Abstract
Background
The value of quantitative computed tomography (QCT) to identify chronic obstructive pulmonary disease (COPD) phenotypes is increasingly appreciated. We hypothesized that QCT-defined emphysema and airway abnormalities relate to St. George's Respiratory Questionnaire (SGRQ) and BODE.
Methods
1,200 COPDGene subjects meeting GOLD criteria for COPD with QCT analysis were included. Total lung emphysema was measured using density mask technique with a -950 HU threshold. An automated program measured mean wall thickness (WT), wall area percent (WA%) and pi10 in six segmental bronchi. Separate multivariate analyses examined the relative influence of airway measures and emphysema on SGRQ and BODE.
Results
In separate models predicting SGRQ score, a one unit standard deviation (SD) increase in each airway measure predicted higher SGRQ scores (for WT, 1.90 points higher, p=0.002; for WA%, 1.52 points higher, p=0.02; for pi10, 2.83 points higher p<0.001). The comparable increase in SGRQ for a one unit SD increase in percent emphysema in these models was relatively weaker, significant only in the pi10 model (for percent emphysema, 1.45 points higher, p=0.01). In separate models predicting BODE, a one unit SD increase in each airway measure predicted higher BODE scores (for WT, 1.07 fold increase, p<0.001; for WA%, 1.20 fold increase, p<0.001; for pi10, 1.16 fold increase, p<0.001). In these models, emphysema more strongly influenced BODE (range 1.24-1.26 fold increase, p<0.001).
Conclusion
Emphysema and airway disease both relate to clinically important parameters. The relative influence of airway disease is greater for SGRQ; the relative influence of emphysema is greater for BODE.
Keywords: Imaging, COPD, emphysema
Introduction
The importance of identifying distinct phenotypes within chronic obstructive pulmonary disease (COPD) is becoming increasingly appreciated, both for the purpose of establishing prognosis but also for identifying appropriate patients for therapies 1. Although multiple methods for describing the heterogeneity intrinsic to this disease have been developed, quantitative computed tomography (QCT) may have significant value as a noninvasive means to identify distinct phenotypes in COPD. To establish the validity of COPD phenotyping based on QCT characteristics, correlations must be made in large groups of comprehensively assessed individuals with COPD. We have previously reported that CT measures of emphysema and airways disease relate to COPD exacerbation frequency, a significant disease outcome for COPD patients 2. The relationship between QCT phenotype and other important patient-centered outcomes is unknown. In the current study, we describe a comprehensive evaluation of 1,200 patients with COPD who underwent clinical, physiological, and radiological evaluation with the goal of assessing the relationship between QCT-defined emphysema severity, airway abnormality and clinically relevant outcomes including the St. George's Respiratory Questionnaire (SGRQ) and BODE index 3,4.
Methods
Patient Selection
The COPDGene Study is a National Heart Lung Blood Institute-funded multicenter investigation to examine the genetic epidemiology of smoking-related lung disease. Subjects were selected for participation in the study based on the following criteria: age 45-80 years, non-smoker or cigarette smoking ≥ 10 pack years, and willingness to undergo study-related testing that included spirometry, CT scan of the chest, and blood collection for biomarker and genetic analysis. Subjects included in our analysis were selected from the first 2,500 dataset from the COPDGene study, April 2010, and included 1,200 subjects who met GOLD criteria 5 for Stages 1–4 fixed airflow obstruction with a post-bronchodilator FEV1/FVC ratio ≤ 0.7 and additionally had complete QCT based emphysema and airway analyses available. All analyses presented utilize the 1,200 subjects except BODE analyses where a smaller subset of 1,179 had complete data available to calculate BODE. All participants provided written informed consent. This research protocol was approved by the institutional review board at each participating institution.
