Abstract
OBJECTIVE
To estimate the multiple dimensions of risk faced by pregnant women and their health care providers when comparing the risks of stillbirth at term with the risk of infant death after birth.
METHODS
This is a retrospective cohort study that included all nonanomalous, term deliveries in the state of California from 1997 to 2006 (N=3,820,826). The study compared infant mortality rates after delivery at each week of term pregnancy with the rates of a composite fetal–infant mortality that would occur after expectant management for 1 additional week.
RESULTS
The risk of stillbirth at term increases with gestational age from 2.1 per 10,000 ongoing pregnancies at 37 weeks of gestation up to 10.8 per 10,000 ongoing pregnancies at 42 weeks of gestation. At 38 weeks of gestation, the risk of expectant management carries a similar risk of death as delivery, but at each later gestational age, the mortality risk of expectant management is higher than the risk of delivery (39 weeks of gestation: 12.9 compared with 8.8 per 10,000; 40 weeks of gestation: 14.9 compared with 9.5 per 10,000; 41 weeks of gestation: 17.6 compared with 10.8 per 10,000).
CONCLUSION
Infant mortality rates at 39, 40, and 41 weeks of gestation are lower than the overall mortality risk of expectant management for 1 week.
The risk of stillbirth after 32 weeks of gestation increases with gestational age, and half of these late fetal deaths occur at term.1 Term stillbirth theoretically can be avoided through the judicious use of labor induction, and stillbirth prevention lies at the heart of many of the accepted indications for labor induction. However, once the child is born, he or she faces new mortality risks, often risks that may be determined partially by gestational age at birth. Determining the optimal time of delivery to minimize the risk of stillbirth necessarily must include considering the mortality risk faced by the child after birth.
For nonanomalous infants born at term, the most common causes of death are asphyxia, infection, and sudden infant death syndrome (SIDS). Rates of infection and SIDS decrease with increasing gestational age at term, with the highest rates at 37 weeks.2 The risk of both neonatal and infant death has been shown in multiple studies to decrease with gestational age at term but then increase again at 41 weeks of gestation.2–5 Part of the relationship between gestational age and infant death is driven by the fact that SIDS deaths decrease with gestational age until 40–41 weeks, after which they begin to increase again; SIDS is the leading cause of postneonatal death in nonanomalous infants.4,6,7
This study attempts to use epidemiologic information to describe the multiple dimensions of risk faced by pregnant women and their health care providers when comparing the risks of stillbirth at term with the risk of infant death after birth, considering that gestational age is one of the many shared risk factors for both stillbirth and infant death.8 Previous studies have attempted to examine the optimal time for delivery by comparing stillbirth risk with a composite of infant morbidity and mortality; in this study, we attempted to develop a risk estimate of mortality alone.9
MATERIALS AND METHODS
We conducted a retrospective cohort study of term births that occurred in California from 1997 to 2006. We obtained institutional review board approval from the Committee on Human Research at the University of California, San Francisco, the institutional review board at Oregon Health and Science University, and the California Office of Statewide Health Planning and Development and the Committee for the Protection of Human Subjects. Because the data are deidentified and part of the public record of vital statistics, informed consent was not required.
The data for these calculations come from the California Vital Statistics Birth Certificate Data, California Patient Discharge Data, Vital Statistics Death Certificate Data, and Vital Statistics Fetal Death File.10 The State of California maintains linked data sets that include maternal antepartum and postpartum hospital records for the 9 months before delivery and 1 year after delivery as well as birth records and all infant admissions occurring within the first year of life. Linkage is performed by the California Office of Statewide Health Planning and Development Healthcare Information Resource Center under the State of California Health and Human Services Agency using a unique “record linkage number” specific to the mother–infant pair.
