Abstract
The development of an effective vaccine preventing HIV-1 infection remains elusive. Thus, the development of novel approaches capable of preventing HIV-1 transmission is of paramount importance. However, this is partly hindered by the lack of an easily accessible small-animal model to rapidly measure viral entry. Here, we report the generation of a human CD4- and human CCR5-expressing transgenic luciferase reporter mouse that facilitates measurement of peritoneal and genitomucosal HIV-1 pseudovirus entry in vivo. We show that antibodies and antiretrovirals mediate preexposure protection in this mouse model and that the serum antibody concentration required for protection from cervicovaginal infection is comparable to that required to protect macaques. Our results suggest that this system represents a model for the preclinical evaluation of prophylactic or vaccine candidates. It further supports the idea that broadly neutralizing antibodies should be evaluated for use as preexposure prophylaxis in clinical trials.
INTRODUCTION
Thirty years after first reports of patients presenting with symptoms of human immunodeficiency virus (HIV) infection (1), AIDS remains one of the leading global health problems (2). While the RV144 trial demonstrated the modest efficacy of the RV144 vaccine in a community risk vaccination setting (3, 4), a highly protective vaccine remains elusive.
Recent studies have shown that antiretroviral therapy (ART) of HIV type 1 (HIV-1)-infected patients can significantly reduce the rate of HIV-1 transmission (5) and decrease the risk of infection when used in seronegative probands (6–8). Other trials, however, failed to demonstrate protective effects for antiretroviral medication, which may be attributable to low drug adherence (9, 10), indicating the need for further research on optimized administration strategies.
Broadly neutralizing antibodies (bnAbs) directed against the envelope protein (Env) of HIV-1 may present an additional option for HIV-1 preexposure prophylaxis. Passive administration of neutralizing antibodies to macaques can provide sterilizing immunity to simian-human immunodeficiency virus (SHIV) infection (11–15) and protect from HIV-1 infection in humanized rodent models (16–20). Many of these studies were, however, performed with earlier-generation neutralizing antibodies. Recently introduced methods for isolation and cloning of monoclonal antibodies have led to the production of human antibodies with increased neutralizing breadth and potency (21–27), which could prove beneficial for clinical use, including therapy (28).
A variety of small-animal models have been developed to study different aspects of HIV-1 infection in vivo, including models with several strains of immunodeficient mice reconstituted with components of the human immune system (reviewed in references 29 to 31). These mice are susceptible to HIV-1 infection, and they can recapitulate the viral life cycle and show long-term viremia. However, infections in individual mice vary, depending on the amount of engraftment, and the mice lack a fully functional immune system, showing only sporadic humoral immune responses to HIV-1 (32–34). The use of transgenic (35, 36) or adenovirus-mediated (19) expression of HIV-1 entry factors represents an alternative approach to rendering rodents susceptible to viral entry but not to efficient replication (37, 38). Mice and rats expressing human CD4 (hCD4) and human CCR5 (hCCR5) were infected with HIV-1 after intravenous injection with HIV-1; however, this route accounts for only a minority of infections in humans (39).
Here, we describe a transgenic reporter mouse expressing hCD4 and hCCR5 under the control of the ubiquitin promoter, allowing in vivo detection of HIV-1 pseudovirus infection after intraperitoneal (i.p.) and intravaginal (i.vag.) application by means of bioluminescence.
MATERIALS AND METHODS
Mice.
Gt(ROSA)26Sortm1(Luc)Kael/J (ROSA-Stop-Luc) mice and Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (ROSA-Stop-tdTomato) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). hCD4/hCCR5 transgenic mice were generated by cloning the hCCR5-2A-hCD4 open reading frame (19) downstream of the human ubiquitin C promoter (40) and injecting a linearized 4.5-kb fragment containing the transgene into fertilized female C57BL/6J mouse pronuclei. A founder line was chosen on the basis of surface expression of hCD4 and hCCR5 on peripheral blood mononuclear cells (PBMCs), as determined by flow cytometry (data not shown). HIV-LucTG mice were produced by breeding hCD4/hCCR5 transgenic mice to ROSA-Stop-Luc mice and backcrossing to ROSA-Stop-Luc mice to obtain mice homozygous for firefly luciferase and were screened by PCR genotyping or were produced by breeding mice homozygous for the transgene and luciferase to ROSA-Stop-Luc mice. Mice homozygous for hCD4 and hCCR5 were obtained by breeding HIV-LucTG mice and selected on the basis of surface expression levels, as determined by flow cytometry. Transgene-negative littermates or ROSA-Stop-Luc mice served as negative controls, as indicated. Mice were bred and maintained at the Comparative Bioscience Center at The Rockefeller University according to guidelines established by the Institutional Animal Committee. Experiments were performed with authorization from the Institutional Review Board (IRB) and the IACUC at The Rockefeller University.
Pseudovirus production.
HIV-1 pseudoviruses were prepared as described previously (19). Briefly, pTripCre, HIV gag-pol, and pSVIIIenvYU-2 (41) or pSVIIIenvJR-FL (42) plasmids were cotransfected in HEK293T cells using X-tremeGENE 9 reagent (Roche) according to the manufacturer's instructions at a 1.42:1.00:1.68 ratio. Supernatants were harvested, cleared of debris by centrifugation at 300 × g, filtered using a 0.45-μm-pore-size filter (Thermo Scientific), concentrated by stirred-cell ultrafiltration using a 300,000-nominal-molecular-weight-limit membrane (Millipore), and stored at −80°C. Viruses pseudotyped with the envelope protein G of the vesicular stomatitis virus (VSVg) were prepared by cotransfection of pTripCre, HIV gag-pol, and pVSVg (43) plasmids at a 3.18:2.24:1.00 ratio either in HEK293T cells using X-tremeGENE 9 and adjusted to a final concentration of 4 μg/ml Polybrene (Sigma) and 50 mM HEPES (Gibco) after harvesting or in HEK293-6E cells using polyethyleneimine (PEI) at a 1.5:1.0 ratio of PEI/DNA and adding sodium butyrate to a final concentration of 5 mM after 12 h. Supernatants were harvested, cleared of debris by centrifugation at 300 × g, filtered using a 0.45-μm-pore-size filter, and stored at −80°C. The p24 content was determined by enzyme-linked immunosorbent assay (ELISA; PerkinElmer) according to the manufacturer's instructions. The 50% tissue culture infective dose (TCID50) was determined using the mouse embryonic fibroblast (MEF)-based assay described below and calculated according to the Reed-Muench method (44). The mean luminescence of replicates of uninfected cells × 1.5 was used as the negative cutoff.
Antibody production.
Anti-VSVg antibodies were purified from supernatants of the I1 hybridoma cell line (ATCC), maintained as per the manufacturer's instructions, using protein G-Sepharose 4 Fast Flow (GE Healthcare). A mouse IgG2a antibody (clone UPC-10, Sigma) dialyzed against phosphate-buffered saline (PBS) was used as an isotype control. Anti-HIV-1 antibodies (24, 45) and the human IgG1 isotype control mGO53 (46) were expressed in HEK293T cells and purified using protein G-Sepharose 4 Fast Flow as described previously (19, 47). Purified antibodies were dialyzed against PBS and sterile filtered through a 0.22-μm-pore-size filter unit.
Cells.