Data Collection and Exacerbation Determination
Demographic data, smoking and medical history were collected via interview or self-administered questionnaires. Dyspnea was quantified using the modified Medical Research Council dyspnea scale (MMRC) 6 which is a 5-point scale that asks respondents to rate dyspnea from 0 (absent) to 4 (dyspnea when dressing/undressing). The SGRQ is a health related quality of life (HRQL), obstructive lung disease-specific instrument with three domains (symptoms, activities and impact), all scored from 0-100. Higher scores correspond to worse HRQL 3. All subjects underwent standard 6-min walk distance (6MWD). BODE index was calculated using 6MWD, forced expiratory volume in one second (FEV1)% predicted, MMRC and body mass index (BMI) as previously described 7.
Physiologic testing
Patients underwent spirometry before and after the administration of short acting bronchodilating medication (albuterol). All spirometric tracings were independently reviewed to ensure that ATS criteria were met 8.
Imaging
Objective analysis of the lung parenchyma and airways was performed on volumetric CT scans of the chest obtained at full inflation. All CT scans were obtained under a standardized protocol2. Parenchymal analysis and airway analyses were performed using VIDA Pulmonary Workstation 2 (www.vidadiagnostics.com). All lung area with a CT attenuation value of less than -950 Hounsfield Units (HU) on the inspiratory scan was considered to be emphysematous tissue. Reconstruction of CT inspiratory images allowed for estimation of total lung capacity (TLC). TLC percent predicted was calculated based on a previously published equation9. Airway morphology was examined in six segmental airways: apical segment, right upper lobe (RB1), lateral segment, right middle lobe (RB4), posterior basal segment, right lower lobe (RB10), apicoposterior segment, left upper lobe (LB1), superior lingular segment (LB4), and posterior basal segment, left lower lobe (LB10) 10. CT-based metrics of airway disease included mean bronchial wall thickness calculated as an average of the six segmental values for each subject, wall area percent (100* wall area/total bronchial area) 11 obtained at the same sites as those used for bronchial wall thickness, and the square root of the wall area of a theoretical airway of 10 mm lumenal perimeter (Pi10), as previously described 12,13.
Statistical Analyses
All statistical analyses were conducted with the SAS statistical analysis package (version 9.2; SAS Institute Inc., Cary, NC). For univariate and multivariate analyses, data are presented for “normalized” and “unnormalized” radiologic parameters. For “normalized” radiologic parameters, airway disease measures and emphysema values have been scaled and centered as follows: (value – mean)/standard deviation. Hence a one unit increase in a normalized measure is one standard deviation. For SGRQ total score, multivariate linear regression models were used adjusted for age, gender, current smoking status, height, FEV1% predicted and scanner type. For BODE, zero-inflated Poisson regression was used adjusted for age, gender, smoking status and scanner type. This model is more appropriate, as the BODE distribution of values in this cohort was skewed with more subjects displaying lower BODE scores (see Figure 1). P-values less than 0.05 were considered to be statistically significant. The three-dimensional, smoothed surface plots of the raw data (Figures 2A and 2C) were created with SAS 9.2 statistical software, using PROC G3GRID, which uses spline interpolation as the smoothing algorithm. The density plots of the raw data (Figures 2B and 2D) were also created with SAS 9.2 statistical software, using PROC KDE which utilizes a nonparametric technique for density estimation.
Figure 1.
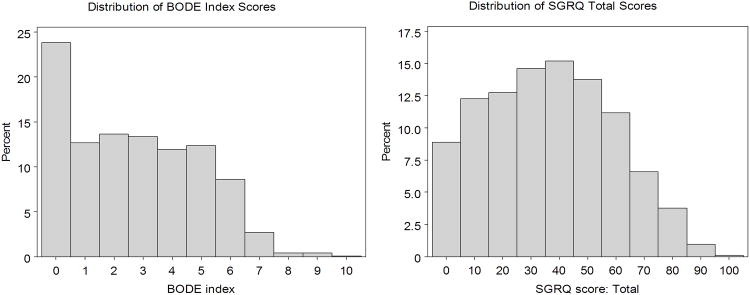
Frequency distribution of BODE index scores (left panel) and St. George's Respiratory Questionnaire (SGRQ) total scores (right panel).