The birth certificate data use last menstrual period as the basis for gestational age dating in days. This gestational age is converted to weeks and treated as an ordered categorical variable. If the last menstrual period was missing or nonsensical, the mother– infant pair was excluded for analysis. This study includes all births from 37 to 42 weeks of gestation; 37 weeks of gestational age included births ranging from 37 0/7 weeks to 37 6/7 weeks, and 42 weeks of gestational age included births from 42 0/7 weeks to 42 6/7 weeks. We excluded multiple gestations, pregnancies complicated by diabetes mellitus (pre-existing or gestational) and chronic hypertension, and infants with congenital anomalies or genetic causes of death based on the International Classification of Diseases (ICD), 9th and 10th Revision codes. Causes of infant death were taken from the ICD, 9th Revision (years 1997–1998) or ICD, 10th Revision (years 1999–2007) codes on death certificates and were grouped into large thematic categories.
The incidence of stillbirth at a given gestational age was calculated as the number of stillbirths (whether antepartum or intrapartum) at that gestational age per 10,000 ongoing pregnancies. Infant mortality at each gestational age was calculated as the number of infants born at this gestational age who die within 1 year of life per 10,000 live births at that same gestational age. For reference, a neonatal death is defined as death within the first 30 days of life, whereas early neonatal death, the metric included in estimates of perinatal mortality, is defined as death within 7 days of life.
The goal of this project was to compare the mortality risks between delivery at a certain gestational age with that of expectant management (ie, continuing the pregnancy for another week and then delivering 1 week later). More specifically, the mortality risk of delivery at a given week was defined as the rate among those infants born at that week of gestation. The mortality risk of 1 week of expectant management was defined as the risk of stillbirth over that week plus the mortality risk experienced by infants born in the subsequent week of gestation. Infant death, rather than neonatal death, was chosen as the preferred metric to examine because of its greater magnitude and persistent correlation with gestational age at delivery. As mentioned previously, infant mortality has been shown to vary with gestational age at term and shares many of the same risk factors as stillbirth.4,6 Although only early neonatal death rates have classically been included in estimates of perinatal risk, as neonatal intensive care improves, a larger proportion of children with complications resulting from gestational age or intrapartum events may be surviving beyond the neonatal period, contributing to the decrease in neonatal mortality rates over time.4,11 Also, recent data demonstrate that term infants who die within the first year of life are more likely to do so within the postneonatal period (age 29–365 days of life) than in the neonatal period.12 Any gestational age-related mortality effect in these children will be better captured by examining infant death rates.
Our calculations rely on the following assumptions: 1) the risks of stillbirth and infant death have a uniform distribution throughout a particular week of gestation; 2) when estimating the risk of delivering at a particular gestational age, the fetus is not at risk for stillbirth beyond that gestational age; therefore, their mortality risk in that week is equal only to the risk of infant death; and 3) all probabilities are conditional rather than cumulative; that is, the risk of stillbirth at 41 weeks of gestation includes the assumption that the pregnancy is viable at that gestational age and has not had a stillbirth in the weeks prior.
The composite risk of expectant management for 1 week represents the sum of the probability of stillbirth during a given week of gestation plus the probability of infant death when birth occurs the subsequent week. This composite risk of expectant management beyond each given week of gestation then was compared with the risk of infant death for children born in the given week of gestation. The “number needed to deliver” was calculated as an analogous measure to the “number needed to treat” by taking the reciprocal of the absolute risk difference between delivery and expectant management.
Statistical calculations were performed with Excel and Stata 12, including proportions, relative risks, and 95% confidence intervals (CIs). Exponential modeling was performed and goodness of fit was reported with the coefficient of determination, R2. Chi square tests were performed to compare proportions of independent variables and analysis of variance was performed to compare means. Statistical significance was reached with a P value of<.05 or if 95% CIs did not overlap. We assumed that the binomial probability distributions of both mortality risks approximated the normal distribution and derived the CI of the composite risk using the sum of the variances plus twice the covariance of the estimates of infant death and stillbirth.
RESULTS
The sample included 3,820,826 nonanomalous term and postterm singleton births delivered in California between 1997 and 2006. Baseline demographic data are displayed in Table 1. The highest risk of stillbirth was seen at 42 weeks with 10.8 per 10,000 ongoing pregnancies (95% CI 9.2–12.4 per 10,000) (Table 2). The risk of stillbirth increased in an exponential fashion with increasing gestational age (R2=0.956) (Fig. 1).
Table 1.
