Abstract
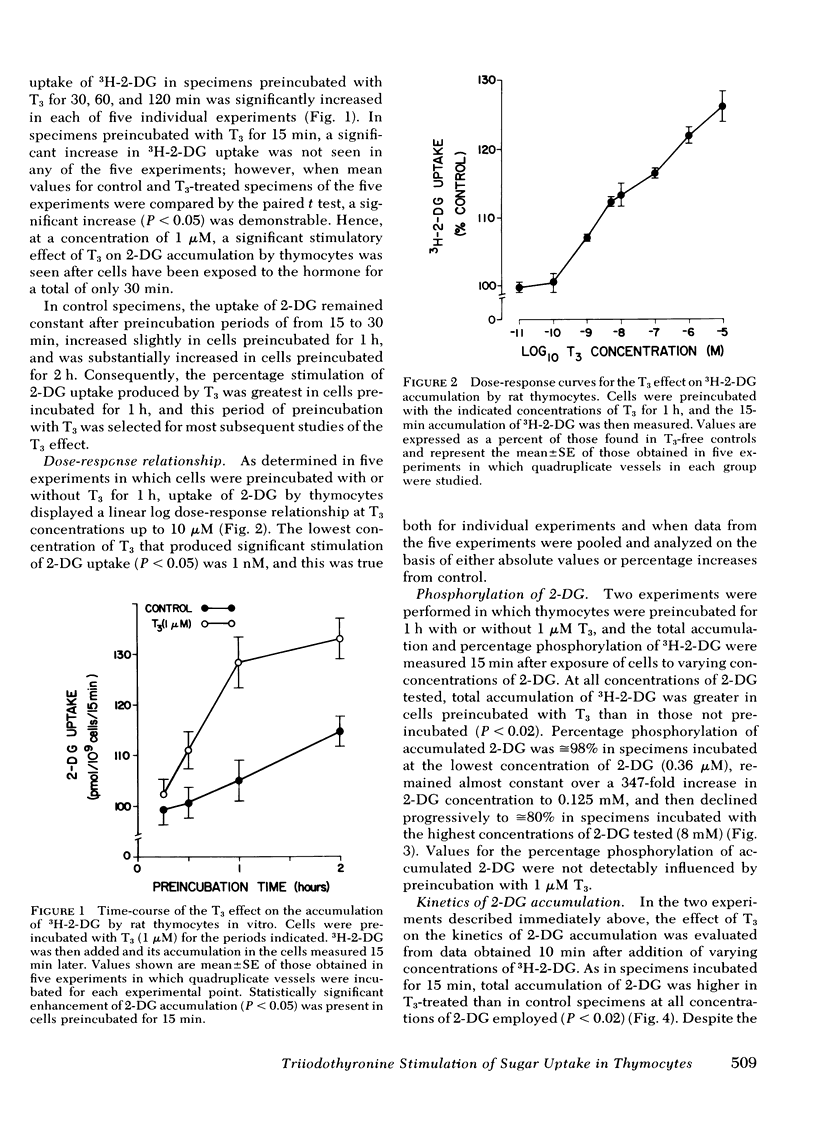
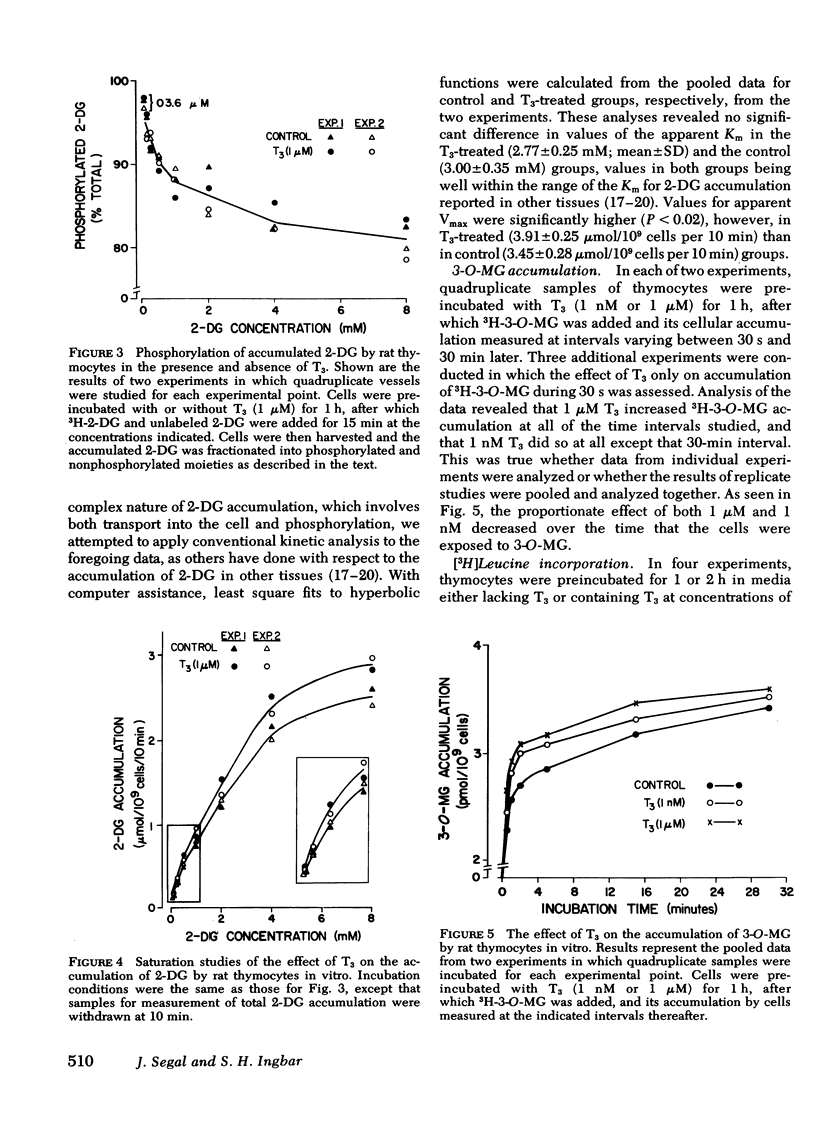
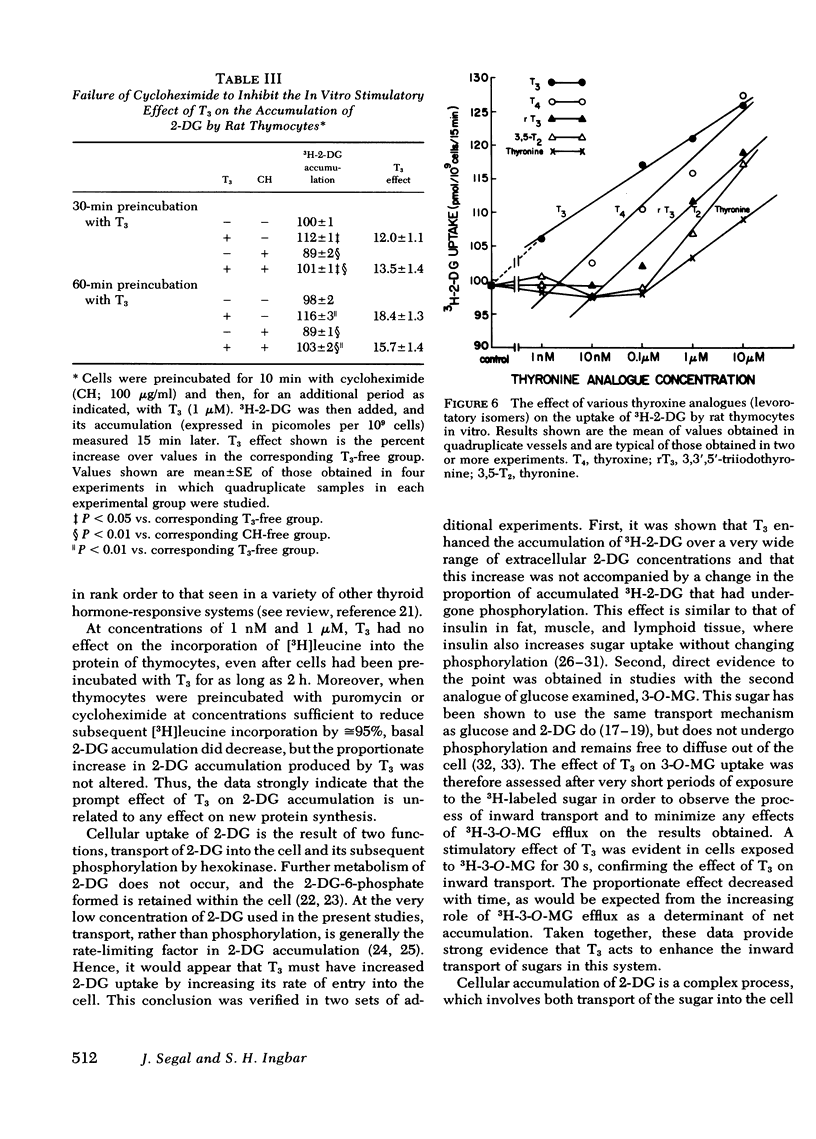
Studies were conducted to ascertain the in vitro effect of 3,5,3′-triiodothyronine (T3) on the accumulation of the glucose analogue, 2-deoxyglucose (2-DG), by thymocytes freshly isolated from weanling rats. At a concentration of 1 μM, T3 stimulated the 15-min uptake of 3H-2-DG after cells had been exposed to T3 for only 30 min. Significant stimulation of 2-DG accumulation was produced by 1 nM T3, with increasing stimulation at doses ranging up to 10 μM. T3 did not alter the fraction of accumulated 2-DG that was phosphorylated, and kinetic studies indicated that its effect was associated with a significant increase in the apparent Vmax of 2-DG accumulation, but not the apparent Km. T3 also enhanced the accumulation by thymocytes of the nonmetabolized glucose analogue, 3-O-methylglucose (3-O-MG), an effect that was evidently the result of an increase in 3-O-MG transport into the cell, because it was seen in cells incubated with 3H-3-O-MG for only 30 s. The proportionate increase in 2-DG accumulation produced by T3 was not altered by preincubating cells with concentrations of puromycin or cycloheximide sufficient to reduce [3H]-leucine incorporation by 95%, and T3 over a period of >2 h had no effect on [3H]leucine incorporation itself.
These results indicate that T3 stimulates the uptake of sugars in rat thymocytes in vitro by an effect on their inward transport. The promptness of the effect and its failure to be inhibited during profound inhibition of protein synthesis further indicate that this effect of T3 is not mediated through a nuclear-dependent mechanism. Rather, the properties of this response, and of the increases in amino acid and 2-DG accumulation produced by T3 in other tissue preparations, strongly suggest that these effects of T3 are mediated at the level of cell membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson L. F., Ingbar S. H. Selective alteration by triiodothyronine of amino acid transport in embryonic bone. Endocrinology. 1967 Dec;81(6):1362–1371. doi: 10.1210/endo-81-6-1362. [DOI] [PubMed] [Google Scholar]
