Abstract
Diabetic nephropathy (DN) represents a major burden to public health cost. Tight glycemic and blood pressure control can dramatically slow but not halt the progression of the disease, and a large number of patients progress towards end-stage renal disease despite the currently available multifactorial interventions. An early and key event in the development of DN is the loss of podocyte function (or glomerular visceral epithelial cells) from the kidney glomerulus, where they contribute to the integrity of the glomerular filtration barrier. The recent evidence that podocyte can be the direct target of circulating hormones, lipids and adipokynes that are affected in diabetes, has prompted us to review the clinical and experimental evidence supporting novel endocrine and metabolic pathways in the pathogenesis of podocyte malfunction and in the development of DN.
Background
Diabetic nephropathy is a chronic progressive disease that affects 20–40% of patients with diabetes mellitus. Clinical trials have demonstrated that multifactorial interventions can slow but not halt the progression of DN (1) (2, 3), a medical condition that is associated with increasing health care costs (4). An early and key event in the development of DN is the loss of podocytes (or glomerular visceral epithelial cells) from the kidney glomerulus (5–9). Podocytes and their foot processes make the outer layer of the kidney ultrafiltration barrier; they are unique cells that form the glomerular slit diaphragm (SD), a complex cellular structure that prevents the development of proteinuria (10, 11) in an actin cytoskeleton dependent manner (12). Although the key elements that compose the SD have initially been thought to be primarily structural molecules, it has become clear that most of them can initiate a cascade of signaling events that affect podocyte function (13). The function and integrity of the SD is affected by a multiplicity of factors, and the more recent evidence that podocytes express receptors for many circulating hormones suggests that a more complex cross talk between the kidney and other organs affected by diabetes may occur in health and disease. The intention of this review article is to summarize the evidence that podocyte can unexpectedly respond to a variety of hormones under physiological condition, and that such responses may be altered in diabetes.
Case vignette
The patient is a 60 year-old Hispanic man diagnosed with type 2 diabetes since age 40 who presents to the nephrology office for the management of diabetic nephropathy. The patient has been married for 42 years, and he has a 36-year-old daughter who is healthy. His 38-year-old son, who was recently diagnosed with type 2 diabetes as well, accompanies him. The patient has never smoked or used illicit drugs, and works as a bank teller. He also has diabetic retinopathy, for which he had several sessions of laser surgeries to both eyes, and diabetic neuropathy. His medications include short and long-acting insulin, an angiotensin converting enzyme inhibitor, a beta-blocker, a loop diuretic, bicarbonate tablets, a phosphate binder, a vitamin d analogue, iron tablets, aspirin, an HMG-CoA reductase inhibitor, and an erythropiesis-stimulating agent. When diagnosed with type 2 diabetes some 20 years ago his renal function had been normal, and a urine albumin-to-creatinine ratio showed an albumin excretion of 22 mg/g. 5 years later microalbuminuria was detected, and his renal function declined steadily over the ensuing years. His blood pressures over the years were consistently around 125–135/75–85 mmHg, and his glycosylated hemoglobin values range between 7.0 and 7.6%. The patient now has stage 4 chronic kidney disease with an estimated glomerular filtration rate of 28 ml/min/1.73 m2 using the modification of diet in renal disease formula, and proteinuria of 1550 mg per day. While the patient at this visit is most interested in learning about transplantation, he and his son also inquire about new treatment options that might prevent the occurrence or slow the progression of diabetic nephropathy.
Pathogenesis of Diabetic Nephropathy: a focus on podocyte
Diabetic nephropathy in humans is histopathologically characterized by glomerular and tubular glomerular basement membrane (GBM) thickening, podocytopenia, mesangial expansion, glomerular and arteriolar hyalinosis and Kimmelstiel-Wilson nodules. The earliest clinical manifestation of DN is microalbuminuria (MA), a strong predictor of renal and cardiovascular disease in both type 1 and type 2 diabetes (14–17). Although predictors for the development of MA have been identified, such as insulin resistance (18–21), HbA1c (22, 23), hypertension or weight gain (24), the identification of causative factors responsible for MA may lead to the identification of novel specific biomarkers of disease initiation and progression.
The study of podocytes and their role in the pathogenesis of diabetic nephropathy has been the subject of intense investigation (25), and several studies have shown that decrease in podocyte density and/or podocyte detachment occurs in diabetic nephropathy in experimental animals and humans (5–9). Podocytepenia correlates with disease progression (5, 26), is inversely related to the control of hypertension and diabetes (8), but can also occur independently of blood pressure (27).