Demographic Characteristics of Women With Singleton, Nonanomalous Gestations Between 37 and 42 Weeks of Gestation in California Between 1997 and 2006
| Characteristic | Stillbirth (n=3,999) |
Infant Death (n=3,879) |
Alive (n=3,812,948) |
P |
|---|---|---|---|---|
| Maternal age (y) | 28.2±6.5 | 25.7±6.2 | 27.6±6.2 | <.001 |
| Race or ethnicity | ||||
| White | 1,356 (34.0) | 1,567 (40.5) | 1,438,897 (37.8) | <.001 |
| Black (non-Hispanic) | 365 (9.2) | 500 (12.9) | 203,057 (5.3) | |
| Hispanic | 1,847 (46.3) | 1,373 (35.5) | 1,685,985 (44.3) | |
| Asian or Pacific Islander | 407 (10.2) | 336 (8.7) | 423,646 (11.1) | |
| Other | 12 (0.3) | 94 (2.4) | 56,773 (1.5) | |
| More than 12 y of education | 1,444 (37.7) | 1,069 (30.1) | 1,431,712 (42.7) | <.001 |
| Private payer | 1,414 (45.8) | 1,046 (37.8) | 1,581,738 (53.2) | <.001 |
| Nulliparous | 862 (38.4) | 1,403 (36.3) | 1,525,096 (40.0) | <.001 |
| Gestational age (wk) | 38.9±1.4 | 39.2±1.4 | 39.3±1.3 | <.001 |
| Birthweight (g) | 2,911±852 | 3,232±658 | 3,431±464 | <.001 |
| Male sex | 1,992 (50) | 2,198 (56.7) | 1,929,958 (51) | <.001 |
Data are mean±standard deviation or n (%) unless otherwise specified.
Table 2.
Risk of Stillbirth, Infant Death, and Expectant Management by Gestational Age
| Gestational Age (wk) |
Deliveries | Stillbirth Total |
Stillbirth/10,000 Ongoing Pregnancies (95% CI) |
Infant Death/10,000 Live Births (95% CI) |
Composite Risk of Expectant Management for 1 wk*/10,000 (95% CI) |
|---|---|---|---|---|---|
| 37 | 336,640 | 807 | 2.1 (2.0–2.3) | 14.4 (13.1–15.7) | 12.6 (11.8–13.3) |
| 38 | 730,908 | 957 | 2.7 (2.6–2.9) | 10.5 (9.7–11.2) | 11.6 (11.0–12.1) |
| 39 | 1,099,469 | 951 | 3.5 (3.2–3.7) | 8.8 (8.3–9.4) | 12.9 (12.3–13.6) |
| 40 | 977,101 | 691 | 4.2 (3.9–4.5) | 9.5 (8.9–10.1) | 14.9 (14.0–15.9) |
| 41 | 508,438 | 411 | 6.1 (5.5–6.7) | 10.8 (9.9–11.7) | 17.6 (15.8–19.3) |
| 42 | 168,270 | 182 | 10.8 (9.2–12.4) | 11.5 (9.9–13.1) | — |
CI, confidence interval.
Composite risk=risk of stillbirth at this gestational age+risk of infant death at the next gestational age week.
