Abstract
Objective
To describe the influence of TTTAn aromatase polymorphism on the relation between obesity and plasma estradiol in obese men.
Design
A 2 years cohort study.
Setting
Clinical research center.
Patients
Severely obese Caucasian men (31 had gastric bypass surgery and 118 controls).
Intervention
Men were genotyped for the TTTAn CYP19A1 polymorphism. Anthropomorphic measures, plasma estradiol and other hormonal levels were determined at baseline and two years follow up.
Main outcomes measures
Relationships between weight and changes in weight and plasma estradiol were examined in relation to the TTTAn polymorphism.
Results
Mean age was 46.5 ± 10.82 years, and BMI was 47.1 ± 8.46 kg/m2. The most common repeats were 7 and 11. TTTAn number did not correlate to plasma estradiol in the univariate analysis. When patients were stratified per weight groups, the correlation between plasma estradiol and weight was seen only among men with higher TTTA repeat at baseline and two years. Similarly, only men with higher TTTA exhibited reduced estradiol levels after weight loss.
Conclusion
Higher TTTA repeat are associated with a strengthened relationship between obesity and estradiol. The well established effect of increased weight on plasma estradiol appears to be absent in men with low TTTA numbers.
Keywords: Obesity, aromatase polymorphism, TTTA repeats, hormones, estradiol, testosterone
Background
Male obesity is associated with increased serum estradiol levels due to the increased peripheral aromatization of androgens that results from increased body mass.(1) Estradiol in men is derived from intra-testicular and peripheral aromatization of C19 androgens (androstenedione, testosterone) under the influence of aromatase, a product of the CYP19 gene (2–4). Multiple studies have reported increased estrogen and reduced free and total testosterone levels among obese men that may result in altered spermatogenesis and reduced fertility. (5–6) We and others showed that weight loss reduces the extent of abnormalities in the reproductive hormonal profile in obese men. (7–8)
Several previous reports showed the existence of polymorphisms in the aromatase enzyme gene or CYP19A1. CYP 19A1 is a single copy gene located on chromosome15q21.2. (2–4, 9) Aromatase polymorphisms were shown to affect risks for various estrogen dependent diseases in women such as breast cancer, endometrial cancer, and osteoporosis. (10–19) In men, aromatase polymorphisms may influence the incidence of osteoporosis and prostate cancer.(4, 20–26) Other studies showed no association between aromatase polymorphism and breast cancer, endometriosis, or osteoporosis in women, and men.(27–33).
The most commonly studied aromatase polymorphism is the tetranucleotide TTTA repeat polymorphism (TTTAn) present in intron 4 of the CYP 19A1 gene. (17, 20–21, 23) This polymorphism is associated with the activity of the aromatase enzyme both in vivo and in vitro. (20)
The nature of the interaction between the TTTAn aromatase polymorphism and obesity in the alteration of the reproductive hormonal profile in men has been controversial and the interaction of the polymorphism on the effects of weight loss on estradiol levels is unknown. (20–21) The purpose of this study was to investigate, 1) the association between variants of the TTTAn aromatase polymorphism and levels of reproductive hormones and 2) the interaction of weight and change in weight with the polymorphism on reproductive hormones in a group of severely obese men.
Material and Methods
Patient population
The Utah Obesity Study aimed at studying the 2-year morbidity of severely obese patientswho underwent Roux-en-Y gastric bypass surgery compared to control groups. The recruitment criteria are detailed in previous publications (34). The Utah obesity study originally recruited 206 Caucasian men (BMI ≥35 kg/m2): 67 men that underwent gastric bypass surgery and 139 controls. Subjects and controls were seen at enrollment and at 2 years follow-up. At baseline, 149 men had sufficient baseline blood and DNA samples available for analysis (31 had gastric bypass surgery and 118 controls). One hundred and twenty five men presented for the follow- up in two years, 24 in the group that had surgery and 101 in the control group. The 24 men who failed to follow up had similar demographic and anthropometric characteristics compared to men who had their 2 years follow up (data not shown).
Anthropometrics
All men had detailed questionnaires and anthropometric measures at baseline and at the two-year follow up. Height was measured using a Harpenden anthropometer (Holtain, Ltd) to the nearest centimeter. Weight was measured in a hospital gown with a Scaletronix scale (model 5100) (Scaletronix Corporation, Wheaton, IL). The scale has an 800 pound capacity and weighing accuracy of 0.1 kilogram. BMI was calculated as body weight divided by height squared (kg/m2). Circumferences (Lufkin metal tape) were measured at the waist (at the top of the iliac crest) and hip (at the largest circumference over the buttocks).