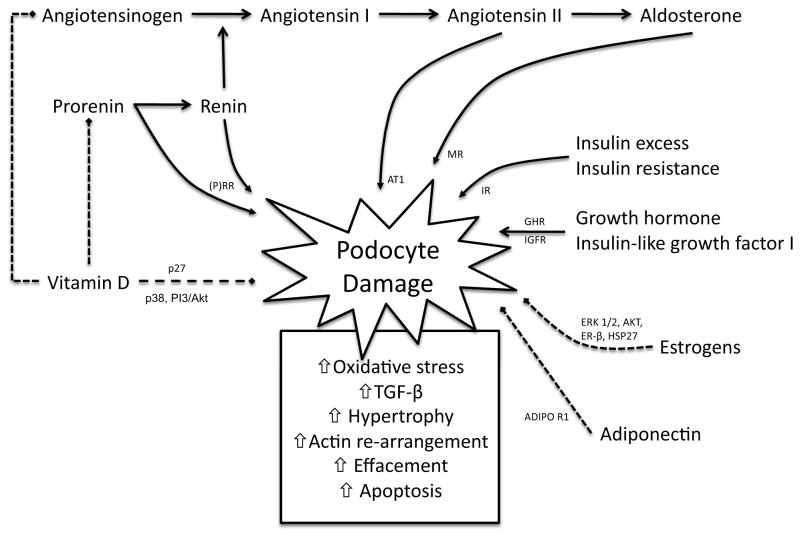
Beside the standard components of the SD that are modulated in diabetes (28, 29), several metabolic and endocrine factors have been recently shown to affect podocyte function (Figure 1). Among them angiotensin II has been the most studied and will not be extensively reviewed herein (30–32). Podocytes have been more recently showed to be the direct target of less traditional components of the renin angiotensin system (RAS), among which aldosterone (33, 34) and prorenin (35, 36). Furthermore, insulin (37, 38), adiponectin (39), sex hormones (40), growth hormone (41–43), and Vitamin D (44) have also been involved in the direct modulation of podocyte function. Here, we propose that an altered hormonal melieu and/or an altered podocytes hormones receptor expression contributes to the podocytopathy observed in DN. We will review the clinical and experimental evidence that hormonal derangements that characterize diabetes may be associated with the severity of MA, which may be caused by a change in the ability of podocytes to respond to circulating hormones. We will focus our attention on hormones for which both podocyte receptor expression and clinical relevance have been suggested. We will solely mention other hormones/parahormones that are most likely additional important modulator of podocytes function, such as free fatty acids (FFA) (45), lipoproteins (46, 47), adrenocorticotropic hormone (ACTH) (48), growth hormone releasing hormone (GHRH) (49), and thyroid hormone (50), as we believe more studies are needed to support their specific function in DN.
Figure 1. Podocyte is the target of several hormones.
Podocyte injury can result from multiple local or systemic endocrine derangements that characterize diabetes, see text for details. Solid arrows represent pathways that promote podoyte injury, while dashed lines indicate protection from podocyte damage. p27: Cyclin-dependent kinase inhibitor 1B; p38: p38 mitogen-activated protein kinase; PI3: Phosphatidylinositol 3-kinase; AKT: Protein kinase B; ERK: Extracellular-signal-regulated kinases; ERβ: Estrogen receptor beta; HSP27: Heat shock 27kDa protein 1; ADIPOR1: Adiponectin receptor 1; GHR: Growth hormone receptor; IGFR: Insulin-like growth factor receptor; IR: Insulin receptor; MR: Mineralocorticoid receptor; AT1: Angiotensin II receptor type 1; (P)RR: (Pro) renin receptor
Recent Advances
Rening Angiotensin-system
Renin-angiotensin system (RAS) blockade has widely established antiproteinuric effects in DN as well as other proteinuric kidney diseases, and it is behind the scope of this study to review the clinical studies supporting the widespread utilization of RAS blockade for renoprotection in DN (1). Although many of the effects of these agents can be attributed to their blood-pressure lowering effects, recent studies have provided valuable insight into the non-hemodynamic actions of Angiotensin II and other components of the RAS in the progression of kidney disease. The renin-angiotensin system plays a key role extracellular matrix (ECM) remodeling (51, 52), podocyte apoptosis (53), local inflammatory response (32, 54) and cell depolarization (55). The presence of a local podocyte specific RAS modulated by glucose has been recently described (30), and RAS blockade may lead to modulation of relevant SD proteins such as nephrin (56, 57) and TRPC6 (58). The importance of the local RAS in the development of proteinuria comes from a mouse model overexpressing Ang II type 1 receptor (AT1R) in podocytes (31). It is interesting to note that RAS blockade may also affect podoycte function through the systemic modulation of adipokines and insulin sensitivity (59). Angiotensin converting enzyme 2 (ACE2), an homologous protease of ACE that negatively regulates RAS, has been localized to podocytes, and in db/db diabetic mice glomerular expression of ACE2 is reduced, suggesting its utility as a potential target for therapeutic interventions to reduce proteinuria and the progression of glomerular disease in DN (60). Therefore, it is likely that both systemic and local effect of RAS blockade contributes to amelioration of podocyte function in DN.
In the past few years, clinical and experimental evidence has been generated on the renoprotection of selective aldosterone blockers (61–63). Because aldosterone has pleiotropic functions, both systemic and local effects may be involved. Interestingly, podocytes express mineralocorticoid receptor (MR), and MT activation in podocytes alters the filtration barrier and results in proteinuria (33). Even more interesting, aldosterone independent MR ligands that are relevant to podocyte function, such as the small GTPase Rac1, have been identified, underlying the complexity of podocyte signaling in DN (34).
