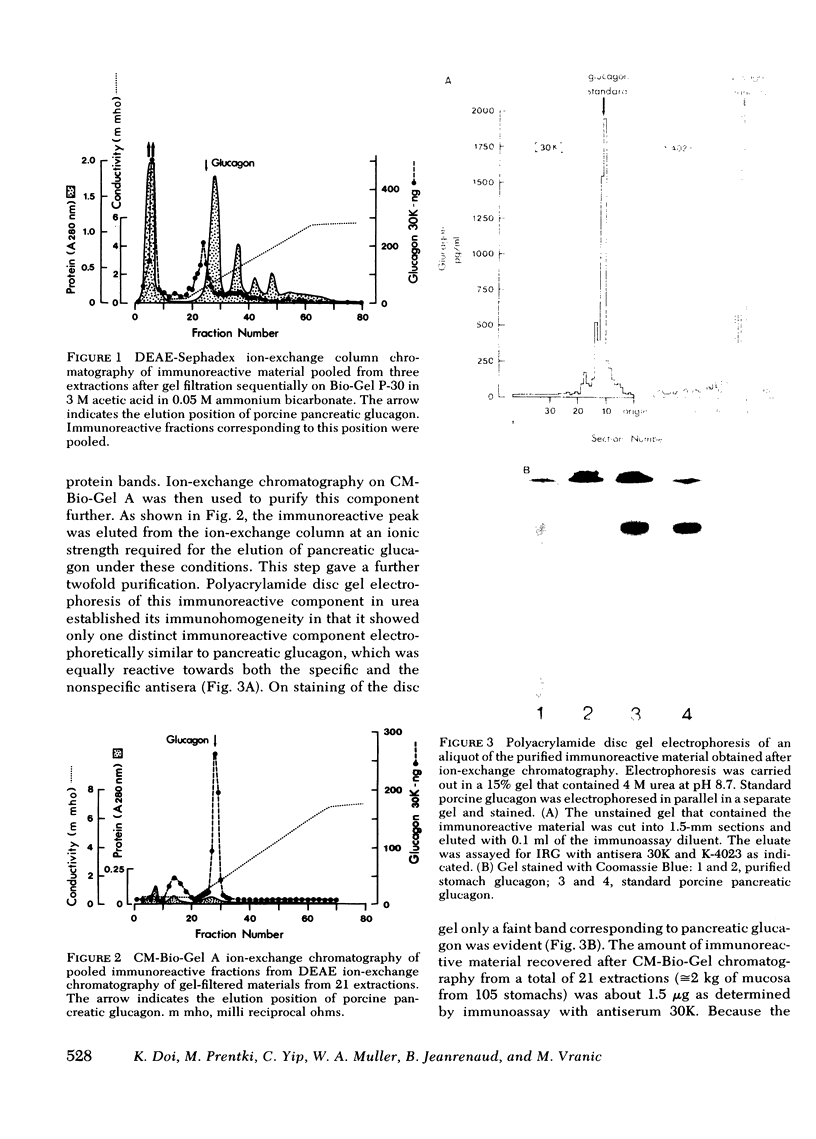
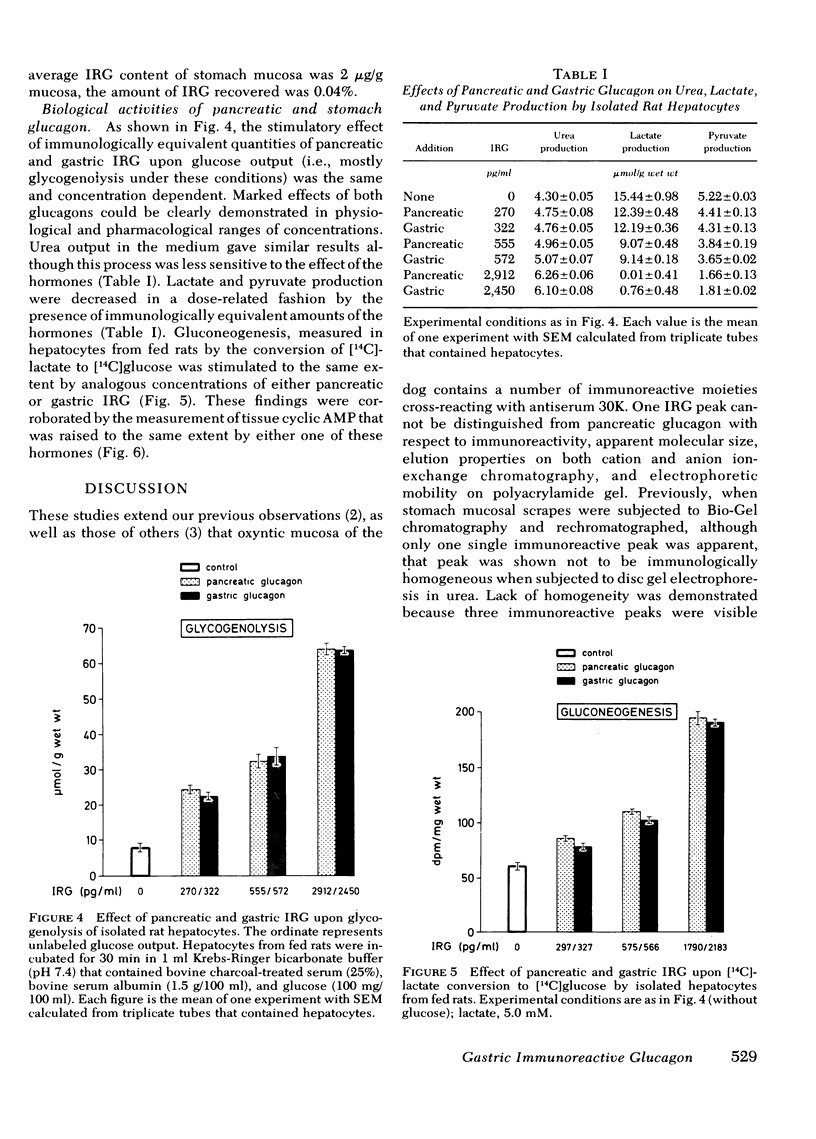
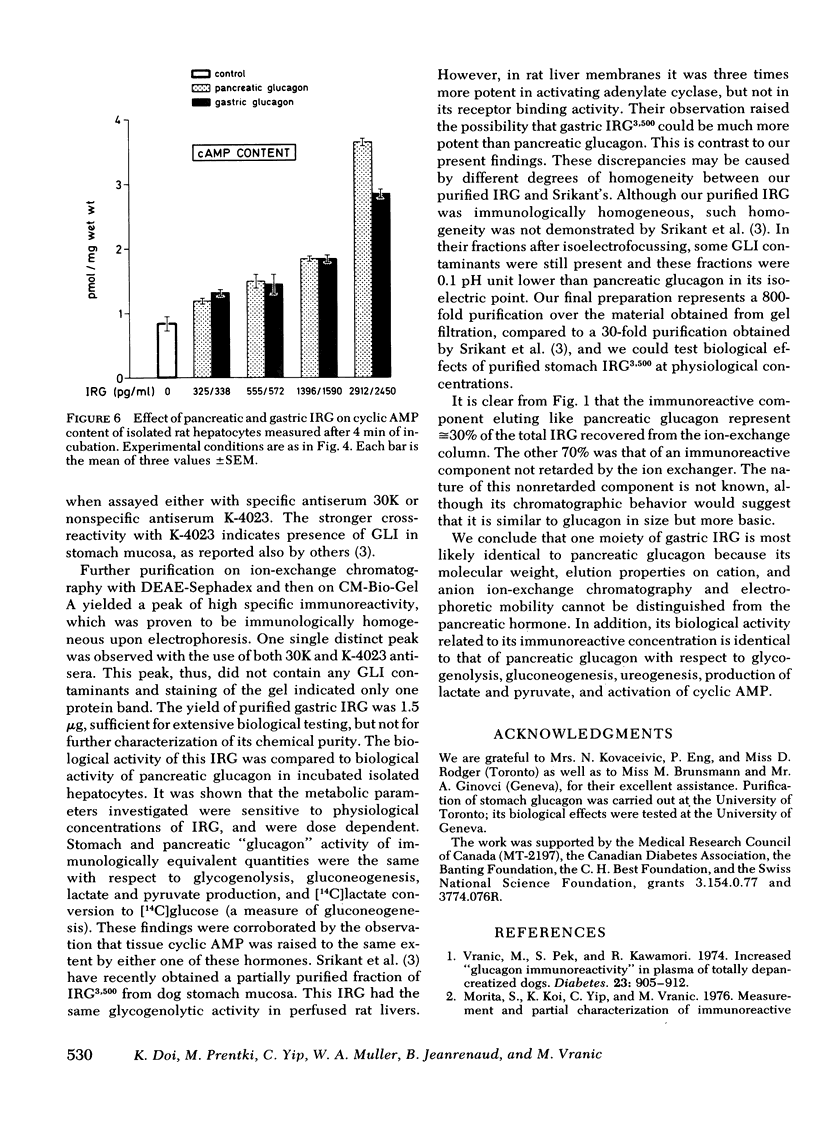
Abstract
Because in the dog, the gastric fundus contains the largest amount of glucagon immunoreactivity (IRG), the IRG of mucosal scrapes of 105 canine stomachs was extracted by acid-ethanol and then precipitated by ether-ethanol. The IRG recovered was measured by antisera 30K, specific for glucagon and K-4023, which cross-reacts with glucagon-like immunoreactivity. Extracts of mucosa of stomach fundus were further purified by gel filtration on Bio-Gel P-30 in 3M acetic acid. One pooled fraction corresponding to marker pancreatic glucagon in its elution volume was then gel-filtered on Bio-Gel P-30 in 0.05 M NH4HCO3 and yielded one IRG peak, which, however, showed three immunoreactive components on polyacrylamide disc gel electrophoresis in urea. In addition, antiserum K-4023 reacted more strongly with that peak than antiserum 30K indicating the presence of glucagon-like immunoreactivity in this fraction. Subsequent ion-exchange column chromatography on DEAE-Sephadex A-25 and then CM-Bio-Gel A allowed purification to a single protein band on disc gel electrophoresis reacting equally to both antisera 30K and K-4023. 1.5 μg of purified gastric glucagon was obtained and its biological effects were compared to those of pancreatic glucagon in isolated rat hepatocytes. When immuno-equivalent amounts (300-2,500 pg/ml) of either type of glucagon were used, the same biological responses with respect to glycogenolysis and gluconeogenesis as well as