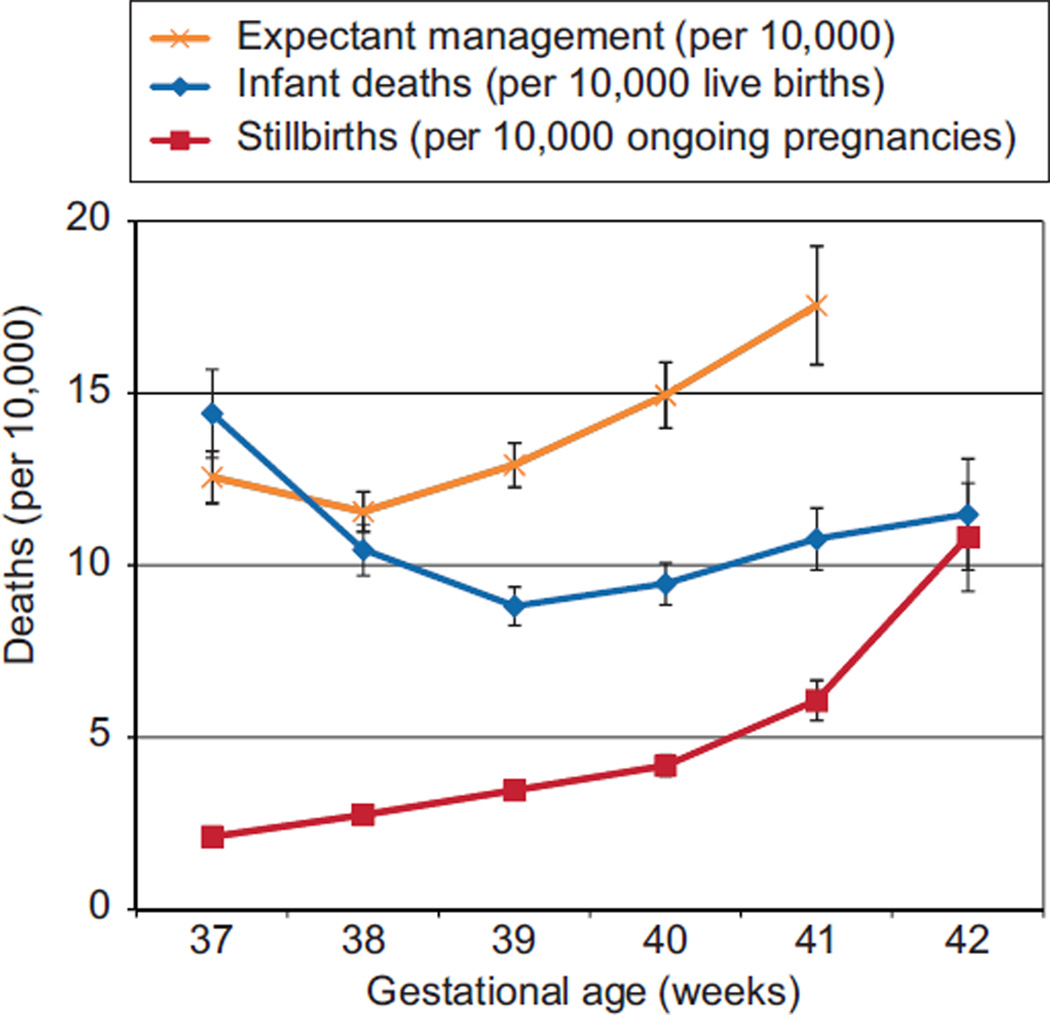
Fig. 1.
This graph compares the risk of delivery (represented by infant death) with the risk of expectant management for 1 week (represented by the stillbirth rate plus the infant death risk at the subsequent gestational age) at each gestational age at term. The stillbirth rate also is displayed graphically to demonstrate its exponential rate of change.
Infant death risk by gestational age at birth had a U-shaped curve, greatest at 37 weeks of gestation with a nadir at 39 weeks (Fig. 1). The highest infant mortality rate was 14.4 per 10,000 (95% CI 13.1–15.7 per 10,000 live births) at 37 weeks of gestation, 1.6 times higher than the rate at 39 weeks of gestation (8.8 per 10,000 live births, 95% CI 8.3–9.4 per 10,000, relative risk 1.63, 95% CI 1.47–1.82) (Tables 2 and 3). The most common cause of infant death was SIDS, comprising 27.7% of all infant deaths among children born between 37 and 42 weeks, followed by accidents (13.6%), complications of labor and delivery (11.7%), and infections (11.0%) (Table 4).
Table 3.
Comparative Risks of Stillbirth, Infant Death, and Expectant Management by Gestational Age
| Gestational Age (wk) |
Stillbirth Risk |
Infant Death Risk |
Expectant Management Risk* |
|---|---|---|---|
| 37 | Referent | 1.6 (1.5–1.8) | Referent |
| 38 | 1.3 (1.2–1.4) | 1.2 (1.1–1.3) | 0.9 (0.8–1.0) |
| 39 | 1.6 (1.5–1.8) | Referent | 1.0 (0.9–1.1) |
| 40 | 2.0 (1.8–2.2) | 1.1 (0.9–1.2) | 1.2 (1.1–1.3) |
| 41 | 2.9 (2.6–3.2) | 1.2 (1.1–1.4) | 1.4 (1.2–1.6) |
| 42 | 5.1 (4.4–6.0) | 1.3 (1.1–1.5) | — |
Data are relative risk (95% confidence interval).