Among other components of the RAS, circulating prorenin is higher in diabetic patients with microvascular complications when compared to well matched diabetic patients without complications (64). Increased prorenin may indeed precede the onset of MA in patients with type 1 diabetes (65). Interestingly, a decoy peptide corresponding to the “handle” region for non proteolitic activation of prorenin inhibits experimental DN (35). The recent evidence that podocytes express the prorenin receptor (66), suggests a direct modulation of podocyte function by prorenin. However, the direct renin inhibitor aliskerin may offer additional renoprotection in patients treated with Ang II type 1 receptor blocker, suggesting that aliskiren may have another effect other than blockade of the traditional renin-angiotensin system (RAS) (67). Indeed aliskerin has been recently shown to block podocyte production of Ang II in a way that is independent of traditional prorenin receptor signaling through extracellular signal-related protein kinase (ERK) (36).
Insulin
Microalbuminuria is a strong predictor of renal disease and cardiovascular outcome in diabetic and non diabetic patients. Altered insulin-signaling correlates with the development of MA in diabetic patients with both type 1 or type 2 diabetes (19, 20, 68, 69), their siblings (18, 70) and non diabetic individuals (71). Furthermore, insulin sensitizing agents of the class of thiazolinideniones (TZD) have been shown to offer a degree of renoprotection that is superior to other agents in patients with diabetes and diabetic nephropathy (DN) (72) as well as in insulinopenic experimental animal models of diabetes and DN (73).
Several studies have correlated insulin signaling pathways with podocytes function (21). Coward et al. showed that podocytes are insulin responsive cells (37), and this responsiveness occurs through their ability to modulated the function of glucose transporters GLUT1 and GLUT4 expressed in podocyte foot processes and regulated by insulin sensitizers (74). We have also demonstrated in the experimental db/db mouse model of DN that the phosphorlylation of AKT, a rate limiting enzyme in the insulin signaling pathway, was lower in glomeruli from diabetic mice when compared to controls, and podocytes isolated from db/db mice prior to the onset of MA are unable to phosphorylate AKT in response to insulin (38). This inability to signal through AKT was associated with an increased susceptibility to cell death. More recently, elegant studies have demonstrated that insulin signaling in podocytes is essential for the preservation of the integrity of the glomerular filtration barrier, as mice with a podocyte specific deletion of insulin receptor demonstrated a glomerular lesion resembling diabetic nephropathy, even in the absence of hyperglycemia (75). Further studies will be needed to understand if podocyte function is dependent on basal and insulin stimulated glucose uptake.
While the clinical and experimental evidence linking podocyte malfunction and insulin resistance in DN is being established, we can not exclude a direct effect of insulin per se on podocyte function, as insulin infusions in normal as well as diabetic individuals have been shown to lead to transient proteinuria (76, 77), and insulin may be inferior to other hypoglycemic agents for the prevention of DN (78). The effect of insulin per se on podocyte function in the setting of preserved insulin signaling warrants further investigation.
Estrogens
Estrogens may provide a protective effect against the development and progression of DN that is lost after menopause (79–81). More recently, the calculated free estradiol was found to be an independent predictor of the progression from MA to ESRD in men with type 1 DM and DN (82).
Podocytes express estrogen receptors, and 17-β estradiol treatment of podocytes can increase ER-β expression (40). Since ER-β is involved in cell survival, it would be intriguing to test if the podocytepenia that characterizes early DN is related to altered signaling of estrogen through ER-β. The estrogen receptor modulator tamoxifen may modify the Erα-Erβ ratio, which in turn may affect the expression of TGF-β, the synthesis of the matrix metalloproteinases and the pathways involved in apoptotic and anti-inflammatory signaling (40). In fact, experimental studies suggested that therapy with 17-β estradiol can affect positively the renal function in DN (83, 84).
Adipocytokines
Recent investigations have focused on the cross talk between adipose tissue and organs affected by macrovascular and microvascular complications of diabetes. Among the various cytokine-like hormones secreted by adipose tissue, also referred to as adipokines, adiponectin is the most abundant (85). Adiponectin is a 30- kDA protein primarily produced by differentiated adypocytes. Adiponectin may circulate as a high molecular weight and a more bioactive low molecular weight form. In humans, adiponectin levels are low in obese subjects and are associated with increased cardiovascular mortality (86–88) and a polymorphism in the adiponectin promoter affecting gene expression has been linked to diabetes and its complications (89).
It is likely that in patients with chronic kidney disease an impaired clearance of adiponectin may occur, as the levels of adiponectin increase in concordance with decreasing renal function (90). High adiponectin levels are also associated with progression to end stage renal disease and with mortality in Type 1 diabetic patients (91–93).
Interestingly, podocytes express both adiponectin receptor 1 and 2 (AR1 and AR2) (39). Adiponectin null mice develop severe proteinuria and podocyte damage through impaired AMPK signaling, which can be reversed by the administration of recombinant adiponectin, which modulates podocyte oxidative stress through an AMPK dependent modulation of the NADPH oxidase Nox 4 in podocytes. We believe this represent a hallmark study, as it establishes a cause-effect relationship between adiponectin and albuminuria, a marker of both insulin resistance and early DN (39). Whether a similar mechanism may be at play in patients with DN remains to be seen. It is possible that modulation of adiponectin production by RAS blockade may provide a unifying explanation for the metabolic, cardiovascular, and renal protection afforded by ACEi and ARB (59).