urea, lactate, and pyruvate production were observed. Liver cyclic AMP was also raised to the same extent by either one of these hormones. We conclude that this moiety of gastric IRG is apparently identical to pancreatic glucagon because (a) their molecular weights, elution properties in ion exchange chromatography, and their electrophoretic mobility are indistinguishable and (b) both hormones elicited identical biological effects in isolated rat hepatocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baetens D., Rufener C., Srikant B. C., Dobbs R., Unger R., Orci L. Identification of glucagon-producing cells (A cells) in dog gastric mucosa. J Cell Biol. 1976 May;69(2):455–464. doi: 10.1083/jcb.69.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez E., Muñoz-Barragan L., Patton G. S., Orci L., Dobbs R. E., Unger R. H. Gastric A-cell function in insulin-deprived depancreatized dogs. Endocrinology. 1976 Nov;99(5):1182–1188. doi: 10.1210/endo-99-5-1182. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Kawamori R., Pek S., Vranic M. Arginine infusion in dogs. Model for the roles of insulin and glucagon in regulating glucose turnover and free fatty acid levels. Diabetes. 1974 Oct;23(10):805–815. doi: 10.2337/diab.23.10.805. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman J. G., Sutherland E. W. Guanyl cyclase, an enzyme catalyzing the formation of guanosine 3',5'-monophosphate from guanosine trihosphate. J Biol Chem. 1969 Dec 10;244(23):6363–6370. [PubMed] [Google Scholar]