Expectant management risk=risk of stillbirth at this gestational age+risk of infant death at the next gestational age week.
Table 4.
Causes of Infant Death by Gestational Age, 37–42 Weeks
| Infant Death Category |
Gestational Age (wk) | ||||||
|---|---|---|---|---|---|---|---|
| 37 | 38 | 39 | 40 | 41 | 42 | Total | |
| SIDS | 133 (27.5) | 210 (27.5) | 274 (28.3) | 254 (27.5) | 151 (27.6) | 51 (26.4) | 1,073 (27.7) |
| Accident | 64 (13.2) | 114 (14.9) | 129 (13.3) | 125 (13.5) | 70 (12.8) | 26 (13.5) | 528 (13.6) |
| Related to labor and delivery | 73 (15.1) | 79 (10.4) | 96 (9.9) | 109 (11.8) | 63 (11.5) | 35 (18.1) | 455 (11.7) |
| Infection | 40 (8.3) | 108 (14.2) | 107 (11.1) | 92 (10.0) | 60 (11.0) | 20 (10.4) | 427 (11.0) |
| Pulmonary | 24 (5.0) | 34 (4.5) | 42 (4.3) | 42 (4.6) | 30 (5.5) | 6 (3.1) | 178 (4.6) |
| Cardiac | 22 (4.6) | 22 (2.9) | 20 (2.1) | 24 (2.6) | 17 (3.1) | 7 (3.6) | 112 (2.9) |
| Neoplasm | 13 (2.7) | 20 (2.6) | 18 (1.9) | 26 (2.8) | 13 (2.4) | 5 (2.6) | 95 (2.5) |
| Other | 85 (17.6) | 141 (18.5) | 222 (22.9) | 190 (20.6) | 100 (18.3) | 31 (16.1) | 769 (19.8) |
| Missing | 30 (6.2) | 35 (4.6) | 60 (6.2) | 62 (6.7) | 43 (7.9) | 12 (6.2) | 242 (6.2) |
| Total | 484 | 763 | 968 | 924 | 547 | 193 | 3,879 |
SIDS, sudden infant death syndrome.
Data are n (%) or n.
A composite death rate was calculated to express the mortality risk associated with expectant management at any given gestational age. This risk is calculated as a sum of the stillbirth probability at a given week of gestation and the probability of infant death at the subsequent week of gestational age. This risk was highest at 41 weeks of gestation (17.6 per 10,000, 95% CI 15.8 –19.3) and lowest at 38 weeks of gestation (11.6 per 10,000, 95% CI 11.0 –12.1).
To determine whether the mortality risk is higher with delivery or with expectant management, the composite death rate related to expectant management was compared with the risk of infant death at each week of term pregnancy. At 37 weeks of gestation, the risk of expectant management is lower than the risk of delivery (12.6 compared with 14.4 per 10,000, relative risk 0.87, 95% CI 0.77– 0.99) (Table 5). At 38 weeks of gestation, the risk of expectant management is higher than the risk of delivery, although the CIs overlap: 11.6 (95% CI, 11.0 –12.1) compared to 10.5 (95% CI, 9.7–11.2 per 10,000). Thereafter, the risk of expectant management is statistically significantly higher than the risk of delivery; at 39 weeks of gestation, the risk of expectant management is 12.9 per 10,000, whereas the risk of delivery is 8.8 per 10,000 (relative risk 1.47, 95% CI 1.35–1.59). These risks continue to diverge substantially at 40 and 41 weeks of gestation, favoring delivery over expectant management when considering the overall risk for either fetal or infant death (Fig. 1). The absolute risk differences, although statistically significant at 39 weeks of gestation and beyond, are small, ranging from 4.1 per 10,000 (95% CI 3.23– 4.97 per 10,000) at 39 weeks of gestation to 6.8 per 10,000 at 41 weeks of gestation (95% CI 5.32– 8.24 per 10,000). To better understand the magnitude of this difference, we can calculate the “number needed to deliver,” which is analogous to the “number needed to treat.” This should be interpreted as the number of women who would need to be delivered at a given gestational age to prevent one excess death. From these data, the number needed to deliver ranged from 2,442 (95% CI 2,014 –3,101) at 39 weeks of gestation to 1,476 (95% CI 1,214 –1,881) at 41 weeks of gestation.