More recently, increased local production of visfatin by podocytes and proximal tubular cells have been demonstrated in early experimental diabetic nephropathy {Kang, #904}. Interestingly, plasma visfatin levels were significantly increased in early stages of diabetic nephropathy and correlates positively with microalbuminuria, suggesting an important paracrine role in the development of DN {Kang, #904}.
Vitamin D
Low level of Vitamin D are associated with progression of CKD and mortality (94), and Vit D treatment not solely reduces cardiovascular mortality (95–97), but also reduces clinical proteinuria (95–97). Furthermore, vitamin D reduces experimental podocyte loss (98) and acts synergistically to AT1 receptor blockers in reducing proteinuria in experimental DN (99, 100) (101), raising the interesting possibility of podocytes being a direct target of Vitamin D. In fact, podocytes express Vitamin D receptor (44), and Vitamin D can act as a strong modulator of the local RAS (102–104), suggesting again that there is a complex interconnection between these hormonal pathways involved in the pathogenesis of DN.
Growth Hormone
Altered Growth Hormone (GH)/Insulin-like Growth Factor (IGF)-1 axis have been described in diabetes and diabetic nephropathy (105). Several studies have suggested a direct relationship between the activity of the GH/IGF-1 axis and certain feature of DN, such as hyperfiltration and MA (106), and somatostatin analogue may be renoprotective in diabetes (107). In experimental models, overexpression of GH results in severe glomerulosclerosis (108), and inhibition of GH action through different mechanisms may improve DN (42, 43, 109). Interestingly, podocytes express GH Receptor (GRH), and signaling through GHR in podocytes affects oxidative stress and actin remodeling, two important features of podocyte biology (41). Taken all together, this solid experimental data suggests that medications targeting the GHR or the GH signaling cascade may represent a novel approach to prevent and/or treat DN. We would also like to mention that a high expression of GHRH has been detected in the kidney (49), but a specific function of GHRH in the kidney remains to be established.
Others players on the pipeline
Few others endocrine derangements that may deserve further investigation as they might contribute to the initiation and progression of DN. In particular, the ability of podocytes to uptake oxidized- Low density lipoprotein (LDL) (46, 47) warrants further investigation, as it has been shown that LDL removal by apheresis in patients with proteinuric DN improves not only the lipid profile but also decreases proteinuria and reduced the loss of podocytes in the urine (110). The role of free fatty acid in the development of MA in DN also remains to be established, as FFA utilization by podocytes has been described (111, 112) and may contribute to the development of podocyte specific insulin resistance (45). The evidence that ACTH deficiency may cause FSGS like lesions (113), together with the evidence that ACTH has been shown to have antiproteinuric properties in membranous nephropathy (114) suggests that further investigation on the role of ACTH axis in DN is needed. Finally, subclinical hypothyroidism is an independent risk factor for DN (115), and levothyroxine treatment in a diabetic patient with renal insufficiency and hypothyroidism prevented progressive renal failure (116), suggesting that further investigation on the kidney as a direct target of thyroid hormone action is warranted.
Summary
Although most of the strategies utilized for the treatment and prevention of DN are currently related to targeting the RAS and improving hyperglycemia, we have reviewed herein additional systemic and local hormonal derangement that characterizes DN and that may lead to novel drug discoveries. We have selected some of the multiple studies suggesting that DN may result from the hormonal derangement that characterizes diabetes. We find this topic of particular interest, as it strongly suggests that a cross talk between distant tissues and organs affected by diabetes may occur. We hope this review will stimulate the reader to initiate novel experimental and clinical research aimed at finding new targets for the treatment of DN.
Acknowledgments
Research support: Dr. Fornoni is supported by the National Institute of Health (DK-82636), by the American Diabetes Association (7-09-JF-23) and by the Diabetes Research Institute Foundation. Dr. Diez-Sampedro is supported by the Florida Biomedical Research Program (08KN-02-17217).
Footnotes
Financial conflicts of interests: Dr Lenz received grants and honoraria from Abbott, Genzyme, Amgen, and Ortho-Biotech. Dr. Fornoni and Dr Diez-Sampedro declare no conflict of interests.