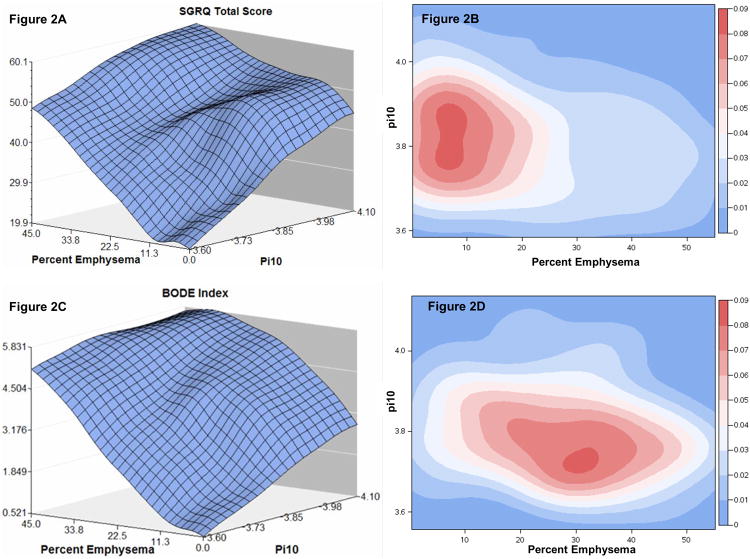
Figure 2.
Figure 2A. Three dimensional plot demonstrating the relationship between increasing pi10, emphysema % and St. George's Respiratory Questionnaire score (left upper panel).
Figure 2B. Density plot of COPD subjects with SGRQ score approximately 90th percentile (score ≥ 64, n=146) demonstrating degree of pi10 and emphysema in this subgroup. Percent of subjects displayed at right, high density in red, low density in blue (right upper panel).
Figure 2C. Three dimensional plot demonstrating the relationship between increasing pi10, emphysema% and BODE index score (left lower panel).
Figure 2D. Density plot of COPD subjects with BODE score approximately 90th percentile (score ≥ 6, n=142) demonstrating the degree of pi10 and emphysema in this subgroup. Percent of subjects displayed at right, high density in red, low density in blue (right lower panel).
Results
Data from 1,200 subjects were available for analysis. Their demographic and physiological data are outlined in Table 1. This dataset is roughly split between men and women. The majority were ex-smokers (63.2%) with moderate COPD by spirometric criteria, median FEV1% predicted 56%. A broad range of disease severity as defined by BODE and SGRQ were also present (see Figure 1).
Table 1. Baseline Demographics & Physiological data.
| Median (IQR) | |
|---|---|
| Number of subjects | 1,200 |
| Male Gender %* | 53.3% |
| Current smokers %* | 36.8% |
| Age in years | 65.0 (47.9, 70.6) |
| FEV1% predicted | 56 (38, 74) |
| FEV1/FVC | 0.53 (0.40, 0.63) |
| Pack-years | 46 (35, 68) |
| Body mass index in kg/m2 | 27.1 (23.5, 31.1) |
| Six minute walk distance (meters) | 381 (288, 470) |
| MMRC | 2 (0, 3) |
| BODE index score | 2 (1, 4) |
| St. George's Respiratory Questionnaire total score | 35.3 (17.2, 52.3) |
| Percent emphysema | 9.7 (3.6, 21.2) |
| Segmental bronchial wall thickness in mm | 1.55 (1.44, 1.68) |
| Segmental wall area percent | 62.34 (60.32, 64.45) |
| Pi10 | 3.46 (3.69, 3.84) |
Reported as mean percentage and not median
In univariate models, airway measures and emphysema demonstrated statistically significant associations with BODE and SGRQ (see Table 2), except segmental wall thickness which did not show a statistically significant relationship with BODE. Because the units for each of these radiologic predictors differs widely with respect to the range of values seen in the population, a better interpretation of the relative importance of each can be seen with the normalized parameter estimates which are also displayed in Table 2. For normalized estimates, a one unit change corresponds to one standard deviation change for that variable. When examining the normalized estimates, the strongest signal for SGRQ is seen for pi10. The strongest signal for BODE is seen with percent emphysema.