Table 5.
Relative and Absolute Risks of Expectant Management Compared With Delivery at 37–41 Weeks of Gestation
| Gestational Age (wk) |
Relative Risk of Expectant Management Compared With Delivery (95% CI) |
Absolute Risk Difference Between Expectant Management and Delivery/10,000 (95% CI) |
No. Needed to Deliver at This Gestational Age to Prevent a Single Excess Death (95% CI) |
|---|---|---|---|
| 37 | 0.87 (0.77–0.99) | −1.84 (−3.59 to −0.09) | — |
| 38 | 1.11 (1.00–1.22) | 1.11 (0.03–2.18) | 9,042 (4,587–316,456) |
| 39 | 1.47 (1.35–1.59) | 4.10 (3.23–4.97) | 2,442 (2,014–3,101) |
| 40 | 1.58 (1.45–1.71) | 5.47 (4.49–6.44) | 1,829 (1,552–2,228) |
| 41 | 1.63 (1.47–1.81) | 6.78 (5.32–8.24) | 1,476 (1,214–1,881) |
CI, confidence interval.
DISCUSSION
Determining the optimal time to deliver a pregnancy necessarily involves balancing risks and benefits. The present analysis examines the risk of fetal death before birth and infant death after birth in an attempt to quantify the mortality risk of delivery at each gestational age at term. Using a novel composite risk estimate composed of the risk of stillbirth plus the risk of infant death to represent the risk of expectant management for 1 additional week, the risk of expectant management carried a lower mortality risk at 37 weeks of gestation than the risk of delivery, equalized at 38 weeks of gestation, and then exceeded the risk of delivery at 39, 40, and 41 weeks of gestation.
In recent years, the idea that delivery at any gestational age from 37 to 42 weeks produces equivalent outcomes has come under scrutiny. Large observational studies of elective deliveries at term have shown small but significant increases in neonatal morbidity among children delivered in weeks 37 and 38 of gestation and in weeks 41 to 42 of gestation compared with those delivered in weeks 39 and 40 of gestation, demonstrating a U-shaped curve similar to the curve representing the risk of infant mortality seen in the current data set.13–15 As a result of these data, increased attention has been paid to decreasing the frequency of elective delivery before 39 weeks of gestation, although none of these studies was designed or powered to measure the stillbirth rate.16 One recent study of a practice change in a large medical center limiting elective delivery to after 39 weeks of gestation did demonstrate decreased neonatal intensive care unit admissions, but this was accompanied by an increase in the incidence of early-term stillbirth, sounding the call for more research in this area.17
One of the strengths of our current study is that the data set is large enough to examine stillbirth rates at each gestational age. The population of California is diverse and represents a wide range of racial and ethnic and socioeconomic groups. Another strength of the study is that infant death rates were used as a comparison metric. Infant death rates include later manifestations of complications of neonatal disease and SIDS, a devastating outcome with known risk factors but without a known cause.
The current study does have some limitations. One is that all antepartum stillbirths were assumed to occur the week that they delivered. Although this was a reasonable assumption because women at term usually see their health care provider weekly to auscultate fetal heart tones, and immediate delivery is recommended if stillbirth is diagnosed, some of the stillbirths might have occurred in the weeks before delivery. If this had occurred, the risk of stillbirth would have been higher at earlier gestational ages. Another limitation is our inability to stratify further than weeks of gestation down to days of gestation. Stratification to a daily level would have improved the accuracy of our estimates but would have severely limited the power of the study and led to confusion to when the stillbirth occurred compared with when the birth occurred. These weaknesses stem from the granularity of our data and not from the method itself; the method could be applied with less restrictive assumptions to future data sets with more detailed dating information and greater statistical power.