- KENNY A. J. Extractable glucagon of the human pancreas. J Clin Endocrinol Metab. 1955 Sep;15(9):1089–1105. doi: 10.1210/jcem-15-9-1089. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Holst J., Håkanson R., Sundler F. Distribution and properties of glucagon immunoreactivity in the digestive tract of various mammals: an immunohistochemical and immunochemical study. Histochemistry. 1975 Sep 29;44(4):281–290. doi: 10.1007/BF00490364. [DOI] [PubMed] [Google Scholar]
- Le Marchand Y., Singh A., Assimacopoulos-Jeannet F., Orci L., Rouiller C., Jeanrenaud B. A role for the microtubular system in the release of very low density lipoproteins by perfused mouse livers. J Biol Chem. 1973 Oct 10;248(19):6862–6870. [PubMed] [Google Scholar]
- Lefèbvre P. J., Luyckx A. S. Factors controlling gastric-glucagon release. J Clin Invest. 1977 Apr;59(4):716–722. doi: 10.1172/JCI108690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S., Doi K., Yip C. C., Vranic M. Measurement and partial characterization of immunoreactive glucagon in gastrointestinal tissues of dogs. Diabetes. 1976 Nov;25(11):1018–1025. doi: 10.2337/diab.25.11.1018. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Girardier L., Seydoux J., Berger M., Renold A. E., Vranic M. Extrapancreatic glucagon and glucagonlike immunoreactivity in depancreatized dogs. A quantitative assessment of secretion rates and anatomical delineation of sources. J Clin Invest. 1978 Jul;62(1):124–132. doi: 10.1172/JCI109096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P., Singh A., Orci L., Jeanrenaud B. Secretory processes, carbohydrate and lipid metabolism in isolated mouse hepatocytes. Aspects of regulation by glucagon and insulin. Biochim Biophys Acta. 1976 Apr 23;428(2):480–494. doi: 10.1016/0304-4165(76)90057-x. [DOI] [PubMed] [Google Scholar]