Table 2.
Univariate associations between unnormalized and normalized radiologic parameters, St. George's Respiratory Questionnaire (SGRQ) total score and BODE.
| SGRQ† | Unnormalized Parameter Estimate (95% CI) | Normalized Parameter Estimate (95% CI) | P-value* |
|---|---|---|---|
| Percent emphysema | 0.53 (0.45, 0.61) | 5.82 (4.91, 6.72) | <0.001 |
| Segmental wall thickness (mm) | 8.72 (2.91, 14.53) | 1.95 (0.65, 3.25) | 0.003 |
| Segmental wall area percent | 1.96 (1.57, 2.35) | 6.27 (5.02, 7.52) | <0.001 |
| Pi10 | 56.28 (46.91, 65.64) | 7.20 (6.00, 8.40) | <0.001 |
| BODE† | |||
| Percent emphysema | 1.02 (1.02, 1.02) | 1.23 (1.20,1.26) | <0.001 |
| Segmental wall thickness (mm) | 1.10 (0.93, 1.29) | 1.02 (0.99, 1.06) | 0.27 |
| Segmental wall area percent | 1.03 (1.02, 1.05) | 1.11 (1.07, 1.16) | <0.001 |
| Pi10 | 2.20 (1.66, 2.91) | 1.11 (1.07, 1.15) | <0.001 |
For SGRQ models, parameter estimate is the increase in SGRQ score for a one unit increase in unnormalized radiologic parameter. For BODE models, parameter estimate is the fold-increase in BODE index score for a one unit increase in the unnormalized radiologic parameter. All models are adjusted for scanner type. For normalized radiologic parameters, airway disease measures and emphysema values have been scaled and centered as follows: (value – mean)/standard deviation. Hence a one unit increase in a normalized measure is one standard deviation.
P-value for significance of parameter estimate in the model which is identical for unnormalized and normalized estimates.
To visualize the relationships between pi10 and emphysema with respect to SGRQ, a three dimensional plot was created (Figure 2A), which demonstrates that regardless of emphysema extent, increasing pi10 is associated with increasing SGRQ score. Similarly, increasing emphysema is also associated with increasing SGRQ score regardless of pi10. In order to understand these relationships further, we selected patients in approximately the highest 10th percentile of SGRQ scores and created density plots, depicting the relationship between emphysema and airway measures (Figure 2B). Although a wide range of pi10 and emphysema values are still represented, for the highest density of subjects depicted in red, normalized pi10 values were higher than the average pi10 (3.78) whereas emphysema values were lower than the group average for percent emphysema (13.9%).
By contrast, a similar three dimensional plot for BODE (Figure 2C), showing the independent relationship between pi10, emphysema and BODE revealed that even at extreme values of pi10 in the absence of emphysema, increases in BODE are modest. A density plot for subjects with approximately the 10th highest percentile BODE scores is depicted in Figure 2D. While a wide range of pi10 and emphysema values are present, the highest density of subjects in red have a much greater amount of emphysema than either the group mean (13.9%) or subjects with SGRQ scores in Figure 2B whereas pi10 values are similar to the group mean (3.78) but lower than subjects with high SGRQ scores in Figure 2B. Additional density plots by airway measure for the entire cohort are presented in Supplemental Figures S1-S3.
Further analysis of the relationships between airway disease measures, emphysema, SGRQ and BODE were accomplished via individual multivariate models including each airway disease measure separately to compare their relative effect to emphysema, adjusting for other relevant confounders. The results of analyses predicting SGRQ are presented in Tables 3A and 3B. In Table 3B, radiologic predictors are normalized such that the relative importance of airway measures to emphysema can be compared.
Table 3.