MEFs from HIV-LucTG and ROSA-Stop-Luc mice were produced by spontaneous immortalization. The placenta, head, and internal organs were thoroughly removed from individual fetuses placed in ice-cold PBS. Fetuses were minced, incubated in 0.025% trypsin-EDTA (Gibco) at 37°C, homogenized by thorough pipetting, filtered using a cell strainer, and washed twice, and cells were maintained in MEF medium (RPMI 1640 supplemented with 1× antibiotic-antimycotic, 2 mM l-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 55 μM β-mercaptoethanol [all from Gibco], 10% fetal bovine serum [FBS; HyClone]). hCD4 and hCCR5 expression was confirmed by flow cytometry. HEK293T cells were maintained in 293T medium (Dulbecco's modified Eagle medium [DMEM; Gibco] supplemented with 1 mM sodium pyruvate, 1× antibiotic-antimycotic, and 10% FBS) and cultured in DMEM supplemented with 1 mM sodium pyruvate and 3% FBS after transfection for pseudovirus production. HEK293-6E cells were maintained in FreeStyle expression medium supplemented with 0.2× penicillin-streptomycin (both from Gibco) on an orbital shaker and cultured in FreeStyle medium without penicillin-streptomycin after transfection. Human healthy control PBMCs were used in accordance with the IRB protocols of The Rockefeller University.
Antiretrovirals.
For use in in vivo experiments, clinical formulations of maraviroc (Pfizer) and efavirenz (Bristol-Myers Squibb) were thoroughly ground using a mortar and pestle, resuspended in H2O, and stored at −20°C. For use in in vitro experiments, maraviroc and efavirenz were acquired through the NIH AIDS Research and Reference Reagent Program, resuspended in dimethyl sulfoxide (DMSO), and stored at −20°C. A clinical formulation of enfuvirtide (Roche) was resuspended in sterile H2O, stored at −20°C, and used for in vivo and in vitro experiments.
Flow cytometry.
Whole blood, healthy human PBMC controls, and MEFs were used to assess expression of hCD4 and hCCR5. MEFs were harvested using enzyme-free dissociation buffer (Gibco). The following antibodies were used for stainings: anti-human CCR5-fluorescein isothiocyanate (FITC) and CD4-allophycocyanin (APC) (BD Pharmingen). Stainings were performed in the presence of mouse FcBlock (2.4G2; Bio X Cell). To determine cells expressing tdTomato, peritoneal cells were acquired by lavage with ice-cold PBS and stained with anti-mouse F4/80-APC and B220-FITC (eBioscience) in the presence of FcBlock. Cells were acquired on a BD LSRFortessa cell analyzer (BD Biosciences) and analyzed with FlowJo software (TreeStar).
In vitro MEF assay.
MEFs were harvested using enzyme-free dissociation buffer (Gibco) and plated in a 24-well plate at a density of 5 × 104 cells/well in MEF medium. Serial dilutions of pseudoviruses were added after 8 to 12 h and incubated for 1 day at 37°C. Pseudoviral supernatants were replaced by fresh MEF medium, and cells were incubated at 37°C for another day. Medium was removed, cells were lysed in 100 μl ONE-Glo luciferase assay reagent (Promega) and 100 μl MEF medium for 5 min, and the lysates were thoroughly homogenized by pipetting. A volume of 150 μl of each sample was transferred to a white 96-well plate, and luminescence was measured using a FLUOstar Omega reader (BMG Labtech). Uninfected cells served as background controls. In some experiments, serial dilutions of antiretroviral drugs were added at the step of pseudovirus infection and DMSO carrier controls were performed (data not shown).
In vivo infections.
For vaginal challenge, mice were pretreated by subcutaneous (s.c.) injection of 3 mg of depot medroxyprogesterone acetate (DMPA; Depo-Provera; Pfizer) 7 days prior to intravaginal application of 40 μl of a commercially available 4.0% nonoxynol-9 (N-9) gel formulation (Conceptrol) 6 h prior to the first viral challenge. A volume of 40 μl of pseudovirus was carefully pipetted into the vagina of isoflurane-anesthetized mice, which were held in an inverted position for 5 min after application to allow adsorption of virus. Pseudovirus application was repeated after 2 and 4 h. For peritoneal challenge, mice were injected in both lower abdominal quadrants. Monoclonal antibodies were injected s.c. 1 day prior to virus challenge. For ART experiments, efavirenz and maraviroc were applied orally twice by gavaging 0.5 mg or 1.5 mg, respectively, 24 h prior to and at the time of viral challenge; a dose of 40 μg of enfuvirtide was applied s.c. every 12 h, starting 24 h prior to vaginal challenge and continuing to up to 12 h after viral challenge. For anti-VSVg experiments, virus was preincubated on ice for 30 min with anti-VSVg antibody I1 or an isotype control at a final concentration of 1 μg/ml each prior to injection. Control medium for VSVg-Cre was DMEM supplemented with 4 μg/ml Polybrene, 50 mM HEPES, and 3% FBS.
Serum antibody ELISA.
The determination of the antibody concentrations in serum was performed as described previously (19). ELISA plates (Corning) were coated with goat anti-human IgG (Jackson ImmunoResearch Laboratories) at 2.5 μg/ml overnight. Plates were blocked with 2 mM EDTA and 0.05% Tween 20 in PBS for 1 h at room temperature. Serial dilutions of mouse serum in PBS were incubated and detected with a horseradish peroxidase-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories) at a 1:1,000 dilution in blocking buffer. Samples were subsequently developed with ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); Invitrogen]. Purified human IgG was included to generate the standard curve.
Animal luciferase assay.
Mice were anesthetized using isoflurane. In mice that received pseudovirus i.p., 150 μl of a 30-mg/ml solution of d-luciferin potassium salt (Regis Technologies) was injected i.p., and peak luminescence was acquired by serial measurements; in mice that received pseudovirus i.vag., 20 μl of the d-luciferin reagent was carefully applied i.vag., and luminescence was acquired immediately thereafter. Luminescence was acquired using an IVIS Lumina II apparatus with an exposure time of 60 s, and regions of interest (ROIs) were analyzed using Living Image software (both from Caliper Life Sciences).
Ex vivo analysis.
For analysis of omental tissues, mice were injected i.p. with 4.5 mg d-luciferin reagent as described above. Mice were sacrificed after 10 min, omenta were dissected and placed into a black 96-well plate, and luminescence was acquired using the IVIS Lumina II apparatus with an exposure time of 120 s.
Statistical analysis.
Statistical analysis was performed using Prism (version 5) software for the Mac OS X operating system (GraphPad Software). Percent infection was calculated using the arithmetic means of non-log-transformed absolute photon counts per second. A two-tailed Mann-Whitney U test was used to compare groups, and tests were not adjusted for multiple comparisons. Data are presented as means ± standard errors of the means (SEMs).