Metabolic and hormonal analysis
At baseline and at the 2-year follow up, study participants underwent a morning venous blood draw after 12 h overnight fasting. The following were measured: total testosterone using Electrochemiluminescent Immunoassay (ECLIA) (Elecsys Testosterone kit, Roche Diagnostics, Se 1.1), sex hormone binding globulin using ECLIA (Elecsys SHBG kit, Roche Diagnostics, Se 0.01, CV% 1–1.1), LH using ECLIA (Elecsys LH kit, Roche Diagnostics, Se 0.011, CV% 1.2–1.5), FSH using ECLIA (Elecsys FSH kit, Roche Diagnostics, Se 0.0036, CV% 1–1.3), estradiol using ECLIA (Architect kit, Abbott Diagnostics Laboratories, sensitivity (Se) 10, CV%: 2.4–6.2), Inhibin B using Enzyme-Linked Immunosorbent Assay (ELISA) (Inhibin B ELISA kit, Diagnostic Systems Laboratory, Se 10, CV% 10), HOMA-IR (glucose and insulin), Leptin using ELISA (Human Leptin ELISA Kit, Linco Research, Se 0.5, CV% 11–24.5) , Adiponectin using ELISA (Human Adiponectin Elisa Kit (K1001-1), B-Bridge International, Se 2, CV% 12.5–13.8). Free testosterone and estradiol was were calculated using the following formula: FT FH = ([TH] - (N × [FH]))/(Kt[SHBG - [TH] + N[FTFH]]) (35). A similar formula was used to calculate free estradiol. All hormonal analyses were performed at the ARUP Institute for Clinical and Experimental pathology. ARUP is a national reference laboratory located in Salt Lake City, Utah.
DNA analysis
The particular polymorphism targeted is the tetranucleotide TTTA repeat polymorphism (TTTAn) present in intron 4 of the Cyp19 A1 gene (36–37). Genomic DNA was extracted from patients' EDTA blood samples using QIAamp DNA blood kit (QIAGEN). The DNA region containing the tetranucleotide polymorphism (TTTAn) at the CYP19A1 gene was genotyped by using a modified PCR and electrophoresis method (20). The primers were designed to yield a 128 bp PCR product with forward primer, 5'-TGATAGAGTCAGAGCCTGTC and reverse primer, 5'-GTAAGCAGGTACTTAGTTAGC. The polymerase chain reaction was performed in 10 ul volume containing 5 ul of 2X FailSafe PCR premix (EPICENTRE Inc), 100 mM Tris-Hci, 100 mM KCl, 5 mM MgCl2, 400uM of each (dNTP and PCR enhancer), 1 ul of 10 pmol for each primer, 1 ul of 1 unit KlenTaq enzyme and 2 ul DNA (20 ng/ul). The PCR temperature profile was as follows: 95°C for 5 minutes followed by 35 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s, and finishing by 10 minutes of incubation at 72 °C. The PCR products were subjected to capillary electrophoresis and were analyzed using QIAxcel multicapillary electrophoresis system (QIAGEN,USA) at ARUP Institute for Clinical and Experimental pathology. Each genotype was verified and confirmed by sequencing at the University of Utah core research facilities.
Previous reports described various classifications for the TTTAn repeats: the number of repeats on each allele was dichotomized into two groups based on the number of repeats such as “7 repeats/ more than 7 repeats” (11, 16–17, 21, 24–25), “8 repeats/ more than 8 repeats “ (19, 32, 38), “9 repeats/ more than 9 repeats” (20, 22, 26, 33), “10 repeats/ more than 10 repeats” (13–14, 28), “11 repeats/ more than 11 repeats” (12) (19) or “12 repeats/ more than 12 repeats” (37). There is accordance of results among these studies that higher number of repeats is associated with higher estrogen levels. For the purpose of this analysis, the number of repeats on each allele was dichotomized to two variables: “7” = 7 repeats and “X”= more than 7 repeats.” This threshold is the most commonly utilized in the literature. (11, 16–17, 21, 24–25) Subsequently, our three genotypic groups were the following: “7-7,” “7-X” and “X-X.”