- Adamson L. F., Ingbar S. H. Some properties of the stimulatory effect of thyroid hormones on amino acid transport by embryonic chick bone. Endocrinology. 1967 Dec;81(6):1372–1378. doi: 10.1210/endo-81-6-1372. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Creagan S., Ingbar S. H., Kipnes R. S. Stimulation of mitochondrial adenosine diphosphate uptake by thyroid hormones. Proc Natl Acad Sci U S A. 1973 Jan;70(1):98–102. doi: 10.1073/pnas.70.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie B. B., Davies J. I., Hynie S., Krishna G., Weiss B. Interrelationships of catecholamines with other endocrine systems. Pharmacol Rev. 1966 Mar;18(1):273–289. [PubMed] [Google Scholar]
- Burns A. H., Reddy W. J. Direct effect of thyroid hormones on glucose oxidation by isolated rat cardiac myocytes. J Mol Cell Cardiol. 1975 Aug;7(8):553–561. doi: 10.1016/0022-2828(75)90114-5. [DOI] [PubMed] [Google Scholar]
- CROFFORD O. B., RENOLD A. E. GLUCOSE UPTAKE BY INCUBATED RAT EPIDIDYMAL ADIPOSE TISSUE. RATE-LIMITING STEPS AND SITE OF INSULIN ACTION. J Biol Chem. 1965 Jan;240:14–21. [PubMed] [Google Scholar]
- Challoner D. R., Allen D. O. An in vitro effect of triiodothyronine on lipolysis, cyclic AMP-C14 accumulation and oxygen consumption in isolated fat cells. Metabolism. 1970 Jul;19(7):480–487. doi: 10.1016/0026-0495(70)90002-8. [DOI] [PubMed] [Google Scholar]
- Corrèze C., Nunez J., Gordon A. Thyroid hormones and lipogenesis from glucose in rat fat cells. Mol Cell Endocrinol. 1977 Dec;9(2):133–144. doi: 10.1016/0303-7207(77)90115-0. [DOI] [PubMed] [Google Scholar]
- Degroot L. J., Refetoff S., Strausser J., Barsano C. Nuclear triiodothyronine-binding protein: partial characterization and binding to chromatin. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4042–4046. doi: 10.1073/pnas.71.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELRICK H., HLAD C. J., Jr, ARAI Y. Influence of thyroid function on carbohydrate metabolism and a new method for assessing response to insulin. J Clin Endocrinol Metab. 1961 Apr;21:387–400. doi: 10.1210/jcem-21-4-387. [DOI] [PubMed] [Google Scholar]
- Friedman Y., Lang M., Burke G. Inhibition of thyroid adenylate cyclase by thyroid hormone: a possible locus for the "short-loop" negative feedback phenomenon. Endocrinology. 1977 Sep;101(3):858–868. doi: 10.1210/endo-101-3-858. [DOI] [PubMed] [Google Scholar]