RESULTS
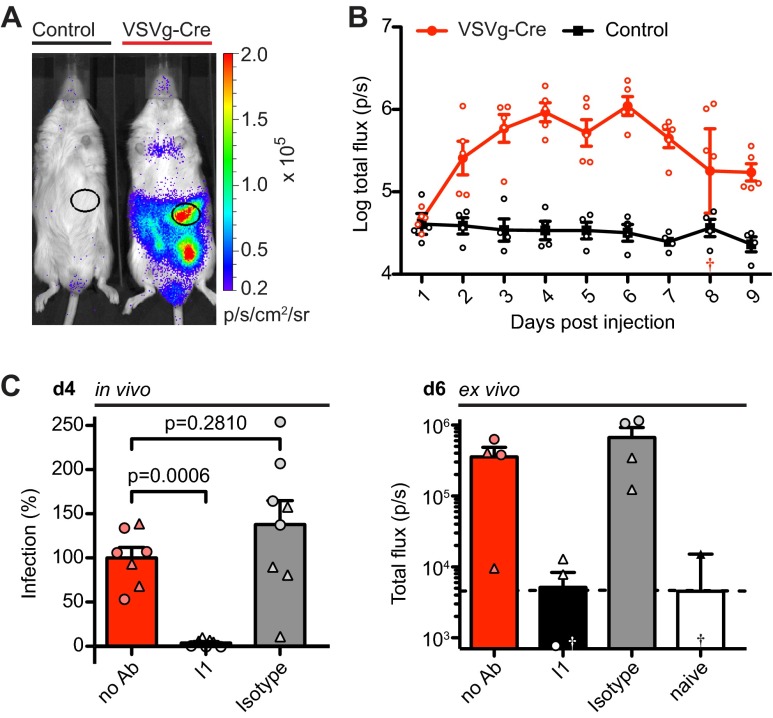
Mice carrying a conditionally transcribed firefly luciferase gene in the ROSA26 locus, regulated by a loxP-flanked transcriptional stop element (ROSA-Stop-Luc) (48), can be used to detect Cre recombinase (Cre) activity by photon emission (48, 49). To test whether ROSA-Stop-Luc mice can be used to measure viral infection by HIV-based viruses, we produced Cre-encoding replication-deficient viruses pseudotyped with the envelope protein G of the vesicular stomatitis virus (VSVg-Cre). Viruses pseudotyped with VSVg are pantropic and can infect a broad range of mammalian target cells (50). Bioluminescence was readily detected in ROSA-Stop-Luc mice after i.p. injection of VSVg-Cre (75 ng p24), while control mice did not show any specific luciferase activity (Fig. 1A and B). The signal in the peritoneal cavity showed a major hot spot at the site of the omentum that peaked 6 days after injection and that had a signal of 1.5 orders of magnitude above that for the control (Fig. 1B; P = 0.0159 at day 6). In addition, bioluminescence could be detected in the mediastinal region, the site of the primary draining lymph nodes of the peritoneal cavity (51); however, the bioluminescence was at a much lower intensity and had higher levels of variability than in the omentum (data not shown). Cre activity results from viral entry mediated by VSVg, as preincubation of VSVg-Cre with the neutralizing anti-VSVg antibody I1 (52) results in nearly complete blockage of infection (P < 0.001), while preincubation with an isotype control did not reduce infection (P = 0.281) (Fig. 1C). In addition, these results were reflected by bioluminescence in ex vivo tissue samples of the omentum (Fig. 1C) and cells harvested by peritoneal lavage (data not shown). We conclude that HIV-based pseudoviruses encoding Cre can be used to detect viral infection in vivo and ex vivo.
Fig 1.
Intraperitoneal injection of VSVg-Cre (75 ng p24) into ROSA-Stop-Luc mice results in bioluminescence in the omentum. (A) Representative luminescence 6 days after i.p. injection of control medium or VSVg-Cre. The unmarked signal in lower left abdomen depicts one site of injection. (B) Time course of photon flux per second for omental ROI in mice i.p. injected with VSVg-Cre (n = 5) or control medium (n = 4). †, off-scale value (3.27). (C) (Left) Preincubation with anti-VSVg antibody I1 (total n = 7) blocks VSVg-Cre infection measured 4 days (d4) after i.p. injection by an omental ROI compared to the result for isotype control mice (total n = 8) and untreated mice (total n = 7), used to define 100% infection (I1 and isotype controls, respectively; final concentration, 1 μg/ml). Data were pooled from two experiments. For each experiment, 0% infection was defined by luminescence of uninfected mice (n = 1 to 2). Symbols depict different individual experiments. (Right) Photon flux per second for omenta dissected after i.p. injection of d-luciferin 6 days (d6) after VSVg-Cre injection (n = 4 per group). Dashed line, mean of naive mice; †, negative off-scale values (for I1 mice, −944.7; for naive mice; −6,075); p/s/cm2/sr, photon flux per second per square centimeter per steradian; Ab, antibody.
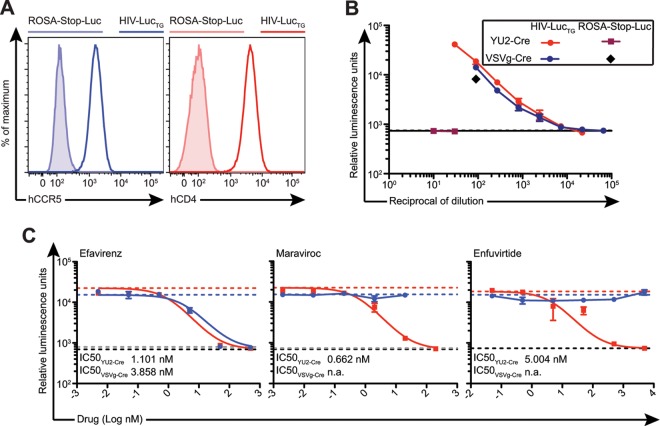
To overcome host restriction for HIV-1 entry, we produced transgenic mice that carry human CD4 (hCD4), the primary receptor for HIV-1 (53, 54), and human CCR5 (hCCR5), the most commonly used coreceptor in initial HIV-1 infection (55–57). Coexpression of hCCR5 and hCD4 was achieved by linking them on a single polyprotein transcript separated by a ribosomal skip 2A peptide sequence (19, 58) driven by the human ubiquitin C promoter (pUP-R plasmid [40]) (Fig. 2A). A founder line was chosen on the basis of cell surface expression of hCCR5 and hCD4 and bred to ROSA-Stop-Luc mice to obtain hCCR5+ hCD4+ ROSA26Luc/Luc mice (termed HIV-LucTG mice). While expression was considerably lower than that on human control PBMCs, hCCR5 and hCD4 could be readily detected on HIV-LucTG bulk PBMCs (Fig. 2B) and on subsets of splenocytes, including T and B cells, dendritic cells, and monocytes/macrophages, as well as on subsets of peritoneal cells, including B cells and macrophages (data not shown).
Fig 2.
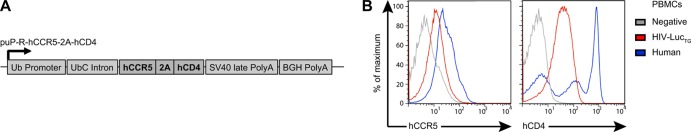
HIV-LucTG mice express hCCR5 and hCD4. (A) Construct used to produce transgenic mice expressing hCCR5 and hCD4 under the control of the human ubiquitin C (UbC) promoter. SV40, simian virus 40; BGH, bovine growth hormone. (B) Representative fluorescence-activated cell sorter analysis of hCCR5 and hCD4 expression on PBMCs of an HIV-LucTG mouse, a transgene-negative littermate, and a human healthy control.