Statistical analysis
The unadjusted means were reported as mean ± SD, the adjusted means of plasma estradiol and various hormonal parameters were reported as mean ± SE. General linear models with polynomial contrasts were used to calculate and compare genotype-specific plasma estradiol means. Covariates for adjustment of the means included age, BMI, and total testosterone levels. Bonferonni correction was used for multiple comparisons. SPSS 16.0 (SPSS Inc, Chicago, IL) was used for statistical analysis.
Results
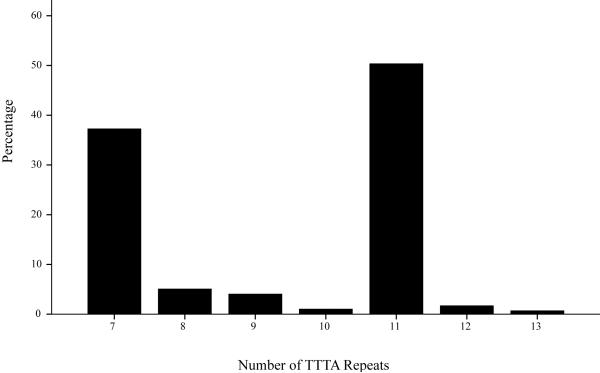
The mean age of our patients was 46.5 ± 10.82 years, the mean BMI was 47.1 ± 8.46 kg/m2 and the mean weight was 337.2 ± 60.87 lb. The distribution of the TTTAn repeats is given in figure 1. The most frequent numbers of repeats in our population were 7 and 11. The distribution of the three genotypes at baseline was as follows: “7-7”= 28.2% (n=42), “7-X”= 18.1% (n=27) and “X-X”= 53.7% (n=80). The distribution of the demographic (age) and anthropometric measures (height, weight, BMI, waist, hip) between the three genotypes at base line and the two years follow up was similar in the three genotype groups (data not shown). The proportion of subjects who would undergo bypass surgery at baseline was similar to the proportion of subjects who had undergone bypass surgery in the group providing 2-year follow-up data.
Figure 1.
Distribution of the different alleles with different numbers of TTTAn repeats
The unadjusted means of the different metabolic and hormonal parameters between the three genotypic groups are given in table 1. The three genotypic groups did not differ for any metabolic and hormonal parameters at baseline and at the 2 years follow up. Higher numbers of TTTAn repeats were associated with higher estradiol levels, but the difference was not statistically different. The levels of free estradiol levels followed the same trends as total estradiol. At baseline the free estradiol levels between the three genotypic groups were as follows: “7-7”: 0.98±0.30 pg/ml, “7-X”: 1.03±0.34 pg/ml and “X-X”: 1.06± 0.39 pg/ml, (p=0.53). Choosing other previously used thresholds for TTTAn repeats such as (both allele= 7 repeats compared to both allele = 11 repeats) and (equal or lower than 9 repeats compared to more than 9 repeats) did not result in a significance difference in estradiol levels.
Table 1.
Unadjusted means of metabolic and hormonal parameters
| 7-7 | 7-X | X-X | P valuea | |
|---|---|---|---|---|
| Baseline | n=42 | n=27 | n=80 | |
| Glucose | 107.1±34.06 | 120.5±35.66 | 110.2±31.05 | 0.620 |
| Insulin | 17.4±11.21 | 18.1±19.58 | 17.3±13.36 | 0.979 |
| Glycosylated Hemoglobin | 6.1±1.26 | 6.5±1.40 | 6.1±0.96 | 0.996 |
| Adiponectin | 7.2±3.53 | 7.0±3.04 | 8.1±6.23 | 0.365 |
| Leptin | 41.5±22.01 | 51.9±28.26 | 48.5±27.42 | 0.164 |
| C reactive Protein (mg/dL) | 0.6±0.63 | 0.6±0.39 | 0.6±0.52 | 0.993 |
| Luteinizing Hormone (IU/L) | 4.2±2.37 | 4.5±2.72 | 4.2±2.38 | 0.983 |
| Follicle Stimulating Hormone(IU/L) | 5.0±4.44 | 6.0±4.45 | 4.6±3.26 | 0.544 |
| Sex hormone Binding globulin (nmol/L) | 24.5±16.87 | 30.0±11.65 | 27.2±16.83 | 0.371 |
| Total testosterone (ng/dL) | 287.7±135.73 | 303.4±131.47 | 332.1±154.68 | 0.112 |
| Free testosterone (pg/mL) | 59.9±24.06 | 57.7±20.52 | 66.9±30.97 | 0.183 |
| Estradiol (pg/mL) | 34.6±9.28 | 38.6±10.82 | 37.7±12.71 | 0.161 |
ANOVA with polynomial contrast and Bonferroni correction.