Table 3A. Multivariate analysis predicting St. George's Respiratory Questionnaire total score using ununnormalized radiologic parameters* (n=1,200).
| Airway Disease Measure | Airway Parameter (95% CI) | % Emphysema Parameter (95% CI) |
|---|---|---|
| Segmental Wall Thickness (mm) | 8.49 (3.19, 13.79) p=0.002 | 0.08 (-0.02, 0.18) p=0.13 |
| Segmental Wall Area Percent | 0.47 (0.08, 0.85) p=0.02 | 0.10 (-0.001, 0.21) p=0.05 |
| Pi10 | 22.13 (12.99, 31.29) p<0.001 | 0.13 (0.03, 0.23) 0.01 |
| Table 3B. Multivariate analysis of St. George's Respiratory Questionnaire total score using normalized radiologic parameters* (n=1,200). | ||
|---|---|---|
| Airway Disease Measure | Airway Parameter (95% CI) | % Emphysema Parameter (95% CI) |
| Segmental Wall Thickness (mm) | 1.90 (0.72, 3.09) p=0.002 | 0.83 (-0.26, 1.93) p=0.13 |
| Segmental Wall Area Percent | 1.52 (0.27, 2.76) p=0.02 | 1.14 (-0.02, 2.30) p=0.05 |
| Pi10 | 2.83 (1.66, 4.00) p<0.001 | 1.45 (0.33, 2.57) p=0.01 |
Models additionally adjusted for age, gender, height, FEV1% predicted, current smoking status and scanner type. Parameter estimate is the increase in SGRQ score for a one unit increase in radiologic parameter. For normalized radiologic parameters, airway disease measures and emphysema values have been scaled and centered as follows: (value – mean)/standard deviation. Hence a one unit increase in a normalized measure is one standard deviation.
In Table 3A, we can see that each airway parameter significantly predicts increasing SGRQ score, where percent emphysema has also been accounted for. A one mm increase in segmental wall thickness results in an 8.49 increase in SGRQ score (95% CI 3.19, 13.79; p=0.002); a one percent increase in wall area percent results in a 0.47 point increase in SGRQ (95% CI 0.08, 0.85; p=0.02); a one unit increase in pi10 results in a 22.13 point increase in SGRQ (95% CI 12.99, 31.29; p<0.001). Only in the model utilizing pi10 is emphysema statistically significant, where a positive association between increasing emphysema and greater SGRQ score is seen. Table 3B further emphasizes the relative importance of airway parameters to emphysema in predicting SGRQ as the normalized airway estimates are all significantly larger than the emphysema estimates. A one unit SD increase in segmental wall thickness results in a 1.90 point increase in SGRQ score (95% CI 0.72, 3.09; p=0.002); a one unit SD increase in WA% results in a 1.52 point increase in SGRQ (95% CI 0.27, 2.76; p=0.02); a one SD unit increase in pi10 results in a 2.83 point increase in SGRQ (95% CI 1.66, 4.00; p<0.001). In the model using pi10, a one unit SD increase in emphysema results in a 1.45 point increase in SGRQ (95% CI 0.33, 2.57; p=0.02). As all parameters in Table 3B are for a SD change for that predictor, the scale of change for each predictor in all models is comparable. To ensure differences in full inspiratory capacity did not bias results, TLC % predicted calculated from the CT was also added to Table 3 models but did not demonstrate any significant change in results (data not shown).
Additional multivariate models for SGRQ subscores using normalized pi10 and emphysema measures are presented in Supplemental Table 1. This analysis demonstrates the strongest relationship for radiologic parameters exists with the activity subscore. A one unit SD increase for pi10 results in a 3.38 point increase in SGRQ activity subscore (95% CI 1.91, 4.84; p<0.001), and a one unit SD increase in emphysema resulting in a 3.06 increase in activity subscore (95% CI 1.54, 4.60; p<0.001.