- GUIDOTTI G., FOA P. P. Development of an insulin-sensitive glucose transport system in chick embryo hearts. Am J Physiol. 1961 Nov;201:869–872. doi: 10.1152/ajplegacy.1961.201.5.869. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Simons C. G., Smith G. J., Ingbar S. H. Cycloleucine transport in isolated rat thymocytes: in vitro effects of triiodothyronine and thyroxine. Endocrinology. 1975 Apr;96(4):1030–1037. doi: 10.1210/endo-96-4-1030. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J., Simons C. G., Ingbar S. H., Jorgensen E. C. Activities of thyroid hormones and related compounds in an in vitro thymocyte assay. J Biol Chem. 1976 Jul 25;251(14):4233–4238. [PubMed] [Google Scholar]
- HENDERSON M. J. THE UPTAKE OF GLUCOSE INTO CELLS AND THE ROLE OF INSULIN IN GLUCOSE TRANSPORT. Can J Biochem. 1964 Jun;42:933–944. doi: 10.1139/o64-105. [DOI] [PubMed] [Google Scholar]
- Hoch F. L., Motta M. V. Reversal of early thyroid hormone action on mitochondria by bovine serum albumin in vitro. Proc Natl Acad Sci U S A. 1968 Jan;59(1):118–122. doi: 10.1073/pnas.59.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IL'IN V. S., KIL'DEMA L. A. [On the effect of cortisone and insulin on the activity of hexokinase in leukocytes]. Vopr Med Khim. 1962 Jul-Aug;8:374–376. [PubMed] [Google Scholar]
- Ichikawa A., Matsumoto H., Sakato N., Tomita K. Effect of thyroid hormones on epinephrine-induced lipolysis in adipose tissue of rats. J Biochem. 1971 Jun;69(6):1055–1064. doi: 10.1093/oxfordjournals.jbchem.a129558. [DOI] [PubMed] [Google Scholar]
- KALANT N., SCHUCHER R. Glucose utilization and insulin responsiveness of leucocytes in diabetes. Can J Biochem Physiol. 1962 Jul;40:899–903. [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. V. The penetration and phosphorylation of 2-deoxyglucose in the rat diaphragm. J Biol Chem. 1959 Jan;234(1):171–177. [PubMed] [Google Scholar]
- Kistler A., Yoshizato K., Frieden E. Binding of thyroxine and triiodothyronine by nuclei of isolated tadpole liver cells. Endocrinology. 1975 Oct;97(4):1036–1042. doi: 10.1210/endo-97-4-1036. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. I. A functional change in the plasma membrane associated with the rate of cell growth. J Biol Chem. 1974 Jun 10;249(11):3366–3374. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. II. Alterations in transport following transformation by a temperature-sensitive mutant of the Rous sarcoma virus. J Biol Chem. 1974 Jun 10;249(11):3375–3382. [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Long-term regulation of adipocyte glucose transport capacity by circulating insulin in rats. J Clin Invest. 