- OKUNO G., PRICE S., GRILLO T. A., FOA P. P. DEVELOPMENT OF PHOSPHORYLASE AND PHOSPHORYLASE-ACTIVATING (GLUCAGON-LIKE) SUBSTANCES IN THE RAT EMBRYO. Gen Comp Endocrinol. 1964 Aug;4:446–451. doi: 10.1016/0016-6480(64)90013-9. [DOI] [PubMed] [Google Scholar]
- Ravazzola M., Baetens D., Engerman R., Kovacevic N., Vranic M., Orci L. Endocrine cells in oxyntic mucosa of a dog 5 years after pancreatectomy. Horm Metab Res. 1977 Nov;9(6):480–483. doi: 10.1055/s-0028-1093504. [DOI] [PubMed] [Google Scholar]
- Srikant C. B., McCorkle K., Unger R. H. Properties of immunoreactive glucagon fractions of canine stomach and pancreas. J Biol Chem. 1977 Mar 25;252(6):1847–1851. [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranic M., Engerman R., Doi K., Morita S., Yip C. C. Extrapancreatic glucagon in the dog. Metabolism. 1976 Nov;25(11 Suppl 1):1469–1473. doi: 10.1016/s0026-0495(76)80169-2. [DOI] [PubMed] [Google Scholar]
- Vranic M., Kawamori R., Pek S., Kovacevic N., Wrenshall G. A. The essentiality of insulin and the role of glucagon in regulating glucose utilization and production during strenuous exercise in dogs. J Clin Invest. 1976 Feb;57(2):245–255. doi: 10.1172/JCI108275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranic M., Pek S., Kawamori R. Increased "glucagon immunoreactivity" in plasma of totally depancreatized dogs. Diabetes. 1974 Nov;23(11):905–912. doi: 10.2337/diab.23.11.905. [DOI] [PubMed] [Google Scholar]