Replication-deficient pseudoviruses are frequently used to assay viral infection and antibody neutralization in vitro. For example, the single-round-infection TZM-bl assay (59) measures luciferase or β-galactosidase activity driven by the viral Tat protein expressed after infection of target cells. To facilitate quantification of infectious particles of HIV-1-enveloped and Cre-encoding but tat-negative pseudoviruses (19), we produced MEFs expressing hCCR5 and hCD4 from HIV-LucTG mice (Fig. 3A). Infection with VSVg-Cre, as quantified by luciferase activity, was readily detected in MEFs generated from both ROSA-Stop-Luc and HIV-LucTG mice (Fig. 3B). In contrast, viruses pseudotyped with the HIV-1 envelope protein gp160 of YU2, a clade B and difficult-to-neutralize tier 2 HIV-1 strain (YU2-Cre) (60), were able to infect only HIV-LucTG MEFs (Fig. 3B). Antiretroviral drugs, which interfere with distinct steps of the viral life cycle, are the cornerstone of HIV therapy (61, 62). They include efavirenz, a nonnucleoside reverse transcriptase inhibitor (NNRTI) (63); maraviroc, an hCCR5 antagonist (64); and enfuvirtide, a peptide fusion inhibitor (65). As expected, infection with YU2-Cre was inhibited in the presence of all three antiretrovirals tested in our MEF-based in vitro assay, with 50% inhibitory concentrations (IC50s) being in the nanomolar range (IC50 of efavirenz, 1.101 nM; IC50 of maraviroc, 0.662 nM; IC50 of enfuvirtide, 5.004 nM), while VSVg-Cre infection was inhibited only by the NNRTI efavirenz (Fig. 3C). We conclude that HIV-LucTG MEFs resemble TZM-bl cells, in that they can be used to titrate viral preparations of Cre-encoding HIV-1 pseudoviruses in vitro, and that the HIV-LucTG MEF assay can be used to assess the effects of antiretroviral drugs on HIV-1 pseudovirus infection in vitro.
Fig 3.
MEFs produced from HIV-LucTG mice can be used for in vitro analysis of HIV-1 pseudovirus infection. (A) hCCR5 and hCD4 expression on MEFs from an HIV-LucTG mouse (solid line) and a ROSA-Stop-Luc mouse (tinted region) determined by flow cytometry. (B) Luminescence after infection of HIV-LucTG and ROSA-Stop-Luc MEFs with serial dilutions of preparations of YU2-Cre or VSVg-Cre. Infection of HIV-LucTG MEFs was performed in quadruplicate; infection of ROSA-Stop-Luc MEFs was performed in duplicate. Results of a representative experiment for different viral preparations are presented. (C) Luminescence after infection of HIV-LucTG MEFs with YU2-Cre (13 TCID50s; red) or VSVg-Cre (35 TCID50s; blue) in the presence of different antiretrovirals. Dashed lines, luminescence in the absence of antiretrovirals (red, YU2-Cre; blue, VSVg-Cre) and for uninfected MEFs (black, YU2-Cre experiments; gray, VSVg-Cre experiments). Infections were performed in duplicate. IC50s were calculated using a nonlinear regression curve. n.a., not applicable.
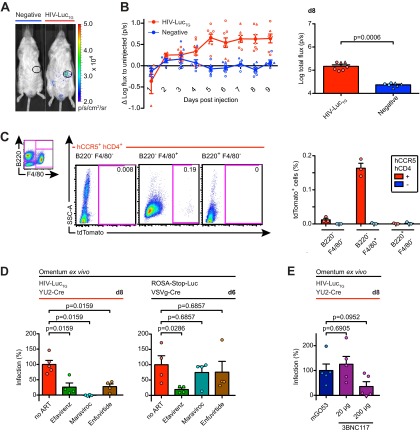
Next, we sought to determine whether HIV-LucTG mice can be used to detect HIV-1 pseudovirus infection in vivo after i.p. injection. Omental luciferase activity after i.p. injection of 585 TCID50s of YU2-Cre increased longitudinally in HIV-LucTG mice, peaking at between days 5 and 8 after injection with a mean signal of ≈0.7 to 0.8 order of magnitude (arithmetic and geometric mean) above that for the hCCR5-negative (hCCR5−)/hCD4-negative (hCD4−) littermates, which remained unaffected (Fig. 4A and B). To determine which cells become infected after i.p. injection of HIV-1 pseudoviruses, we bred HIV-LucTG mice to mice harboring a loxP-flanked tdTomato gene in the ROSA26 locus (66). Peritoneal lavage performed 6 days after injection of YU2-Cre revealed infection to be relatively inefficient, with nearly all of the infected cells being of a macrophage-like phenotype (F4/80-positive B220-negative), as determined by flow cytometry (Fig. 4C). To test whether HIV-LucTG mice can be used as a model for preexposure prophylaxis, mice were pretreated with antiretroviral drugs or the broadly neutralizing anti-CD4-binding-site antibody 3BNC117 (24), and dissected omenta were analyzed 8 (YU2-Cre) or 6 (VSVg-Cre) days after i.p. injection of the respective virus. Pretreatment with efavirenz (0.5 mg twice per os [p.o.]), maraviroc (1.5 mg twice p.o.), or enfuvirtide (40 μg four times s.c.), starting 1 day prior to viral challenge, significantly reduced infection with YU2-Cre (585 TCID50s; P = 0.0159), while only the NNRTI efavirenz showed a significant effect on VSVg-Cre infection (2,190 TCID50s; P = 0.0286) (Fig. 4D). In addition, while s.c. injection of 20 μg 3BNC117 1 day prior to challenge with YU2-Cre failed to show protection (P = 0.6905), injection of 200 μg 3BNC117, while not reaching a level of statistical significance, showed clear protective effects in three of five treated mice compared to the effect of the isotype control mGO53 (46) (P = 0.0952) (Fig. 4E). We conclude that HIV-LucTG mice can serve as a tool to rapidly monitor HIV-1 entry after i.p. injection, primarily by infection of macrophage-like cells, and that infection can be blocked by antiretrovirals and neutralizing antibodies.
Fig 4.
HIV-LucTG mice become infected with HIV-1 pseudovirus after i.p. injection. (A) Representative luminescence 8 days after i.p. injection. (B) Time course of changes in photon flux per second for an omental ROI for HIV-LucTG mice and transgene-negative littermates injected i.p. with YU2-Cre (585 TCID50s) compared to the luminescence of uninjected mice (left) and photon flux per second 8 days after injection (right). Pooled data from two experiments depicted by individual symbols are presented (total n starting on day 3, 7 to 8 [one mouse died during the course of the experiment] for HIV-LucTG mice, 8 for negative mice, and 2 for uninjected mice). (C) Detection of tdTomato-positive (tdTomato+) cells in peritoneal lavage fluid obtained 6 days after i.p. injection of YU2-Cre (585 TCID50s) in hCCR5+ hCD4+ ROSA26tdTomato/Luc mice or transgene-negative littermates. Representative tdTomato expression found in different cellular subsets of an hCCR5+/hCD4+ mouse is shown. (Left) Gating strategy for cells pregated on the basis of forward scatter and sideward scatter (SSC); (right) mean frequency (n = 3 per group) of tdTomato-positive cells in cellular subsets of hCCR5+/hCD4+ mice and negative littermates. (D) Ex vivo luminescence analysis of dissected omenta after i.p. d-luciferin injection. Analysis was performed 8 days after i.p. YU2-Cre injection (585 TCID50s) in HIV-LucTG mice (left) and 6 days after i.p. VSVg-Cre injection (2,190 TCID50s) in ROSA-Stop-Luc mice (right). Mice were pretreated with antiretroviral compounds starting 24 h prior to viral challenge (efavirenz, 0.5 mg twice p.o.; maraviroc, 1.5 mg twice p.o.; enfuvirtide, 40 μg four times s.c.) or untreated. The log differences in the arithmetic means of photon flux per second defining 0% and 100% infection are 1.2 (YU2) and 3.0 (VSVg). (E) Ex vivo luminescence analysis of dissected omenta after i.p. d-luciferin injection. Analysis was performed 8 days after i.p. YU2-Cre (585 TCID50s) injection in HIV-LucTG mice. Mice were s.c. pretreated with 3BNC117 or 200 μg mGO53 24 h prior to viral challenge. The log difference in the arithmetic mean of photon flux per second defining 0% and 100% infection is 0.74. For panels D and E, untreated or isotype-treated mice were used to define 100% infection, respectively, and naive mice were used to define 0% infection (n ≥ 4 for virus-injected groups, n = 2 to 3 for naive mice).