Multivariate analyses for predicting BODE index are reported in Tables 4A and 4B, unnormalized and normalized radiologic parameters respectively. In 4A, each of the three airway parameters predict increasing BODE index when additionally adjusted for emphysema. A one mm increase in segmental wall thickness results in a 1.36 fold increase in BODE index score (95% CI 1.14, 1.62; p<0.001); a one percent increase in wall area percent results in a 1.06 fold increase in BODE index score (95% CI 1.05, 1.07; p<0.001); a one unit increase in pi10 results in a 3.30 fold increase in BODE index score (95% CI 2.48, 4.40; p<0.001). In all three models, an increase in emphysema is also associated with approximately a 1.02 fold increase in BODE index score (p<0.001 for all models). Once again, all parameters in Table 4B are for a SD change in that predictor such that the scale of change for each predictor in all models is comparable. Here we see that opposed to the model for SGRQ, the emphysema parameter is larger than the airway parameters for every model and statistically significant.
Table 4.
Table 4A. Multivariate analysis predicting BODE index score using unnormalized radiologic parameters* (n=1.179).
| Airway Disease Measure | Airway Parameter Estimate expressed as Fold Change in BODE (95% CI) | % Emphysema Parameter Estimate expressed as Fold Change in BODE (95% CI) |
|---|---|---|
| Segmental Wall Thickness (mm) | 1.36 (1.14, 1.62) p<0.001 | 1.02 (1.02, 1.02) p<0.001 |
| Segmental Wall Area Percent | 1.06 (1.05, 1.07) p<0.001 | 1.02 (1.02, 1.02) p<0.001 |
| Pi10 | 3.30 (2.48, 4.40) p<0.001 | 1.02 (1.02, 1.02) p<0.001 |
| Table 4B. Multivariate analysis of BODE index score using normalized radiologic parameters*. (n=1,179). | ||
|---|---|---|
| Airway Disease Measure | Airway Parameter Estimate expressed as Fold Change in BODE Normalized | % Emphysema Parameter Estimate expressed as Fold Change in BODE Normalized |
| Segmental Wall Thickness (mm) | 1.07 (1.03, 1.11) p<0.001 | 1.24 (1.20, 1.27) p<0.001 |
| Segmental Wall Area Percent | 1.20 (1.15, 1.25) p<0.001 | 1.26 (1.23, 1.30) p<0.001 |
| Pi10 | 1.16 (1.12, 1.21) p<0.001 | 1.25 (1.21, 1.29) p<0.001 |
Model additionally adjusted for age, gender, current smoking status, and scanner type. Parameter estimate is the fold change in BODE index score for a one unit increase in radiologic parameter. For normalized radiologic parameters, airway disease measures and emphysema values have been scaled and centered as follows: (value– mean)/standard deviation. Hence a one unit increase in a normalized measure is one standard deviation.
The fold increase in BODE score for a one SD increase in emphysema ranges between 1.24 and 1.26 (p<0.001) in all three models, exceeding the upper bound of the 95% confidence interval for the fold-changes seen in airway parameters. The relative weight for the three airway parameters is less, yet still independently predictive when adjusted for emphysema. A one unit SD increase in segmental wall thickness results in a 1.07 fold increase in BODE index score (p<0.001), a one unit SD increase in segmental wall area percent results in a 1.20 fold increase in BODE index score (p<0.001); a one unit SD increase in pi10 results in a 1.16 fold increase in BODE index score (p<0.001). To correct for differences in inspiratory capacity during the imaging maneuver, Table 4 models were additionally adjusted for TLC % predicted that was calculated from reconstruction of inspiratory CT images. This did not result in significant change in results (data not shown).
To better understand the relationship between radiologic parameters and BODE components, Spearman's correlations were performed between radiologic parameters and BODE components. These results are displayed in Supplemental Table 2. Emphysema correlates most strongly with FEV1% predicted (r = -0.54, p<0.001). Pi10 correlates most strongly with FEV1% predicted, r = -0.37, p<0.001. Supplemental Table 3 displays additional analyses subdividing the cohort based on presence (≥ 75th percentile) or absence (≤25th percentile) of emphysema and airways disease (utilizing pi10 as a representative airway metric). These analyses further highlight the relationship between increasing emphysema and BODE as well as increasing airway disease and SGRQ.