References
- 1.Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661. doi: 10.1053/ajkd.2000.16225. [DOI] [PubMed] [Google Scholar]
- 2.Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1258–1264. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 3.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 4.USRDS. USRDS 2007 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]
- 5.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 6.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 7.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 8.Verzola D, Gandolfo MT, Ferrario F, et al. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007 doi: 10.1038/sj.ki.5002531. [DOI] [PubMed] [Google Scholar]
- 9.White KE, Bilous RW, Marshall SM, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51:3083–3089. doi: 10.2337/diabetes.51.10.3083. [DOI] [PubMed] [Google Scholar]
- 10.Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- 11.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 12.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Benzing T. Signaling at the slit diaphragm. J Am Soc Nephrol. 2004;15:1382–1391. doi: 10.1097/01.asn.0000130167.30769.55. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Stampfer MJ, Castelli WP, Verter J. The prognostic significance of proteinuria: the Framingham study. Am Heart J. 1984;108:1347–1352. doi: 10.1016/0002-8703(84)90763-4. [DOI] [PubMed] [Google Scholar]
- 15.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310:356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 16.Yuyun MF, Dinneen SF, Edwards OM, Wood E, Wareham NJ. Absolute level and rate of change of albuminuria over 1 year independently predict mortality and cardiovascular events in patients with diabetic nephropathy. Diabet Med. 2003;20:277–282. doi: 10.1046/j.1464-5491.2003.00940.x. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi N, Bandinelli S, Mangili R, Penno G, Rottiers RE, Fuller JH. Microalbuminuria in type 1 diabetes: rates, risk factors and glycemic threshold. Kidney Int. 2001;60:219–227. doi: 10.1046/j.1523-1755.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- 18.Forsblom CM, Eriksson JG, Ekstrand AV, Teppo AM, Taskinen MR, Groop LC. Insulin resistance and abnormal albumin excretion in non-diabetic first-degree relatives of patients with NIDDM. Diabetologia. 1995;38:363–369. doi: 10.1007/BF00400643. [DOI] [PubMed] [Google Scholar]
- 19.Groop L, Ekstrand A, Forsblom C, et al. Insulin resistance, hypertension and microalbuminuria in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:642–647. doi: 10.1007/BF00404074. [DOI] [PubMed] [Google Scholar]
- 20.Parvanova AI, Trevisan R, Iliev IP, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55:1456–1462. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 21.Jauregui A, Mintz DH, Mundel P, Fornoni A. Role of altered insulin signaling pathways in the pathogenesis of podocyte malfunction and microalbuminuria. Curr Opin Nephrol Hypertens. 2009;18:539–545. doi: 10.1097/MNH.0b013e32832f7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 23.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 24.Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. Bmj. 2004;328:1105. doi: 10.1136/bmj.38070.450891.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 26.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koya D, Haneda M, Nakagawa H, et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. Faseb J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 28.Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 29.Li JJ, Kwak SJ, Jung DS, et al. Podocyte biology in diabetic nephropathy. Kidney Int Suppl. 2007:S36–42. doi: 10.1038/sj.ki.5002384. [DOI] [PubMed] [Google Scholar]
- 30.Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol. 2008;294:F830–839. doi: 10.1152/ajprenal.00266.2007. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol. 2004;15:1475–1487. doi: 10.1097/01.asn.0000127988.42710.a7. [DOI] [PubMed] [Google Scholar]
- 32.Reiser J, Mundel P. Dual effects of RAS blockade on blood pressure and podocyte function. Curr Hypertens Rep. 2007;9:403–408. doi: 10.1007/s11906-007-0074-7. [DOI] [PubMed] [Google Scholar]
- 33.Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- 34.Shibata S, Nagase M, Yoshida S, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–1376. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 35.Ichihara A, Hayashi M, Kaneshiro Y, et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114:1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakoda M, Ichihara A, Kurauchi-Mito A, et al. Aliskiren Inhibits Intracellular Angiotensin II Levels Without Affecting (Pro)renin Receptor Signals in Human Podocytes. Am J Hypertens. doi: 10.1038/ajh.2009.273. [DOI] [PubMed] [Google Scholar]
- 37.Coward RJ, Welsh GI, Yang J, et al. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54:3095–3102. doi: 10.2337/diabetes.54.11.3095. [DOI] [PubMed] [Google Scholar]
- 38.Tejada T, Catanuto P, Ijaz A, et al. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;73:1385–1393. doi: 10.1038/ki.2008.109. [DOI] [PubMed] [Google Scholar]