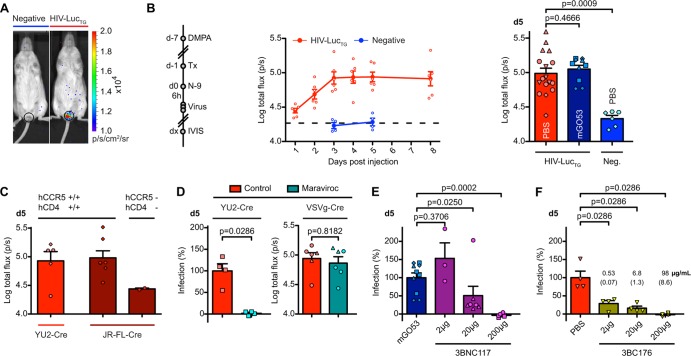
Cervicovaginal exposure accounts for the majority of global HIV-1 infections (39). To establish whether HIV-LucTG mice can be used to monitor genital mucosal infection, we adapted protocols used in macaques and mice and pretreated mice with 3 mg of DMPA (13, 14, 67–70). Seven days later, a volume of 40 μl of a 4% N-9 gel formulation (71, 72) was applied to the vagina, 6 h prior to three vaginal challenges with 160 TCID50s of YU2-Cre every 2 h. In vivo imaging revealed a luminescent signal in HIV-LucTG mice that was not seen in controls, peaking at an ≈0.7 order of magnitude (arithmetic and geometric means) above that for the controls at between 3 and 5 days after application, and that was not affected by pretreatment with mGO53 24 h prior to viral challenge (Fig. 5A and B). Similar results were obtained when mice homozygous for hCCR5 and hCD4 were i.vag. subjected to viruses pseudotyped with the JR-FL gp160 (MEF titer, >1:270), an additional clade B tier 2 isolate (73), highlighting the flexibility of the system for infection by different HIV-1 Env pseudotypes (Fig. 5C). To determine whether HIV-LucTG mice can be used to examine antiretrovirals or anti-HIV-1 antibodies for prophylaxis, we administered maraviroc, 3BNC117, or the broadly neutralizing antibody 3BC176, which targets a not yet precisely defined conformational Env epitope (45), before viral challenge. Pretreatment with maraviroc (1.5 mg twice p.o.) nearly completely blocked infection with YU2-Cre (P = 0.0286), while infection with VSVg-Cre (three challenges with 175 TCID50s) was not significantly reduced (P = 0.8182) (Fig. 5D). Subcutaneous injection of 3BNC117 1 day prior to virus application led to a dose-dependent reduction of the level of YU2-Cre infection compared to that after injection of mGO53 (Fig. 5E). While an injected dose of 2 μg of 3BNC117 failed to prevent YU2-Cre infection (P = 0.3706), a dose of 20 μg reduced infection by a mean of 49% (P = 0.025) or 75%, when one unprotected outlier is excluded, and a dose of 200 μg led to complete protection (≥95% reduction of infection) (P = 0.0002). Compared to the infection in PBS-treated mice, we found a reduction of YU2-Cre infection by a mean of 71% after s.c. pretreatment with as little as 2 μg of 3BC176 1 day prior to viral challenge (P = 0.0286), corresponding to a serum concentration of 0.5 μg/ml at the time of viral challenge, and complete protection (≥96% reduction of infection) after pretreatment with 200 μg (P = 0.0286) (Fig. 5F). We conclude that HIV-LucTG mice can be used to assay cervicovaginal infection of HIV-1 pseudoviruses in vivo and to measure prophylaxis mediated by antiretroviral drugs and neutralizing antibodies.
Fig 5.
HIV-LucTG mice become infected with HIV-1 pseudoviruses after intravaginal application. (A) Representative luminescence in HIV-LucTG mice and transgene-negative littermates 5 days after i.vag. YU2-Cre application (three times with 160 TCID50s). (B) Timeline for experiments (left; d, day; Tx, treatment) and time course of photon flux per second for vaginal ROI after i.vag. application of YU2-Cre (three times with 160 TCID50s each time) (middle) in PBS-treated HIV-LucTG (n = 6) mice and transgene-negative littermates (n = 4). Dashed line, luminescence 5 days after application in ROSA-Stop-Luc mice (n = 3). (Right) Pooled luminescence 5 days (d5) after infection for PBS-treated (total n = 17) or mGO53-treated (200 μg s.c., total n = 10) HIV-LucTG mice and transgene-negative littermates (total n = 6). Symbols depict individual experiments (n = 7). (C) Photon flux per second 5 days after i.vag. application of JR-FL-Cre (MEF titer > 1:270; three times with 40 μl each time) and YU2-Cre (three times with 160 TCID50s each time) in mice homozygous for hCCR5 and hCD4 compared to transgene-negative littermates. Pooled data from two experiments are presented (total n for hCCR5+/hCD4+ mice, ≥5; total n for negative mice, 2). (D) Infection in mice pretreated with maraviroc (1.5 mg twice p.o.) or controls 5 days after i.vag. application of pseudovirus. (Left) YU2-Cre infection (three times with 160 TCID50s each time) in HIV-LucTG mice (n = 4). mGO53-pretreated mice (200 μg s.c., n = 4) (red bar) and ROSA-Stop-Luc mice (n = 3; see panel B) from the same experiment were used to define 100% and 0% infection, respectively. (Right) Total photon flux per second in ROSA-Stop-Luc mice after VSVg-Cre application (three times with 175 TCID50s each time). Pooled data from two individual experiments are presented (total n = 6 per group). (E) Infection 5 days after intravaginal application of YU2-Cre (three times with 160 TCID50s each time) in HIV-LucTG mice s.c. pretreated with 3BNC117 (for 2 μg, total n = 3; for 20 μg, total n = 7; for 200 μg, total n = 6) or 200 μg mGO53 (total n = 10; see panel B) 24 h prior to viral challenge. Pooled mGO53-treated mice and pooled transgene-negative littermates (total n = 6; see panel B) were used to define 100% and 0% infection, respectively. Pooled data from a total of five experiments depicted by individual symbols are presented. (F) Infection 5 days after intravaginal application of YU2-Cre (three times with 160 TCID50s each time) in HIV-LucTG mice s.c. pretreated with 3BC176 or PBS (n = 4 per group) 24 h prior to viral challenge. PBS-treated mice and pooled transgene-negative littermates (total n = 6; see panel B) were used to define 100% and 0% infection, respectively. Numbers show human IgG serum concentration (n = 5 per group) in μg/ml at the time of viral challenge, and SEMs are in parentheses.