Discussion
The results of the current study establish a relationship between QCT metrics and two indices of disease impact, a specific measure of respiratory health status (SGRQ) and a multidimensional index of COPD severity, BODE, that has been correlated with mortality. In multivariate models, increases in both QCT measures of airway disease and emphysema were associated with higher SGRQ and BODE scores. However, in models that were centered and scaled such that the relative weight of airway disease measures and of emphysema measures could be compared, a one unit standard deviation increase in airway disease measures had a greater relative effect on increasing SGRQ total score than a one unit standard deviation increase in emphysema. In contrast, a similar analysis performed on BODE index indicated greater relative increase in BODE index scores for a one unit standard deviation increase in emphysema as opposed to airway measures. The color-coded density plots of subjects with high SGRQ and BODE scores also emphasize that an airway disease predominant phenotype is associated with more impaired higher SGRQ whereas an emphysema predominant phenotype is associated with higher BODE index scores.
This analysis extends the findings of others regarding an association between QCT measures of airway disease and symptoms in COPD. In a prior report of the COPDGene cohort, segmental airway wall thickening but not emphysema was associated with the presence of cough and sputum 14. Grydeland, et al. previously reported that in COPD, both pi10 and emphysema were related to dyspnea, but only pi10 was associated with cough and wheeze 15. Here we broaden these prior findings by examining the relationships between QCT measures and SGRQ. While cough, phlegm production and shortness of breath are all important items assessed by the SGRQ, the SGRQ is an important independent measure of health related quality of life in COPD 3. In the present study, we help to demonstrate a significant relationship between increasing airways disease and emphysema with SGRQ, but also through normalizing the QCT parameters, demonstrate that the relative influence of airway disease on SGRQ is significantly stronger than that of emphysema.
While univariate analysis suggests a relationship between disease severity as quantified by emphysema and SGRQ score does exist, the multivariate analyses data suggest that airway disease plays the relatively stronger role. In our multivariate analysis, a 0.20 increase in pi10 would correlate with a 4 point increase in SGRQ, the generally accepted minimal important difference for SGRQ16. The relationship between pi10 and the activity subscore of the SGRQ appears to be the strongest. A ready explanation for this is not obvious, although it may be that patients who have more significant airway disease represent a unique subgroup of subjects who either due to pulmonary physiology or comorbid conditions experience greater decrements in functional status. The current results differ from our prior analysis of 156 COPD subjects, which demonstrated a relationship between increasing emphysema and SGRQ score but not right upper lobe wall area percent 17. That cohort, however, was not only very small but also composed of a unique study population who were being evaluated for lung surgery (tumor resection, lung volume reduction surgery, or lung transplantation). This current analysis is significantly larger and includes a significantly more representative sample of the general COPD patient population.
While we and others have documented a relationship between QCT emphysema measures and BODE score 17,18, in the current analysis we demonstrated a relationship between both airway disease measures and emphysema with BODE score. However, we are also able to examine the relative relationship between airway disease, emphysema and BODE. The relationship between emphysema and BODE is stronger than the relationship between airway disease and BODE. This is suggested not only by the relative weight of the normalized parameter estimates in the multivariate models (Table 4B), but also the density plot (Figure 2D) where it is evident that patients with high BODE scores have a much greater burden of emphysema than airway disease. To put this in perspective, our multivariate analysis suggests that a 10% increase in emphysema would correlate with a 1.23 fold increase in BODE score. While an MID for BODE is difficult to define with certainty, a 1.19 fold increase in BODE has been reported with pulmonary rehabilitation which was also associated with improved survival19. The relationship between emphysema and BODE appears to be driven most strongly by its relationship to FEV1, but not surprisingly a significant relationship between emphysema and the other BODE components including BMI, MMRC and 6MWD are also seen. Weight loss20 and hyperinflation21 are known associations with emphysema that likely contribute to these relationships.