- 39.Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catanuto P, Doublier S, Lupia E, et al. 17 beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 2009;75:1194–1201. doi: 10.1038/ki.2009.69. [DOI] [PubMed] [Google Scholar]
- 41.Reddy GR, Kotlyarevska K, Ransom RF, Menon RK. The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens. 2008;17:32–36. doi: 10.1097/MNH.0b013e3282f2904d. [DOI] [PubMed] [Google Scholar]
- 42.Flyvbjerg A, Bennett WF, Rasch R, Kopchick JJ, Scarlett JA. Inhibitory effect of a growth hormone receptor antagonist (G120K-PEG) on renal enlargement, glomerular hypertrophy, and urinary albumin excretion in experimental diabetes in mice. Diabetes. 1999;48:377–382. doi: 10.2337/diabetes.48.2.377. [DOI] [PubMed] [Google Scholar]
- 43.Thirone AC, Scarlett JA, Gasparetti AL, et al. Modulation of growth hormone signal transduction in kidneys of streptozotocin-induced diabetic animals: effect of a growth hormone receptor antagonist. Diabetes. 2002;51:2270–2281. doi: 10.2337/diabetes.51.7.2270. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Zhou J, Minto AW, et al. Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int. 2006;70:882–891. doi: 10.1038/sj.ki.5001624. [DOI] [PubMed] [Google Scholar]
- 45.Lennon R, Pons D, Sabin MA, et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transplant. 2009;24:3288–3296. doi: 10.1093/ndt/gfp302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bussolati B, Deregibus MC, Fonsato V, et al. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;16:1936–1947. doi: 10.1681/ASN.2004080629. [DOI] [PubMed] [Google Scholar]
- 47.Gutwein P, Abdel-Bakky MS, Schramme A, et al. CXCL16 is expressed in podocytes and acts as a scavenger receptor for oxidized low-density lipoprotein. Am J Pathol. 2009;174:2061–2072. doi: 10.2353/ajpath.2009.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodman HC, Sellers AL, Smith S, 3rd, Marmorston J. Endocrine influences on proteinuria in the rat; effect of ACTH. Proc Soc Exp Biol Med. 1951;77:725–728. doi: 10.3181/00379727-77-18907. [DOI] [PubMed] [Google Scholar]
- 49.Havt A, Schally AV, Halmos G, et al. The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci U S A. 2005;102:17424–17429. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu H, Suzuki S, Sellitti DF, et al. Expression of a thyroglobulin (Tg) variant in mouse kidney glomerulus. Biochem Biophys Res Commun. 2009;389:269–273. doi: 10.1016/j.bbrc.2009.08.129. [DOI] [PubMed] [Google Scholar]
- 51.Morano S, Cipriani R, Cerrito MG, et al. Angiotensin-converting enzyme inhibition modulates high-glucose-induced extracellular matrix changes in mouse glomerular epithelial cells. Nephron Exp Nephrol. 2003;95:e30–35. doi: 10.1159/000073021. [DOI] [PubMed] [Google Scholar]
- 52.Lenz O, Elliot SJ, Stetler-Stevenson WG. Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol. 2000;11:574–581. doi: 10.1681/ASN.V113574. [DOI] [PubMed] [Google Scholar]
- 53.Sharma K, Ziyadeh FN, Alzahabi B, et al. Increased renal production of transforming growth factor-beta1 in patients with type II diabetes. Diabetes. 1997;46:854–859. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- 54.Blanco S, Vaquero M, Gomez-Guerrero C, Lopez D, Egido J, Romero R. Potential role of angiotensin-converting enzyme inhibitors and statins on early podocyte damage in a model of type 2 diabetes mellitus, obesity, and mild hypertension. Am J Hypertens. 2005;18:557–565. doi: 10.1016/j.amjhyper.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 55.Gloy J, Henger A, Fischer KG, et al. Angiotensin II depolarizes podocytes in the intact glomerulus of the Rat. J Clin Invest. 1997;99:2772–2781. doi: 10.1172/JCI119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benigni A, Tomasoni S, Gagliardini E, et al. Blocking angiotensin II synthesis/activity preserves glomerular nephrin in rats with severe nephrosis. J Am Soc Nephrol. 2001;12:941–948. doi: 10.1681/ASN.V125941. [DOI] [PubMed] [Google Scholar]
- 57.Bonnet F, Cooper ME, Kawachi H, Allen TJ, Boner G, Cao Z. Irbesartan normalises the deficiency in glomerular nephrin expression in a model of diabetes and hypertension. Diabetologia. 2001;44:874–877. doi: 10.1007/s001250100546. [DOI] [PubMed] [Google Scholar]
- 58.Winn MP, Conlon PJ, Lynn KL, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 59.Lenz O, Fornoni A. Renin-angiotensin system blockade and diabetes: moving the adipose organ from the periphery to the center. Kidney Int. 2008;74:851–853. doi: 10.1038/ki.2008.391. [DOI] [PubMed] [Google Scholar]
- 60.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 61.Epstein M, Williams GH, Weinberger M, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1:940–951. doi: 10.2215/CJN.00240106. [DOI] [PubMed] [Google Scholar]
- 62.Epstein M. Re-examining RAS-blocking treatment regimens for abrogating progression of chronic kidney disease. Nat Clin Pract Nephrol. 2009;5:12–13. doi: 10.1038/ncpneph0980. [DOI] [PubMed] [Google Scholar]
- 63.Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150:776–783. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M. Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N Engl J Med. 1985;312:1412–1417. doi: 10.1056/NEJM198505303122202. [DOI] [PubMed] [Google Scholar]