DISCUSSION
Although significant progress in HIV-1 prevention has been made, further research is required to establish an efficient vaccine or optimized pharmaceutical prevention strategies. While studies in nonhuman primates are the current “gold standard” for late-stage preclinical studies, the limited number of animals available and the cost of these experiments make them unsuitable for large-scale early preclinical studies.
Several small-animal models have been developed to allow assessment of HIV-1 infection. Humanized mice partially reconstituted with cellular components of the human immune system allow HIV-1 infection, including mucosal transmission, viral replication, and long-term viremia resembling the clinical situation (29–31). However, these mice fail to mount a robust immune response to HIV-1, rendering them unsuitable for vaccination studies. In addition, the need for reconstitution can lead to differences between individual mice. Immunocompetent transgenic rodent models have been established, but to date their use has been restricted to extramucosal routes of infection (35, 36, 74), which account for a minority of human HIV-1 infections (39).
HIV-LucTG mice and cells obtained from these mice allow rapid in vivo and in vitro detection of infection with HIV-1 pseudoviruses. These mice differ from humanized mice, in that they should be immunologically intact and could potentially be used for vaccine studies. They also differ from previously reported transgenic models, in that they can be used to assay infection by mucosal exposure. We have shown that HIV-LucTG mice can be used to detect infection and prophylaxis mediated by antiretrovirals and anti-HIV-1 antibodies after both intraperitoneal and cervicovaginal application of HIV-1 pseudoviruses.
In this study, we show that passive transfer of neutralizing anti-HIV-1 antibodies can protect HIV-LucTG mice from cervicovaginal infection with HIV-1 pseudoviruses. In previous experiments, we have determined an in vitro IC50 of 0.054 μg/ml for the highly active agonistic anti-CD4-binding-site antibody (HAAD) 3BNC117 against YU2 (24) and measured the 3BNC117 serum concentration in ROSA-Stop-Luc mice 1 day after subcutaneous injection (2-μg dose, 0.4 ± 0.03 μg/ml; 20-μg dose, 5.0 ± 0.6 μg/ml; 200-μg dose, 24.3 ± 1.8 μg/ml [mean ± SEM, n = 3]) (19). Here, 3BNC117 reduces intravaginal infection by ≈50 to 75% after administration of 20 μg, corresponding to a serum concentration of ≈5 μg/ml at the time of viral challenge (19), which is roughly 100-fold higher than the in vitro IC50 determined by the TZM-bl assay. These results are in good agreement with those of previous macaque studies of vaginal SHIV challenge, indicating that 50% protection in vivo requires serum antibody concentrations to be 100-fold or more of those needed for 50% neutralization in vitro (14, 69, 75). Pretreatment with 3BC176, targeting a conformational epitope found on cell surface-expressed gp160 (45), led to an ≈85% reduction of infection at a serum concentration of ≈6.8 μg/ml, only 23-fold higher than the IC50 against YU2 determined in vitro (0.29 μg/ml) (45). Interestingly, a significantly reduced infection (≈70%) could also be detected in the presence of a serum concentration of as little as 0.53 μg/ml of 3BC176. These results are consistent with those of work in macaques showing that antibody-mediated protection from vaginal SHIV challenge can be achieved at relatively low titers (76, 77). However, it stands in contrast to the findings of antibody therapy experiments in YU2-infected humanized mice, where 3BC176 was ineffective at a serum concentration of up to more than 100 μg/ml (28). Thus, the requirements for prevention and therapy of HIV-1 infection by antibodies may differ significantly.
Establishing infection with HIV-1 pseudoviruses after vaginal application required pretreatment with both DMPA and N-9, which may alter the mucosal epithelia and milieu in a way that does not reflect the human cervicovaginal anatomy and its role in HIV-1 transmission. Progesterone is frequently used in vaginal infection models to synchronize estrous cycles and to increase susceptibility by thinning out the vaginal layer, including models of simian immunodeficiency virus/SHIV infection in nonhuman primates (13, 14, 67, 69, 70, 78) and mouse models of HIV-1 (68, 79), human papillomavirus (HPV) (80), and herpes simplex virus 2 (HSV-2) (81) infection. Increased susceptibility to viral infection after mucosal N-9 application has been described in models of HPV infection (80) and both vaginal and rectal HSV-2 infection (72, 82, 83). In addition, an increased frequency of HIV-1 infection has been found in female sex workers after application of high doses of N-9 (84). Lesions in cervical and vaginal epithelia described after application of N-9 are one likely contributor to these observations (71, 72), and induction of proinflammatory conditions with an influx of inflammatory cells may increase the number of potential target cells for HIV-1 infection (72, 85). While the hCCR5 antagonist maraviroc, which shows only minor inhibitory activity against murine CCR5 (86), may have reduced a murine chemokine-mediated influx of cells possibly targeted by pseudoviruses in our mouse model (87), in contrast to infection with YU2-Cre, infection with VSVg-Cre was not markedly reduced in the presence of maraviroc.
Expression of hCCR5 and hCD4 in HIV-LucTG mice is driven by the ubiquitin promoter, rendering virtually all murine cell types potential target cells for HIV-1 pseudoviruses. While both entry factors could be detected on a number of splenocyte and peritoneal cell subsets, different cell types may show different susceptibilities to both pseudovirus infection and protection mediated by antiretroviral compounds and antibodies. In addition, expression of entry factors in HIV-LucTG mice is markedly lower than that on human CD4+ and CCR5+ cells, likely leading to lower rates of entry factor-dependent infection in HIV-LucTG mice (88). Fluorescent analysis of individual cells revealed that murine macrophage-like cells are the main target of HIV-1 pseudoviruses after intraperitoneal challenge. While hCCR5-expressing macrophages are considered to be among the target cells for primary HIV-1 infection of R5-tropic HIV-1 in humans, it remains unclear how murine macrophages compare to their human counterpart. Further studies will be required to determine whether specific cells are targeted after vaginal application of HIV-1 pseudoviruses in HIV-LucTG mice and if they can serve as a model system for rectal transmission of HIV-1. With a signal approximately 0.7 order of magnitude above that for the controls for both i.p. and i.vag. infection, the dynamic range of our system is relatively narrow, and there is some considerable variation in the amount of infection between individual animals, which may result in limited sensitivity for the detection of minor differences between individual antibodies or doses. More importantly, while our study focused on the use of passively transferred, broadly neutralizing, and highly potent antibodies for antibody-mediated protection, vaccination strategies so far have not resulted in the generation of antibodies with comparable breadth and potency, and the dynamic range of our system may not be sufficient to detect the protective effects of antibodies with more limited potency.