Another weakness of our study is the fact that the gestational ages used for analysis were based on last menstrual period alone, according to current California Department of Health practices. Studies show that relying solely on last menstrual period dating rather than ultrasonographic judgment, clinical judgment, or both is less accurate because it is subject to recall bias and the assumption that conception occurred 14 days after the last menstrual period. Pregnancies dated by last menstrual period alone have been shown to be more likely to be classified as “postterm.”18 –21 This bias should be distributed evenly among the stillbirth and infant death populations and would bias the results toward the null because many “term” infants (at 37, 38, 39, and 40 weeks of gestation) would be more likely to be classified as “postterm” (41 and 42 weeks of gestation), bringing the results closer together.
Despite these limitations, the current study suggests that delivery carries a greater mortality risk than expectant management at 37 weeks of gestation, carries equivalent risk at 38 weeks of gestation, but becomes advantageous at 39 weeks of gestation and beyond. However, it is difficult to make recommendations for clinical policy based only on retrospective data. Although the current study does not consider neonatal or maternal morbidity, other studies of neonatal morbidity suggest that these rates are lowest at 39 weeks of gestation.14,15,22 Although the mortality benefit of delivery at 39 weeks of gestation is thought-provoking, the absolute risks of either stillbirth or infant death are very low as is the risk difference between delivery and expectant management. A policy of delivery at 39 weeks of gestation solely to decrease mortality in low-risk pregnancies would require hundreds of thousands of inductions with uncertain effects on cost, maternal morbidity, and the cesarean delivery rate. A recent analysis suggested that routine induction of labor at 41 weeks of gestation would be a cost-effective intervention that would reduce stillbirth among other outcomes; such a study should be conducted at 39 weeks of gestation as well.23
Rather than suggesting a practice change based on these data alone, this study provides a novel methodology to examine perinatal morbidity and mortality at different gestational ages. This strategy may prove useful in the ongoing endeavor to determine the optimal time for delivery at term. Further research should be directed toward refining these risk estimates and customizing estimates for groups with higher risk for stillbirth.
Acknowledgments
Dr. Cheng is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant # HD01262, as a Women’s Reproductive Health Research Scholar.
Footnotes
Presented at the 31st Annual Meeting of the Society for Maternal Fetal Medicine, February 7–12, 2011, San Francisco, California.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth throughout pregnancy in the United States. Am J Obstet Gynecol. 2006;195:764–770. doi: 10.1016/j.ajog.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Kramer MS. Variations in mortality and morbidity by gestational age among infants born at term. J Pediatr. 2009;154:358–362. 362.e1. doi: 10.1016/j.jpeds.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Bruckner TA, Cheng YW, Caughey AB. Increased neonatal mortality among normal-weight births beyond 41 weeks of gestation in California. Am J Obstet Gynecol. 2008;199:421, e1–e7. doi: 10.1016/j.ajog.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko CW, Willinger M. Term pregnancy: a period of heterogeneous risk for infant mortality. Obstet Gynecol. 2011;117:1279–1287. doi: 10.1097/AOG.0b013e3182179e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donovan EF, Besl J, Paulson J, Rose B, Iams J Ohio Perinatal Quality Collaborative. Infant death among Ohio resident infants born at 32 to 41 weeks of gestation. Am J Obstet Gynecol. 2010;203:58, e1–e5. doi: 10.1016/j.ajog.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 6.Smith GC, Pell JP, Dobbie R. Risk of sudden infant death syndrome and week of gestation of term birth. Pediatrics. 2003;111:1367–1371. doi: 10.1542/peds.111.6.1367. [DOI] [PubMed] [Google Scholar]
