivity of the budded virus of gp64-null Autographa californica multinucleocapsid nucleopolyhedrovirus pseudotyped with baculovirus envelope fusion protein F. J. Virol. 82:9800–9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lung OY, Cruz-Alvarez M, Blissard GW. 2003. Ac23, an envelope fusion protein homolog in the baculovirus Autographa californica multicapsid nucleopolyhedrovirus, is a viral pathogenicity factor. J. Virol. 77:328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng X, Krell P, Arif B. 2001. P34.8 (GP37) is not essential for baculovirus replication. J. Gen. Virol. 82:299–305 [DOI] [PubMed] [Google Scholar]
- 39. Chen L, Hu XL, Xiang XW, Yu SF, Yang R, Wu XF. 2012. Autographa californica multiple nucleopolyhedrovirus odv-e25 (Ac94) is required for budded virus infectivity and occlusion-derived virus formation. Arch. Virol. 157:617–625 [DOI] [PubMed] [Google Scholar]
- 40. Hong T, Summers MD, Braunagel SC. 1997. N-terminal sequences from Autographa californica nuclear polyhedrosis virus envelope proteins ODV-E66 and ODV-E25 are sufficient to direct reporter proteins to the nuclear envelope, intranuclear microvesicles and the envelope of occlusion derived virus. Proc. Natl. Acad. Sci. U. S. A. 94:4050–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Braunagel SC, He H, Ramamurthy P, Summers MD. 1996. Transcription, translation, and cellular localization of three Autographa californica nuclear polyhedrosis virus structural proteins: ODV-E18, ODV-E35, and ODV-EC27. Virology 222:100–114 [DOI] [PubMed] [Google Scholar]
- 42. McCarthy CB, Theilmann DA. 2008. AcMNPV ac143 (odv-e18) is essential for mediating budded virus production and is the 30th baculovirus core gene. Virology 375:277–291 [DOI] [PubMed] [Google Scholar]
- 43. Reilly LM, Guarino LA. 1996. The viral ubiquitin gene of Autographa californica nuclear polyhedrosis virus is not essential for viral replication. Virology 218:243–247 [DOI] [PubMed] [Google Scholar]
- 44. Blissard GW, Wenz JR. 1992. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane-fusion. J. Virol. 66:6829–6835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu JC, Maruniak JE. 1999. Molecular characterization of genes in the GP41 region of baculoviruses and phylogenetic analysis based upon GP41 and polyhedrin genes. Virus Res. 64:187–196 [DOI] [PubMed] [Google Scholar]
- 46. Faulkner P, Kuzio J, Williams GV, Wilson JA. 1997. Analysis of p74, a PDV envelope protein of Autographa californica nucleopolyhedrovirus required for occlusion body infectivity in vivo. J. Gen. Virol. 78:3091–3100 [DOI] [PubMed] [Google Scholar]
- 47. Kuzio J, Jaques R, Faulkner P. 1989. Identification of p74, a gene essential for virulence of baculovirus occlusion bodies. Virology 173:759–763 [DOI] [PubMed] [Google Scholar]
- 48. Yao LG, Zhou WK, Xu H, Zheng Y, Qi YP. 2004. The Heliothis armigera single nucleocapsid nucleopolyhedrovirus envelope protein P74 is required for infection of the host midgut. Virus Res. 104:111–121 [DOI] [PubMed] [Google Scholar]
- 49. Kikhno I, Gutierrez S, Croizier L, Croizier G, Ferber ML. 2002. Characterization of pif, a gene required for the per os infectivity of Spodoptera littoralis nucleopolyhedrovirus. J. Gen. Virol. 83:3013–3022 [DOI] [PubMed] [Google Scholar]
- 50. Pijlman GP, Pruijssers AJ, Vlak JM. 2003. Identification of pif-2, a third conserved baculovirus gene required for per os infection of insects. J. Gen. Virol. 84:2041–2049 [DOI] [PubMed] [Google Scholar]