While protection in macaque or humanized mouse studies is usually defined by the absence of viral RNA in peripheral blood, HIV-LucTG mice do not support efficient viral replication (35, 37), and therefore, this model is limited to studies involving the entry phase of the viral life cycle. Despite these caveats, HIV-LucTG mice can serve as a valuable tool for preclinical assessment of preexposure prophylaxis in HIV-1 infection. They should be suitable for vaccination studies, and, as shown in this study, they can be readily used to assess and compare the protective effects of both small-molecule antiretroviral drugs and potent anti-HIV-1 neutralizing antibodies.
ACKNOWLEDGMENTS
We thank Johannes F. Scheid for providing antibody plasmids of 3BNC117, Florian Klein for providing antibody plasmids of 3BC176 and antiretrovirals, Jose Romero for providing DMPA, Gerd Fätkenheuer for providing antiretrovirals, David Bosque for help with mouse colonies, Joseph Sodroski for providing pSVIIIenvYU-2 and pSVIIIenvJR-FL plasmids, and members of the M.C.N. laboratory for helpful discussions.
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH): pT4B from Richard Axel, pc.CCR-5 from Nathaniel Landau, efavirenz, and maraviroc (catalog no. 11580).
This work was supported by grants from the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery (to J.V.R. and M.C.N.) and NIH grant AI081677, Novel Approaches to Vaccine Design (to J.V.R. and M.C.N.). H.G. was supported by the German National Academic Foundation. A.P. is a recipient of the Astella Young Investigator Award of the Infectious Diseases Society of America and of the Liver Scholar Award from the American Liver Foundation. M.C.N. is a Howard Hughes Medical Institute investigator.
Footnotes
Published ahead of print 29 May 2013
REFERENCES
- 1. Centers for Disease Control 1981. Pneumocystis pneumonia—Los Angeles. MMWR Morb. Mortal. Wkly. Rep. 30:250–252 [PubMed] [Google Scholar]
- 2. UNAIDS 2012. Global report: UNAIDS report on the global AIDS epidemic 2012. UNAIDS, Geneva, Switzerland: http://www.unaids.org/en/resources/campaigns/20121120_globalreport2012/globalreport/ [Google Scholar]
- 3. Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 5. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR. 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT. 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N. Engl. J. Med. 367:423–434 [DOI] [PubMed] [Google Scholar]
- 9. Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. 2012. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 367:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marrazzo J, Ramjee G, Nair G, Palanee T, Mkhize B, Nakabiito C, Taljaard M, Piper J, Gomez Feliciano K, Chirenje M. 2013. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE study (MTN 003), paper 26LB. Abstr. 20th Conf. Retroviruses Opportunistic Infect http://www.retroconference.org/2013b/Abstracts/47951.htm [Google Scholar]
- 11. Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15:951–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. 2010. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 84:1302–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210 [DOI] [PubMed] [Google Scholar]
- 14. Parren PWHI, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204–210 [DOI] [PubMed] [Google Scholar]
- 16. Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. 2012. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481:81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gauduin MC, Parren PW, Weir R, Barbas CF, Burton DR, Koup RA. 1997. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 3:1389–1393 [DOI] [PubMed] [Google Scholar]
- 18. Parren PWHI, Ditzel HJ, Gulizia RJ, Binley JM, Barbas CF, III, Burton DR, Mosier DE. 1995. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS 9:F1–F6 [DOI] [PubMed] [Google Scholar]
- 19. Pietzsch J, Gruell H, Bournazos S, Donovan BM, Klein F, Diskin R, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. A mouse model for HIV-1 entry. Proc. Natl. Acad. Sci. U. S. A. 109:15859–15864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veselinovic M, Neff CP, Mulder LR, Akkina R. 2012. Topical gel formulation of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 confers protection against HIV-1 vaginal challenge in a humanized mouse model. Virology 432:505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diskin R, Scheid JF, Marcovecchio PM, West AP, Jr, Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, Bjorkman PJ. 2011. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334:1289–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PN, Spencer DI, Seaman MS, Schuitemaker H, Feizi T, Nussenzweig MC, Bjorkman PJ. 2012. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. U. S. A. 109:E3268–E3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio PM, Incesu RB, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492:118–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berges BK, Rowan MR. 2011. The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology 8:65. 10.1186/1742-4690-8-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borkow G. 2005. Mouse models for HIV-1 infection. IUBMB Life 57:819–823 [DOI] [PubMed] [Google Scholar]
- 31. Denton PW, Garcia JV. 2011. Humanized mouse models of HIV infection. AIDS Rev. 13:135–148 [PMC free article] [PubMed] [Google Scholar]
- 32. Baenziger S, Tussiwand R, Schlaepfer E, Mazzucchelli L, Heikenwalder M, Kurrer MO, Behnke S, Frey J, Oxenius A, Joller H, Aguzzi A, Manz MG, Speck RF. 2006. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−γc−/− mice. Proc. Natl. Acad. Sci. U. S. A. 103:15951–15956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, Shin HS, Brooks SF, Knight HL, Eichbaum Q, Yang YG, Sykes M, Walker BD, Freeman GJ, Pillai S, Westmoreland SV, Brander C, Luster AD, Tager AM. 2009. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J. Virol. 83:7305–7321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, Shiozawa Y, Dewan MZ, Yu Z, Ito M, Morio T, Shimizu N, Honda M, Yamamoto N. 2007. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rγnull mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 109:212–218 [DOI] [PubMed] [Google Scholar]
- 35. Browning J, Horner JW, Pettoello-Mantovani M, Raker C, Yurasov S, DePinho RA, Goldstein H. 1997. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc. Natl. Acad. Sci. U. S. A. 94:14637–14641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keppler OT, Welte FJ, Ngo TA, Chin PS, Patton KS, Tsou CL, Abbey NW, Sharkey ME, Grant RM, You Y, Scarborough JD, Ellmeier W, Littman DR, Stevenson M, Charo IF, Herndier BG, Speck RF, Goldsmith MA. 2002. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J. Exp. Med. 195:719–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bieniasz PD, Cullen BR. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74:9868–9877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zheng YH, Yu HF, Peterlin BM. 2003. Human p32 protein relieves a post-transcriptional block to HIV replication in murine cells. Nat. Cell Biol. 5:611–618 [DOI] [PubMed] [Google Scholar]
- 39. Hladik F, McElrath MJ. 2008. Setting the stage: host invasion by HIV. Nat. Rev. Immunol. 8:447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. 2001. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cell. Immunol. 214:110–122 [DOI] [PubMed] [Google Scholar]