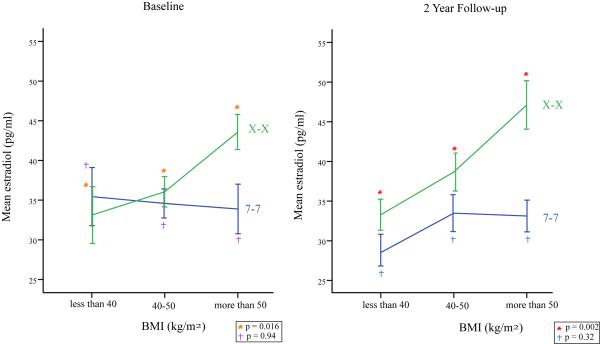
In contrast to the effect of TTTA repeat on estradiol, Weight was strongly associated with estradiol levels at baseline and at the 2 years follow up (data not shown). The absence of association in the raw data between TTTAn and estradiol indicates a much larger and important role for weight in the determination of estradiol level in obese men. To determine whether the TTTAn polymorphism modulate the effect of weight on estradiol levels, the estradiol levels were averaged per weight category in the groups with high and low number of TTTA repeats, Figure 2. Higher TTTAn repeats were associated with an enhancement of the interaction between weight and estradiol levels. The correlation between weight and estradiol level is clearly and statistically significantly seen among men homozygous for higher TTTAn repeat numbers both at baseline and at two years, whereas no such correlation is evident for men homozygous for lower numbers of repeats.
Figure 2.
Estradiol levels (pg/ml) at baseline and the 2 years follow up between the BMI groups (mean + 1 SD). P value is testing for linear trend in the three categories.
Estradiol levels (pg/ml) between the three TTTAn repeats genotypes at base line and 2 years follow up per BMI groups (mean + 1 SD). P value is testing for linear trend in the three categories.
The apparent relationship between higher TTTAn repeats and strengthened relationship between weight and estradiol levels was next evaluated in relation to the interaction between the TTTAn polymorphism with the effect of weight loss on estradiol levels. At the 2 years follow up and after an average weight loss of 43.0± 66.00 (−255.00 – +49.00) lb, one hundred and twenty five men presented for the follow- up, 24 in the group that had surgery and 101 in the control group. The average percent weight loss in our patient population was 11% with a range of a loss of 52 % to a gain of 14%. The percent weight loss after follow up was divided into three categories: minimal change in weight (weight loss or gain of less than 10%) n=68, moderate weight loss (weight loss more than 10 % of the original weight but less or equal to 25%) n= 11 and severe weight loss (weight loss more than 25% of the original weight) n=37. The rest of patients had weight gain of more than 10% of the original weight and were part of the control group, they were subsequently excluded from the analysis of the effect of weight loss on estradiol levels. The percent of change in estradiol in response to change in weight among patients with low and high TTTAn repeats is presented in Figure 3. After correction for gastric bypass surgery, men with higher TTTAn repeats (X-X) reduced dramatically their estradiol in response to weight loss, p=0.003. In men with lower TTTAn repeats, the change in estradiol was not associated with degree of weight loss (p=0.84).
Figure 3.
Change in estradiol levels (pg/ml) between the baseline and the 2 years follow up by category of weight loss in the two group of men with higher number of TTTAn repeats (X-X) and Lower number of TTTAn repeats (7-7) of the aromatase polymorphism (mean + 1 SD). The percent weight loss after follow up was divided into three categories: minimal change in weight (weight loss or gain of less than 10%) n=68, moderate weight loss (weight loss more than 10 % of the original weight but less or equal to 25%) n= 11 and severe weight loss (weight loss more than 25% of the original weight) n=37. P value is testing for linear trend in the three categories.