- 65.Deinum J, Ronn B, Mathiesen E, Derkx FH, Hop WC, Schalekamp MA. Increase in serum prorenin precedes onset of microalbuminuria in patients with insulin-dependent diabetes mellitus. Diabetologia. 1999;42:1006–1010. doi: 10.1007/s001250051260. [DOI] [PubMed] [Google Scholar]
- 66.Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Itoh H. The (pro)renin receptor and the kidney. Semin Nephrol. 2007;27:524–528. doi: 10.1016/j.semnephrol.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 68.Ekstrand AV, Groop PH, Gronhagen-Riska C. Insulin resistance precedes microalbuminuria in patients with insulin-dependent diabetes mellitus. Nephrol Dial Transplant. 1998;13:3079–3083. doi: 10.1093/ndt/13.12.3079. [DOI] [PubMed] [Google Scholar]
- 69.Yip J, Mattock MB, Morocutti A, Sethi M, Trevisan R, Viberti G. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;342:883–887. doi: 10.1016/0140-6736(93)91943-g. [DOI] [PubMed] [Google Scholar]
- 70.Yip J, Mattock M, Sethi M, Morocutti A, Viberti G. Insulin resistance in family members of insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;341:369–370. doi: 10.1016/0140-6736(93)90167-f. [DOI] [PubMed] [Google Scholar]
- 71.Mykkanen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47:793–800. doi: 10.2337/diabetes.47.5.793. [DOI] [PubMed] [Google Scholar]
- 72.Miyazaki Y, Cersosimo E, Triplitt C, DeFronzo RA. Rosiglitazone decreases albuminuria in type 2 diabetic patients. Kidney Int. 2007;72:1367–1373. doi: 10.1038/sj.ki.5002516. [DOI] [PubMed] [Google Scholar]
- 73.Ohtomo S, Izuhara Y, Takizawa S, et al. Thiazolidinediones provide better renoprotection than insulin in an obese, hypertensive type II diabetic rat model. Kidney Int. 2007;72:1512–1519. doi: 10.1038/sj.ki.5002570. [DOI] [PubMed] [Google Scholar]
- 74.Lennon R, Welsh GI, Singh A, et al. Rosiglitazone enhances glucose uptake in glomerular podocytes using the glucose transporter GLUT1. Diabetologia. 2009 doi: 10.1007/s00125-009-1423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Welsh GI, Hale LJ, Eremina V, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 12:329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hegedus L, Christensen NJ, Mogensen CE, Gundersen HJ. Oral glucose increases urinary albumin excretion in normal subjects but not in insulin-dependent diabetics. Scand J Clin Lab Invest. 1980;40:479–482. doi: 10.3109/00365518009101871. [DOI] [PubMed] [Google Scholar]
- 77.Mogensen CE, Christensen NJ, Gundersen HJ. The acute effect of insulin on heart rate, blood pressure, plasma noradrenaline and urinary albumin excretion. The role of changes in blood glucose. Diabetologia. 1980;18:453–457. doi: 10.1007/BF00261700. [DOI] [PubMed] [Google Scholar]
- 78.Savage S, Estacio RO, Jeffers B, Schrier RW. Increased complications in noninsulin-dependent diabetic patients treated with insulin versus oral hypoglycemic agents: a population study. Proc Assoc Am Physicians. 1997;109:181–189. [PubMed] [Google Scholar]
- 79.Iliescu R, Reckelhoff JF. Sex and the kidney. Hypertension. 2008;51:1000–1001. doi: 10.1161/HYPERTENSIONAHA.107.101345. [DOI] [PubMed] [Google Scholar]
- 80.Maric C. Sex, diabetes and the kidney. Am J Physiol Renal Physiol. 2009;296:F680–688. doi: 10.1152/ajprenal.90505.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med. 2008;5 (Suppl A):S3–S10. doi: 10.1016/j.genm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Maric C, Forsblom C, Thorn L, Waden J, Groop PH. Association between testosterone, estradiol and sex hormone binding globulin levels in men with type 1 diabetes with nephropathy. Steroids. doi: 10.1016/j.steroids.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mankhey RW, Bhatti F, Maric C. 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288:F399–405. doi: 10.1152/ajprenal.00195.2004. [DOI] [PubMed] [Google Scholar]
- 84.Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol. 2004;15:1546–1556. doi: 10.1097/01.asn.0000128219.65330.ea. [DOI] [PubMed] [Google Scholar]
- 85.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006;110:267–278. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 86.Jalovaara K, Santaniemi M, Timonen M, et al. Low serum adiponectin level as a predictor of impaired glucose regulation and type 2 diabetes mellitus in a middle-aged Finnish population. Metabolism. 2008;57:1130–1134. doi: 10.1016/j.metabol.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 87.Takemura Y, Walsh K, Ouchi N. Adiponectin and cardiovascular inflammatory responses. Curr Atheroscler Rep. 2007;9:238–243. doi: 10.1007/s11883-007-0025-4. [DOI] [PubMed] [Google Scholar]
- 88.Zoccali C, Mallamaci F. Obesity, diabetes, adiponectin and the kidney: a podocyte affair. Nephrol Dial Transplant. 2008;23:3767–3770. doi: 10.1093/ndt/gfn517. [DOI] [PubMed] [Google Scholar]
- 89.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guebre-Egziabher F, Bernhard J, Funahashi T, Hadj-Aissa A, Fouque D. Adiponectin in chronic kidney disease is related more to metabolic disturbances than to decline in renal function. Nephrol Dial Transplant. 2005;20:129–134. doi: 10.1093/ndt/gfh568. [DOI] [PubMed] [Google Scholar]