- 7.Halloran DR, Alexander GR. Preterm delivery and age of SIDS death. Ann Epidemiol. 2006;16:600–606. doi: 10.1016/j.annepidem.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Spong CY, Iams J, Goldenberg R, Hauck FR, Willinger M. Disparities in perinatal medicine: preterm birth, stillbirth, and infant mortality. Obstet Gynecol. 2011;117:948–955. doi: 10.1097/AOG.0b013e318211726f. [DOI] [PubMed] [Google Scholar]
- 9.Hutcheon JA, Lisonkova S, Magee LA, Von Dadelszen P, Woo HL, Liu S, et al. Optimal timing of delivery in pregnancies with pre-existing hypertension. BJOG. 2011;118:49–54. doi: 10.1111/j.1471-0528.2010.02754.x. [DOI] [PubMed] [Google Scholar]
- 10.California Department of Health Services. Sacramento (CA): California Department of Health Services; 2006. Center for Health Statistics Birth cohort public use file 1999–2003. [Google Scholar]
- 11.Oestergaard MZ, Inoue M, Yoshida S, Mahanani WR, Gore FM, Cousens S, et al. Neonatal mortality levels for 193 countries in 2009 with trends since 1990: a systematic analysis of progress, projections, and priorities. PLoS Med. 2011;8:e1001080. doi: 10.1371/journal.pmed.1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HY, Chauhan SP, Ward TC, Mori N, Gass ET, Cisler RA. Aberrant fetal growth and early, late, and postneonatal mortality: an analysis of Milwaukee births 1996–2007. Am J Obstet Gynecol. 2011;204:261, e1–e10. doi: 10.1016/j.ajog.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 13.Tita AT, Landon MB, Spong CY, Lai Y, Leveno KJ, Varner MW, et al. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360:111–120. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng YW, Nicholson JM, Nakagawa S, Bruckner TA, Washington AE, Caughey AB. Perinatal outcomes in low-risk term pregnancies: do they differ by week of gestation? Am J Obstet Gynecol. 2008;199:370, e1–e7. doi: 10.1016/j.ajog.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Clark SL, Miller DD, Belfort MA, Dildy GA, Frye DK, Meyers JA. Neonatal and maternal outcomes associated with elective term delivery. Am J Obstet Gynecol. 2009;200:156, e1–e4. doi: 10.1016/j.ajog.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 16.Greene MF. Making small risks even smaller. N Engl J Med. 2009;360:183–184. doi: 10.1056/NEJMe0810120. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenthal DB, Hoffman MK, Jiang X, Ostrum G. Neonatal outcomes after implementation of guidelines limiting elective delivery before 39 weeks of gestation. Obstet Gynecol. 2011;118:1047–1055. doi: 10.1097/AOG.0b013e3182319c58. [DOI] [PubMed] [Google Scholar]
- 18.Ananth CV. Menstrual versus clinical estimate of gestational age dating in the United States: temporal trends and variability in indices of perinatal outcomes. Paediatr Perinat Epidemiol. 2007;21(suppl 2):22–30. doi: 10.1111/j.1365-3016.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 19.Dietz PM, England LJ, Callaghan WM, Pearl M, Wier ML, Kharrazi M. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Paediatr Perinat Epidemiol. 2007;21(suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 20.Pearl M, Wier ML, Kharrazi M. Assessing the quality of last menstrual period date on California birth records. Paediatr Perinat Epidemiol. 2007;21(suppl 2):50–61. doi: 10.1111/j.1365-3016.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- 21.Savitz DA, Terry JW, Jr, Dole N, Thorp JM, Jr, Siega-Riz AM, Herring AH. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187:1660–1666. doi: 10.1067/mob.2002.127601. [DOI] [PubMed] [Google Scholar]
- 22.Caughey AB, Washington AE, Laros RK., Jr Neonatal complications of term pregnancy: rates by gestational age increase in a continuous, not threshold, fashion. Am J Obstet Gynecol. 2005;192:185–190. doi: 10.1016/j.ajog.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 23.Kaimal AJ, Little SE, Odibo AO, Stamilio DM, Grobman WA, Long EF, et al. Cost-effectiveness of elective induction of labor at 41 weeks in nulliparous women. Am J Obstet Gynecol. 2011;204:137, e1–e9. doi: 10.1016/j.ajog.2010.08.012. [DOI] [PubMed] [Google Scholar]