Discussion
Our data show that the numbers of TTTAn repeats in the aromatase polymorphism may have a small contribution when compared to weight on estradiol levels in obese men. Failure to demonstrate a more clear effect of the polymorphism on estradiol levels appears attributable to an interaction between the effects of weight and the polymorphism on estradiol levels. In this regard, we found that the effect of increasing degrees of obesity on estradiol levels depends on the number of TTTAn repeats in the aromatase polymorphism, as does the decline in estradiol levels with weight loss. Thus, the interaction of weight and estradiol levels in men varies according to this polymorphism. Our population included only obese men, in another population that includes normal and overweight men in addition to obese men, the effect of TTTAn repeats may be more pronounced. Another limitation that precluded the statistical significance is the small sample in our study.
The effect of aromatase polymorphism on the estrogen metabolism in men has been reported in previous work. Gennari et al studied the effect of aromatase polymorphism in bone metabolism. found that men with higher numbers of TTTAn repeats (more than 9) on both alleles had higher estradiol value and higher estradiol to testosterone ratio when compared with men lower number of TTTAn repeats on both alleles. This difference in hormonal profile at baseline was also reflected in a higher lumbar bone mineral density in men with higher numbers of TTTAn repeats (more than 9) on both alleles. This difference was reduced when the authors corrected for BMI, suggesting a stronger effect of obesity on estradiol and bone density than the aromatase polymorphism.(20) These results are consistent with our observations although our study population included no men of normal weight. Our data suggest further, however, that the effect of weight on estradiol is more pronounced in the high TTTAn repeat group than in the low TTTAn repeat group, such that correcting for BMI might be expected to have different outcomes for different allelic groups for the TTTAn polymorphism. A previous study by Van Pottelbergh et al, who did not find a correlation between aromatase polymorphism and reproductive hormones until he they excluded men with the lowest (25th percentile) and highest (75th percentile) BMIs. In this same study, men who were carrier of the polymorphism with the higher number of TTTAn repeats were less likely, after an average 3.4 years of follow-up, to reduce their bone mineral density at the total distal forearm and mid-subregion when compared to men with the shorter TTTAn repeat allelic variant.(21) Our data suggest that the correlation between weight and estradiol among subjects in Van Pottlebergh's data may have been obscured by the “subpopulation of men with lower TTTAn repeats for whom the weight-E2 relationship is damped. Together our data taken with these studies shows that the effect of obesity dominates the effect of the TTTAn repeats on estradiol levels, but to a lesser degree for men with lower repeat numbers among whom the weight to estradiol relationship is blunted.
The polymorphism studied in this our paper is located in intron 4 of the CYP19 gene. A biological plausibility of the relation between TTTAn repeats and aromatase activity may be attributed to a possible linkage to other polymorphisms in the promoter or other regulatory area of the CYP 19A1 gene. In factThere are several SNPs that were described in the aromatase genes such as the (3'UTR) C>T change (rs10046)(36–37), and the deletion/insertion polymorphism located in the intron 4, IVS4 [-/TCT](rs11575899), Arg264Cys and Val80Val (G/A). (36, 39) These polymorphisms have been linked to various clinical conditions with conflicting results. (40) The deletion/insertion polymorphism [-/TCT] has been suggested to affect the relation between TTTAn polymorphism and estrogen levels. We have screened a group of men from the same population for the deletion/insertion polymorphism [-/TCT] and found that it did not strengthen the relation between the TTTA polymorphism and estradiol levels (data not shown). Other polymorphisms are of uncertain clinical significance. There are 2 coding SNPs that have been described, Arg264Cys and Val80Val (G/A), these polymorphism were not associated estrogen-dependent end points.(40) Another coding SNP, Trp39Arg, was associated with less active aromatase protein in a Japanese population. (14) A recent report found that the (rs2470152) SNP (G→A) located in intron 1 of the CYP 19A1 gene to be highly correlated to estrogen levels in in two populations of 1041 young and 4490 older men. (41).
A direct role of the TTTAn polymorphism on transcriptional or post transcriptional pathways of the aromatase enzyme remains to be demonstrated. The aromatase enzyme is known to have several tissue specific promoters.(4) The expression of CYP19A1 in adipose tissue is dependent on promoter I.4 regulated by class I cytokines and other activators such as TNFα and glucocortcoids.(42–43) Our data show that the various allelic forms of the TTTAn polymorphism account for differential effects of weight on estradiol levels; this may, for instance, imply that this polymorphism may be related to differential expression of aromatase in fatty tissue through activation or repression of fat tissue specific promoter. In conclusion, the TTTAn aromatase polymorphism may influences the levels of estradiol in men by differentially modulating the quantitatively greater effects of obesity.