- 91.Jorsal A, Tarnow L, Frystyk J, et al. Serum adiponectin predicts all-cause mortality and end stage renal disease in patients with type I diabetes and diabetic nephropathy. Kidney Int. 2008;74:649–654. doi: 10.1038/ki.2008.201. [DOI] [PubMed] [Google Scholar]
- 92.Saraheimo M, Forsblom C, Fagerudd J, et al. Serum adiponectin is increased in type 1 diabetic patients with nephropathy. Diabetes Care. 2005;28:1410–1414. doi: 10.2337/diacare.28.6.1410. [DOI] [PubMed] [Google Scholar]
- 93.Saraheimo M, Forsblom C, Thorn L, et al. Serum adiponectin and progression of diabetic nephropathy in patients with type 1 diabetes. Diabetes Care. 2008;31:1165–1169. doi: 10.2337/dc07-2306. [DOI] [PubMed] [Google Scholar]
- 94.Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 95.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168:397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 96.Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008;19:1613–1619. doi: 10.1681/ASN.2007111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tentori F, Hunt WC, Stidley CA, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 98.Kuhlmann A, Haas CS, Gross ML, et al. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol. 2004;286:F526–533. doi: 10.1152/ajprenal.00316.2003. [DOI] [PubMed] [Google Scholar]
- 99.Deb DK, Sun T, Wong KE, et al. Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int. doi: 10.1038/ki.2010.22. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci U S A. 2008;105:15896–15901. doi: 10.1073/pnas.0803751105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Z, Sun L, Wang Y, et al. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 2008;73:163–171. doi: 10.1038/sj.ki.5002572. [DOI] [PubMed] [Google Scholar]
- 102.Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 2003;88:327–331. doi: 10.1002/jcb.10343. [DOI] [PubMed] [Google Scholar]
- 103.Deb DK, Chen Y, Zhang Z, et al. 1,25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-{kappa}B pathway. Am J Physiol Renal Physiol. 2009;296:F1212–1218. doi: 10.1152/ajprenal.00002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yuan W, Pan W, Kong J, et al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282:29821–29830. doi: 10.1074/jbc.M705495200. [DOI] [PubMed] [Google Scholar]
- 105.Flyvbjerg A. The role of growth hormone in the pathogenesis of diabetic kidney disease. Pediatr Endocrinol Rev. 2004;1 (Suppl 3):525–529. [PubMed] [Google Scholar]
- 106.Cummings EA, Sochett EB, Dekker MG, Lawson ML, Daneman D. Contribution of growth hormone and IGF-I to early diabetic nephropathy in type 1 diabetes. Diabetes. 1998;47:1341–1346. doi: 10.2337/diab.47.8.1341. [DOI] [PubMed] [Google Scholar]
- 107.Serri O, Beauregard H, Brazeau P, et al. Somatostatin analogue, octreotide, reduces increased glomerular filtration rate and kidney size in insulin-dependent diabetes. Jama. 1991;265:888–892. [PubMed] [Google Scholar]
- 108.Doi T, Striker LJ, Quaife C, et al. Progressive glomerulosclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulinlike growth factor-1. Am J Pathol. 1988;131:398–403. [PMC free article] [PubMed] [Google Scholar]
- 109.Bellush LL, Doublier S, Holland AN, Striker LJ, Striker GE, Kopchick JJ. Protection against diabetes-induced nephropathy in growth hormone receptor/binding protein gene-disrupted mice. Endocrinology. 2000;141:163–168. doi: 10.1210/endo.141.1.7284. [DOI] [PubMed] [Google Scholar]
- 110.Nakamura T, Kawagoe Y, Ogawa H, et al. Effect of low-density lipoprotein apheresis on urinary protein and podocyte excretion in patients with nephrotic syndrome due to diabetic nephropathy. Am J Kidney Dis. 2005;45:48–53. doi: 10.1053/j.ajkd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 111.Mayrhofer C, Krieger S, Huttary N, et al. Alterations in fatty acid utilization and an impaired antioxidant defense mechanism are early events in podocyte injury: a proteomic analysis. Am J Pathol. 2009;174:1191–1202. doi: 10.2353/ajpath.2009.080654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sieber J, Lindenmeyer MT, Kampe K, et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol. 299:F821–829. doi: 10.1152/ajprenal.00196.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yamada S, Bandai S, Masutani K, et al. Focal segmental glomerulosclerosis in a patient with isolated ACTH deficiency and reversible hypothyroidism. Clin Exp Nephrol. 2009 doi: 10.1007/s10157-009-0228-9. [DOI] [PubMed] [Google Scholar]
- 114.Rauen T, Michaelis A, Floege J, Mertens PR. Case series of idiopathic membranous nephropathy with long-term beneficial effects of ACTH peptide 1–24. Clin Nephrol. 2009;71:637–642. doi: 10.5414/cnp71637. [DOI] [PubMed] [Google Scholar]
- 115.Chen HS, Wu TE, Jap TS, et al. Subclinical hypothyroidism is a risk factor for nephropathy and cardiovascular diseases in Type 2 diabetic patients. Diabet Med. 2007;24:1336–1344. doi: 10.1111/j.1464-5491.2007.02270.x. [DOI] [PubMed] [Google Scholar]
- 116.Miura M, Nomoto Y, Sakai H. Prevention of progressive renal failure by levothyroxine sodium in a diabetic patient with renal insufficiency and hypothyroidism. Intern Med. 1992;31:251–255. doi: 10.2169/internalmedicine.31.251. [DOI] [PubMed] [Google Scholar]