- 51. Ohkawa T, Washburn JO, Sitapara R, Sid E, Volkman LE. 2005. Specific binding of Autographa californica M nucleopolyhedrovirus occlusion-derived virus to midgut cells of Heliothis virescens larvae is mediated by products of pif genes Ac119 and Ac022 but not by Ac115. J. Virol. 79:15258–15264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiang XW, Chen L, Hu XL, Yu SF, Yang R, Wu XF. 2011. Autographa californica multiple nucleopolyhedrovirus odv-e66 is an essential gene required for oral infectivity. Virus Res. 158:72–78 [DOI] [PubMed] [Google Scholar]
- 53. Whitford M, Faulkner P. 1992. Nucleotide-sequence and transcriptional analysis of a gene encoding gp41, a structural glycoprotein of the baculovirus Autographa californica nuclear polyhedrosis virus. J. Virol. 66:4763–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Whitford M, Faulkner P. 1992. A structural polypeptide of the baculovirus Autographa californica nuclear polyhedrosis virus contains O-linked N-acetylglucosamine. J. Virol. 66:3324–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Olszewski J, Miller LK. 1997. A role for baculovirus GP41 in budded virus production. Virology 233:292–301 [DOI] [PubMed] [Google Scholar]
- 56. Braunagel SC, Elton DM, Ma H, Summers MD. 1996. Identification and analysis of an Autographa californica nuclear polyhedrosis virus structural protein of the occlusion-derived virus envelope: ODV-E56. Virology 217:97–110 [DOI] [PubMed] [Google Scholar]
- 57. Theilmann DA, Chantler JK, Stewart S, Flipsen HTM, Vlak JM, Crook NE. 1996. Characterization of a highly conserved Baculovirus structural protein that is specific for occlusion-derived virions. Virology 218:148–158 [DOI] [PubMed] [Google Scholar]
- 58. Wu WB, Lin TH, Pan LJ, Yu M, Li ZF, Pang Y, Yang K. 2006. Autographa californica multiple nucleopolyhedrovirus nucleocapsid assembly is interrupted upon deletion of the 38K gene. J. Virol. 80:11475–11485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McCarthy CB, Dai X, Donly C, Theilmann DA. 2008. Autographa californica multiple nucleopolyhedrovirus ac142, a core gene that is essential for BV production and ODV envelopment. Virology 372:325–339 [DOI] [PubMed] [Google Scholar]
- 60. Yuan M, Wu W, Liu C, Wang Y, Hu Z, Yang K, Pang Y. 2008. A highly conserved baculovirus gene p48 (ac103) is essential for BV production and ODV envelopment. Virology 379:87–96 [DOI] [PubMed] [Google Scholar]
- 61. Liu C, Li Z, Wu W, Li L, Yuan M, Pan L, Yang K, Pang Y. 2008. Autographa californica multiple nucleopolyhedrovirus ac53 plays a role in nucleocapsid assembly. Virology 382:59–68 [DOI] [PubMed] [Google Scholar]
- 62. Hu ZY, Yuan MJ, Wu WB, Liu C, Yang K, Pang Y. 2010. Autographa californica multiple nucleopolyhedrovirus ac76 is involved in intranuclear microvesicle formation. J. Virol. 84:7437–7447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alfonso V, Maroniche GA, Reca SR, Lopez MG, del Vas M, Taboga O. 2012. AcMNPV core gene ac109 is required for budded virion transport to the nucleus and for occlusion of viral progeny. PLoS One 7:e46146. 10.1371/journal.pone.0046146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lehiy CJ, Wu W, Berretta MF, Passarelli AL. 2013. Autographa californica M nucleopolyhedrovirus open reading frame 109 affects infectious budded virus production and nucleocapsid envelopment in the nucleus of cells. Virology 435:442–452 [DOI] [PubMed] [Google Scholar]
- 65. Takagi I, Yamada K, Sato T, Hanaichi T, Iwamoto T, Jin L. 1990. Penetration and sustainability of modified Sato's lead staining solution. J. Electron Microsc. (Tokyo) 39:67–68 [PubMed] [Google Scholar]