Acknowledgments
Supported by a grant (DK-55006) from the National Institute of Diabetes and Digestive and Kidney Diseases and a grant (M01-RR00064) from the National Center for Research Resources;
References
- 1.Tchernof A, Despres JP, Belanger A, Dupont A, Prud'homme D, Moorjani S, et al. Reduced testosterone and adrenal C19 steroid levels in obese men. Metabolism. 1995;44:513–9. doi: 10.1016/0026-0495(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 2.Ma CX, Adjei AA, Salavaggione OE, Coronel J, Pelleymounter L, Wang L, et al. Human aromatase: gene resequencing and functional genomics. Cancer Res. 2005;65:11071–82. doi: 10.1158/0008-5472.CAN-05-1218. [DOI] [PubMed] [Google Scholar]
- 3.Sebastian S, Bulun SE. A highly complex organization of the regulatory region of the human CYP19 (aromatase) gene revealed by the Human Genome Project. J Clin Endocrinol Metab. 2001;86:4600–2. doi: 10.1210/jcem.86.10.7947. [DOI] [PubMed] [Google Scholar]
- 4.Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol. 2003;86:219–24. doi: 10.1016/s0960-0760(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen R, Wilcox A, Skjaerven R, DD B. Men's body mass index and infertility. Hum Reprod. 2007;17 doi: 10.1093/humrep/dem139. [DOI] [PubMed] [Google Scholar]
- 6.Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Sex hormones and sexual function in obese men losing weight. Obes Res. 2003;11:689–94. doi: 10.1038/oby.2003.98. [DOI] [PubMed] [Google Scholar]
- 8.Hammoud A, Gibson M, Hunt SC, Adams TD, Carrell DT, Kolotkin RL, et al. Effect of Roux-en-Y Gastric Bypass Surgery on the Sex Steroids and Quality of Life in Obese Men. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2008-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polymeropoulos MH, Xiao H, Rath DS, Merril CR. Tetranucleotide repeat polymorphism at the human aromatase cytochrome P-450 gene (CYP19) Nucleic Acids Res. 1991;19:195. doi: 10.1093/nar/19.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talbog KE, Gammon MD, Kibriya MG, Chen Y, Teitelbaum SL, Long CM, et al. A CYP19 (aromatase) polymorphism is associated with increased premenopausal breast cancer risk. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9794-2. [DOI] [PubMed] [Google Scholar]
- 11.Huang CS, Kuo SH, Lien HC, Yang SY, You SL, Shen CY, et al. The CYP19 TTTA repeat polymorphism is related to the prognosis of premenopausal stage I–II and operable stage III breast cancers. Oncologist. 2008;13:751–60. doi: 10.1634/theoncologist.2007-0246. [DOI] [PubMed] [Google Scholar]
- 12.Ahsan H, Whigemore AS, Chen Y, Senie RT, Hamilton SP, Wang Q, et al. Variants in estrogen-biosynthesis genes CYP17 and CYP19 and breast cancer risk: a family-based genetic association study. Breast Cancer Res. 2005;7:R71–81. doi: 10.1186/bcr951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxter SW, Choong DY, Eccles DM, Campbell IG. Polymorphic variation in CYP19 and the risk of breast cancer. Carcinogenesis. 2001;22:347–9. doi: 10.1093/carcin/22.2.347. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi Y, Iwao K, Ikeda N, Egawa C, Noguchi S. Breast cancer risk associated with polymorphism in CYP19 in Japanese women. Int J Cancer. 2000;89:325–8. doi: 10.1002/1097-0215(20000720)89:4<325::aid-ijc2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Kristensen VN, Andersen TI, Lindblom A, Erikstein B, Magnus P, Borresen-Dale AL. A rare CYP19 (aromatase) variant may increase the risk of breast cancer. Pharmacogenetics. 1998;8:43–8. doi: 10.1097/00008571-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Paynter RA, Hankinson SE, Colditz GA, Kraj P, Hunter DJ, De Vivo I. CYP19 (aromatase) haplotypes and endometrial cancer risk. Int J Cancer. 2005;116:267–74. doi: 10.1002/ijc.21041. [DOI] [PubMed] [Google Scholar]