1978 Jul;62(1):73–81. doi: 10.1172/JCI109116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki T., Yamakawa S. Effects of dibutyryl cyclic adenosine 3':5' monophosphate and theophylline on 2-deoxy-D-glucose and 2-aminoisobutyric acid uptake by hamster embryo cells. Int J Cancer. 1974 Jul 15;14(1):32–39. doi: 10.1002/ijc.2910140105. [DOI] [PubMed] [Google Scholar]
- NARAHARA H. T., OZAND P. Studies of tissue permeability. IX. The effect of insulin on the penetration of 3-methylglucose-H3 in frog muscle. J Biol Chem. 1963 Jan;238:40–49. [PubMed] [Google Scholar]
- Olefsky J. M. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975 Dec;56(6):1499–1508. doi: 10.1172/JCI108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliam N. B., Goldfine I. D. High affinity thyroid hormone binding sites on purified rat liver plasma membranes. Biochem Biophys Res Commun. 1977 Nov 7;79(1):166–172. doi: 10.1016/0006-291x(77)90075-4. [DOI] [PubMed] [Google Scholar]
- Porterfield S. P., Hendrich C. E. The effect of maternal hypothyroidism on maternal and fetal tissue glucose-1-14C incorporation in rats. Horm Res. 1975;6(4):236–246. doi: 10.1159/000178697. [DOI] [PubMed] [Google Scholar]
- Porterfield S. P. The effects of maternal hypothyroidism on the in vitro metabolism of [1(-14)C]-glucose in rats. Horm Metab Res. 1977 Nov;9(6):502–506. doi: 10.1055/s-0028-1093510. [DOI] [PubMed] [Google Scholar]
- Primack M. P., Buchanan J. L., Tapley D. F. Early stimulation of mitochondrial protein synthesis in livers from triiodothyronine-injected mice. Endocrinology. 1970 Dec;87(6):1355–1357. doi: 10.1210/endo-87-6-1355. [DOI] [PubMed] [Google Scholar]
- Primack M. P., Tapley D. F., Buchanan J. Stimulation of mitochondrial protein synthesis and oxygen consumption by thyroxine in vitro without deiodination to triiodothyronine. Biochim Biophys Acta. 1971 Aug 19;244(2):349–352. doi: 10.1016/0304-4165(71)90236-4. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3488–3492. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J., Gordon A. The effect 3,5,3'-triiodo-L-thyronine on the kinetic parameters of sugar transport in cultured chick embryo heart cells. Endocrinology. 1977 Nov;101(5):1468–1474. doi: 10.1210/endo-101-5-1468. [DOI] [PubMed] [Google Scholar]