- 41. Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. 1995. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J. Virol. 69:4413–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choe H, Farzan M, Konkel M, Martin K, Sun Y, Marcon L, Cayabyab M, Berman M, Dorf ME, Gerard N, Gerard C, Sodroski J. 1998. The orphan seven-transmembrane receptor apj supports the entry of primary T-cell-line-tropic and dualtropic human immunodeficiency virus type 1. J. Virol. 72:6113–6118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. U. S. A. 100:7271–7276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reed LJ, Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 45. Klein F, Gaebler C, Mouquet H, Sather DN, Lehmann C, Scheid JF, Kraft Z, Liu Y, Pietzsch J, Hurley A, Poignard P, Feizi T, Morris L, Walker BD, Fatkenheuer G, Seaman MS, Stamatatos L, Nussenzweig MC. 2012. Broad neutralization by a combination of antibodies recognizing the CD4 binding site and a new conformational epitope on the HIV-1 envelope protein. J. Exp. Med. 209:1469–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. 2003. Predominant autoantibody production by early human B cell precursors. Science 301:1374–1377 [DOI] [PubMed] [Google Scholar]
- 47. Mouquet H, Klein F, Scheid JF, Warncke M, Pietzsch J, Oliveira TY, Velinzon K, Seaman MS, Nussenzweig MC. 2011. Memory B cell antibodies to HIV-1 gp140 cloned from individuals infected with clade A and B viruses. PLoS One 6:e24078. 10.1371/journal.pone.0024078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Safran M, Kim WY, Kung AL, Horner JW, DePinho RA, Kaelin WG., Jr 2003. Mouse reporter strain for noninvasive bioluminescent imaging of cells that have undergone Cre-mediated recombination. Mol. Imaging 2:297–302 [DOI] [PubMed] [Google Scholar]
- 49. Dorner M, Horwitz JA, Robbins JB, Barry WT, Feng Q, Mu K, Jones CT, Schoggins JW, Catanese MT, Burton DR, Law M, Rice CM, Ploss A. 2011. A genetically humanized mouse model for hepatitis C virus infection. Nature 474:208–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. U. S. A. 90:8033–8037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marco AJ, Domingo M, Ruberte J, Carretero A, Briones V, Dominguez L. 1992. Lymphatic drainage of Listeria monocytogenes and Indian ink inoculated in the peritoneal cavity of the mouse. Lab. Anim. 26:200–205 [DOI] [PubMed] [Google Scholar]
- 52. Lefrancois L, Lyles DS. 1982. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology 121:157–167 [PubMed] [Google Scholar]
- 53. Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763–767 [DOI] [PubMed] [Google Scholar]
- 54. Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767–768 [DOI] [PubMed] [Google Scholar]
- 55. Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666 [DOI] [PubMed] [Google Scholar]
- 56. Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667–673 [DOI] [PubMed] [Google Scholar]
- 57. Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. 2004. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat. Biotechnol. 22:589–594 [DOI] [PubMed] [Google Scholar]
- 59. Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li Y, Kappes JC, Conway JA, Price RW, Shaw GM, Hahn BH. 1991. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J. Virol. 65:3973–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. De Clercq E. 2007. The design of drugs for HIV and HCV. Nat. Rev. Drug Discov. 6:1001–1018 [DOI] [PubMed] [Google Scholar]
- 62. Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Gunthard HF, Hammer SM, Reiss P, Richman DD, Rizzardini G, Thomas DL, Jacobsen DM, Volberding PA. 2012. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society—USA Panel. JAMA 308:387–402 [DOI] [PubMed] [Google Scholar]
- 63. Young SD, Britcher SF, Tran LO, Payne LS, Lumma WC, Lyle TA, Huff JR, Anderson PS, Olsen DB, Carroll SS. 1995. L-743,726 (DMP-266): a novel, highly potent nonnucleoside inhibitor of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 39:2602–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49:4721–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wild C, Greenwell T, Matthews T. 1993. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res. Hum. Retroviruses 9:1051–1053 [DOI] [PubMed] [Google Scholar]
- 66. Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci. U. S. A. 108:11181–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khanna KV, Whaley KJ, Zeitlin L, Moench TR, Mehrazar K, Cone RA, Liao Z, Hildreth JEK, Hoen TE, Shultz L, Markham RB. 2002. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J. Clin. Invest. 109:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. 2012. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci. U. S. A. 109:18921–18925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343–346 [DOI] [PubMed] [Google Scholar]
- 71. Catalone BJ, Kish-Catalone TM, Budgeon LR, Neely EB, Ferguson M, Krebs FC, Howett MK, Labib M, Rando R, Wigdahl B. 2004. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob. Agents Chemother. 48:1837–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cone RA, Hoen T, Wong X, Abusuwwa R, Anderson DJ, Moench TR. 2006. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect. Dis. 6:90. 10.1186/1471-2334-6-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819–822 [DOI] [PubMed] [Google Scholar]
- 74. Goffinet C, Allespach I, Keppler OT. 2007. HIV-susceptible transgenic rats allow rapid preclinical testing of antiviral compounds targeting virus entry or reverse transcription. Proc. Natl. Acad. Sci. U. S. A. 104:1015–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Willey R, Nason MC, Nishimura Y, Follmann DA, Martin MA. 2010. Neutralizing antibody titers conferring protection to macaques from a simian/human immunodeficiency virus challenge using the TZM-bl assay. AIDS Res. Hum. Retroviruses 26:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5:e1000433. 10.1371/journal.ppat.1000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Watkins JD, Siddappa NB, Lakhashe SK, Humbert M, Sholukh A, Hemashettar G, Wong YL, Yoon JK, Wang W, Novembre FJ, Villinger F, Ibegbu C, Patel K, Corti D, Agatic G, Vanzetta F, Bianchi S, Heeney JL, Sallusto F, Lanzavecchia A, Ruprecht RM. 2011. An anti-HIV-1 V3 loop antibody fully protects cross-clade and elicits T-cell immunity in macaques mucosally challenged with an R5 clade C SHIV. PLoS One 6:e18207. 10.1371/journal.pone.0018207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084–1089 [DOI] [PubMed] [Google Scholar]
- 79. Di Fabio S, Giannini G, Lapenta C, Spada M, Binelli A, Germinario E, Sestili P, Belardelli F, Proietti E, Vella S. 2001. Vaginal transmission of HIV-1 in hu-SCID mice: a new model for the evaluation of vaginal microbicides. AIDS 15:2231–2238 [DOI] [PubMed] [Google Scholar]
- 80. Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 13:857–861 [DOI] [PubMed] [Google Scholar]
- 81. Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 77:4558–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Galen BT, Martin AP, Hazrati E, Garin A, Guzman E, Wilson SS, Porter DD, Lira SA, Keller MJ, Herold BC. 2007. A comprehensive murine model to evaluate topical vaginal microbicides: mucosal inflammation and susceptibility to genital herpes as surrogate markers of safety. J. Infect. Dis. 195:1332–1339 [DOI] [PubMed] [Google Scholar]
- 83. Phillips DM, Zacharopoulos VR. 1998. Nonoxynol-9 enhances rectal infection by herpes simplex virus in mice. Contraception 57:341–348 [DOI] [PubMed] [Google Scholar]
- 84. Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Tshibaka LM, Ettiègne-Traoré V, Uaheowitchai C, Karim SSA, Mâsse B, Perriëns J, Laga M. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971–977 [DOI] [PubMed] [Google Scholar]
- 85. Milligan GN, Dudley KL, Bourne N, Reece A, Stanberry LR. 2002. Entry of inflammatory cells into the mouse vagina following application of candidate microbicides: comparison of detergent-based and sulfated polymer-based agents. Sex. Transm. Dis. 29:597–605 [DOI] [PubMed] [Google Scholar]
- 86. Saita Y, Kondo M, Shimizu Y. 2007. Species selectivity of small-molecular antagonists for the CCR5 chemokine receptor. Int. Immunopharmacol. 7:1528–1534 [DOI] [PubMed] [Google Scholar]
- 87. Liu J. 2005. Interferon regulatory factor 1 is an essential and direct transcriptional activator for interferon γ-induced RANTES/CCl5 expression in macrophages. J. Biol. Chem. 280:24347–24355 [DOI] [PubMed] [Google Scholar]
- 88. Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]