- 17.Tojeng CL, Kindmark A, Brandstrom H, Abrahamsen B, Petersen S, Stiger F, et al. Polymorphisms in the CYP19 and AR genes--relation to bone mass and longitudinal bone changes in postmenopausal women with or without hormone replacement therapy: The Danish Osteoporosis Prevention Study. Calcif Tissue Int. 2004;74:25–34. doi: 10.1007/s00223-002-2158-3. [DOI] [PubMed] [Google Scholar]
- 18.Dick IM, Devine A, Prince RL. Association of an aromatase TTTA repeat polymorphism with circulating estrogen, bone structure, and biochemistry in older women. Am J Physiol Endocrinol Metab. 2005;288:E989–95. doi: 10.1152/ajpendo.00550.2004. [DOI] [PubMed] [Google Scholar]
- 19.Masi L, Becherini L, Gennari L, Amedei A, Colli E, Falchek A, et al. Polymorphism of the aromatase gene in postmenopausal Italian women: distribution and correlation with bone mass and fracture risk. J Clin Endocrinol Metab. 2001;86:2263–9. doi: 10.1210/jcem.86.5.7450. [DOI] [PubMed] [Google Scholar]
- 20.Gennari L, Masi L, Merlok D, Picariello L, Falchek A, Tanini A, et al. A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: effects on bone metabolism. J Clin Endocrinol Metab. 2004;89:2803–10. doi: 10.1210/jc.2003-031342. [DOI] [PubMed] [Google Scholar]
- 21.Van Pogelbergh I, Goemaere S, Kaufman JM. Bioavailable estradiol and an aromatase gene polymorphism are determinants of bone mineral density changes in men over 70 years of age. J Clin Endocrinol Metab. 2003;88:3075–81. doi: 10.1210/jc.2002-021691. [DOI] [PubMed] [Google Scholar]
- 22.Czajka-Oraniec I, Zgliczynski W, Kurylowicz A, Mikula M, Ostrowski J. Association between gynecomastia and aromatase (CYP19) polymorphisms. Eur J Endocrinol. 2008;158:721–7. doi: 10.1530/EJE-07-0556. [DOI] [PubMed] [Google Scholar]
- 23.Kastelan D, Grubic Z, Kraljevic I, Polasek O, Dusek T, Stingl K, et al. The role of estrogen receptor-alpha gene TA polymorphism and aromatase gene TTTA polymorphism on peak bone mass againment in males: is there an additive negative effect of certain allele combinations? J Bone Miner Metab. 2009;27:198–204. doi: 10.1007/s00774-008-0029-3. [DOI] [PubMed] [Google Scholar]
- 24.Huang YC, Chen M, Lin MW, Chung MY, Chang YH, Huang WJ, et al. CYP19 TCT tri-nucleotide Del/Del genotype is a susceptibility marker for prostate cancer in a Taiwanese population. Urology. 2007;69:996–1000. doi: 10.1016/j.urology.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya N, Wang L, Suzuki H, Segawa T, Fukuda H, Narita S, et al. Impact of IGF-I and CYP19 gene polymorphisms on the survival of patients with metastatic prostate cancer. J Clin Oncol. 2006;24:1982–9. doi: 10.1200/JCO.2005.02.9439. [DOI] [PubMed] [Google Scholar]
- 26.Lorentzon M, Swanson C, Eriksson AL, Mellstrom D, Ohlsson C. Polymorphisms in the aromatase gene predict areal BMD as a result of affected cortical bone size: the GOOD study. J Bone Miner Res. 2006;21:332–9. doi: 10.1359/JBMR.051026. [DOI] [PubMed] [Google Scholar]
- 27.Cussenot O, Azzouzi AR, Nicolaiew N, Fromont G, Mangin P, Cormier L, et al. Combination of polymorphisms from genes related to estrogen metabolism and risk of prostate cancers: the hidden face of estrogens. J Clin Oncol. 2007;25:3596–602. doi: 10.1200/JCO.2007.11.0908. [DOI] [PubMed] [Google Scholar]
- 28.Okobia MN, Bunker CH, Zmuda JM, Ezeome ER, Anyanwu SN, Uche EE, et al. Simple tandem repeat (TTTA)n polymorphism in CYP19 (aromatase) gene and breast cancer risk in Nigerian women. J Carcinog. 2006;5:12. doi: 10.1186/1477-3163-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Healey CS, Dunning AM, Durocher F, Teare D, Pharoah PD, Luben RN, et al. Polymorphisms in the human aromatase cytochrome P450 gene (CYP19) and breast cancer risk. Carcinogenesis. 2000;21:189–93. doi: 10.1093/carcin/21.2.189. [DOI] [PubMed] [Google Scholar]