- Segal J., Gordon A. The effects of actinomycin D, puromycin, cycloheximide and hydroxyurea on 3',5,3-triiodo-L-thyronine stimulated 2-deoxy-D-glucose uptake in chick embryo heart cells in vitro. Endocrinology. 1977 Jul;101(1):150–156. doi: 10.1210/endo-101-1-150. [DOI] [PubMed] [Google Scholar]
- Segal J., Schwartz H., Gordon A. The effect of triiodothyronine on 2-deoxy-D-(1-3H)glucose uptake in cultured chick embryo heart cells. Endocrinology. 1977 Jul;101(1):143–149. doi: 10.1210/endo-101-1-143. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Gorski J. Extrogen control of uterine glucose metabolism. An analysis based on the transport and phosphorylation of 2-deoxyglucose. J Biol Chem. 1968 Aug 25;243(16):4169–4174. [PubMed] [Google Scholar]
- Spaulding S. W., Noth R. H. Thyroid-catecholamine interactions. Med Clin North Am. 1975 Sep;59(5):1123–1131. doi: 10.1016/s0025-7125(16)31962-9. [DOI] [PubMed] [Google Scholar]
- Spindler B. J., MacLeod K. M., Ring J., Baxter J. D. Thyroid hormone receptors. Binding characteristics and lack of hormonal dependency for nuclear localization. J Biol Chem. 1975 Jun 10;250(11):4113–4119. [PubMed] [Google Scholar]
- Sterling K., Milch P. O., Brenner M. A., Lazarus J. H. Thyroid hormone action: the mitochondrial pathway. Science. 1977 Sep 2;197(4307):996–999. doi: 10.1126/science.196334. [DOI] [PubMed] [Google Scholar]
- Vaughan M. An in vitro effect of triiodothyronine on rat adipose tissue. J Clin Invest. 1967 Sep;46(9):1482–1491. doi: 10.1172/JCI105640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinten J., Gliemann J., Osterlind K. Exchange of 3-O-methylglucose in isolated fat cells. Concentration dependence and effect of insulin. J Biol Chem. 1976 Feb 10;251(3):794–800. [PubMed] [Google Scholar]
- Waldstein S. S. Thyroid-catecholamine interrelations. Annu Rev Med. 1966;17:123–132. doi: 10.1146/annurev.me.17.020166.001011. [DOI] [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- Winnick S. Response of hepatic glucokinase and glucose-6-phosphatase activities in juvenile and adult hyperthyroid mice. Endocrinology. 1970 Jul;87(1):124–128. doi: 10.1210/endo-87-1-124. [DOI] [PubMed] [Google Scholar]
- Yu S., Friedman Y., Richman R., Burke G. Altered thyroidal responsivity to thyrotropin induced by circulating thyroid hormones. A "short-loop" regulatory mechanism? J Clin Invest. 1976 Mar;57(3):745–755. doi: 10.1172/JCI108333. [DOI] [PMC free article] [PubMed] [Google Scholar]