- 30.Hur SE, Lee S, Lee JY, Moon HS, Kim HL, Chung HW. Polymorphisms and haplotypes of the gene encoding the estrogen-metabolizing CYP19 gene in Korean women: no association with advanced-stage endometriosis. J Hum Genet. 2007;52:703–11. doi: 10.1007/s10038-007-0174-x. [DOI] [PubMed] [Google Scholar]
- 31.Kado N, Kitawaki J, Obayashi H, Ishihara H, Koshiba H, Kusuki I, et al. Association of the CYP17 gene and CYP19 gene polymorphisms with risk of endometriosis in Japanese women. Hum Reprod. 2002;17:897–902. doi: 10.1093/humrep/17.4.897. [DOI] [PubMed] [Google Scholar]
- 32.Salmen T, Heikkinen AM, Mahonen A, Kroger H, Komulainen M, Pallonen H, et al. Relation of aromatase gene polymorphism and hormone replacement therapy to serum estradiol levels, bone mineral density, and fracture risk in early postmenopausal women. Ann Med. 2003;35:282–8. doi: 10.1080/07853890310006370. [DOI] [PubMed] [Google Scholar]
- 33.Remes T, Vaisanen SB, Mahonen A, Huuskonen J, Kroger H, Jurvelin JS, et al. Aerobic exercise and bone mineral density in middle-aged finnish men: a controlled randomized trial with reference to androgen receptor, aromatase, and estrogen receptor alpha gene polymorphisms small star, filled. Bone. 2003;32:412–20. doi: 10.1016/s8756-3282(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 34.Adams TD, Avelar E, Cloward T, Crosby RD, Farney RJ, Gress R, et al. Design and rationale of the Utah obesity study. A study to assess morbidity following gastric bypass surgery. Contemp Clin Trials. 2005;26:534–51. doi: 10.1016/j.cct.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 36.Dunning AM, Dowseg M, Healey CS, Tee L, Luben RN, Folkerd E, et al. Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J Natl Cancer Inst. 2004;96:936–45. doi: 10.1093/jnci/djh167. [DOI] [PubMed] [Google Scholar]
- 37.Kristensen VN, Harada N, Yoshimura N, Haraldsen E, Lonning PE, Erikstein B, et al. Genetic variants of CYP19 (aromatase) and breast cancer risk. Oncogene. 2000;19:1329–33. doi: 10.1038/sj.onc.1203425. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki K, Nakazato H, Matsui H, Koike H, Okugi H, Ohtake N, et al. Association of the genetic polymorphism of the CYP19 intron 4[TTTA]n repeat with familial prostate cancer risk in a Japanese population. Anticancer Res. 2003;23:4941–6. [PubMed] [Google Scholar]
- 39.Siegelmann-Danieli N, Buetow KH. Constitutional genetic variation at the human aromatase gene (Cyp19) and breast cancer risk. Br J Cancer. 1999;79:456–63. doi: 10.1038/sj.bjc.6690071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez-Lorca R, Grilo A, Martinez-Larrad MT, Manzano L, Serrano-Hernando FJ, Moron FJ, et al. Sex and body mass index specific regulation of blood pressure by CYP19A1 gene variants. Hypertension. 2007;50:884–90. doi: 10.1161/HYPERTENSIONAHA.107.096263. [DOI] [PubMed] [Google Scholar]
- 41.Eriksson AL, Lorentzon M, Vandenput L, Labrie F, Lindersson M, Syvanen AC, et al. Genetic Variations in Sex Steroid-Related Genes as Predictors of Serum Estrogen Levels in Men. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2008-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahendroo MS, Mendelson CR, Simpson ER. Tissue-specific and hormonally controlled alternative promoters regulate aromatase cytochrome P450 gene expression in human adipose tissue. J Biol Chem. 1993;268:19463–70. [PubMed] [Google Scholar]
- 43.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2009;30:343–75. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]