Previously we had reported no significant relationship between airway disease as measured by right upper lobe WA% and BODE. However, this prior analysis was a performed on a small, highly selected COPD cohort as compared to the significantly larger cohort described here and also used a very localized measure of airway disease (right upper lobe) as opposed to the measures used here that are averaged from six separate bronchial segments throughout the lung.
The current analysis also demonstrates that three of the QCT airway measures currently in use do not behave identically in multivariate modeling. Currently no gold standard airway disease measure has been identified and hence all three are included here. As the derivation of airway measures differ significantly, it is reasonable to expect that they each may contribute different information. For instance, because WA% reflects wall thickness relative to airway size, that measure will not be changed if both elements increase. Both WA% and pi10 also have a stronger relationship to FEV1 than wall thickness. Determining which airway measure best helps characterize COPD patients is certainly an issue that deserves further attention so that we can better understand which aspects of airway dimension relate to characteristics intrinsic to normal development as opposed to disease.
These data also raise several questions about the interplay between QCT phenotypes and clinical descriptors of disease. The SGRQ was developed and has been tested in large numbers of patients and can safely be assumed to be a valid indicator of quality of life in COPD for patients with a wide variety of phenotypes. However, our data would suggest that the relationship between SGRQ and pathologic abnormality is complex as SGRQ appears to be more influenced by changes in airway disease than emphysema. Hence therapies targeted at different aspects of the disease may expect relative changes in SGRQ to be different which should be considered when planning clinical trials and determining appropriate outcome measures. Hypothetically speaking, a therapy targeted at slowing emphysema, for instance, may not be expected to have the same influence on SGRQ as therapies targeted at airways disease. While improving quality of life for patients with COPD is an important goal by itself, these data underscore the complexity of inferring a relationship between SGRQ and disease pathology in patient populations that are not well phenotyped.
An additional question raised is whether the relationship between BODE and mortality is driven by the presence of emphysema. Would emphysema itself be a better predictor of mortality? Emphysema is associated with systemic inflammation, and interestingly, lung volume reduction surgery in patients with upper lobe predominant emphysema results not only in improved survival but also a decrease in inflammatory mediators including TNF-alpha and IL-8 as well as an increase in alpha-1-antitrypsin and BMI 22,23. Another question raised by this analysis is whether the BODE index is the best predictor of mortality among patients with an airway predominant disease phenotype. Although longitudinal data will be required to answer these questions definitively, previously published data underscore the potential importance of CT characterization to identify subsets of COPD for specific therapies. Emphysema distribution helps to identify appropriate patients for lung volume reduction therapy 23. We have also previously demonstrated that QCT can be used to identify airway disease predominant and emphysema predominant patient populations with increased risk for exacerbations 2.
A limitation to our analysis is that while emphysema and airway disease measures were adjusted for scanner model, these CT data were not corrected for variation within any particular scanner model due to tube calibration or other factors. This is a difficulty associated with performing QCT studies across multiple sites and scanners. Methodology to do this is currently being developed through the use of CT phantoms to standardize images, but the algorithms to perform such corrections are still being researched. Such corrections become even more critically important when performing longitudinal studies when both intrascanner and interscanner variations may risk obscuring signal detection.
In summary, our analyses demonstrate that radiologic indices of both airway disease and emphysema influence BODE and SGRQ. Airway disease, however, appears to be more closely associated with higher SGRQ scores and emphysema appears to be more closely associated with BODE. These analyses all demonstrate the ability of QCT measures to relate to clinically relevant outcomes and add to our ability to understand the heterogeneity implicit to COPD. Future prospective studies will allow us to better understand the predictive value of radiologic indices as compared to composite indices such as the SGRQ and BODE.
Supplementary Material
Figure S1. Cohort density plot demonstrating distribution of subjects respect to pi10 and percent emphysema.
Figure S2. Cohort density plot demonstrating distribution of subjects respect to segmental wall area percent and percent emphysema.
Figure S3. Cohort density plot demonstrating distribution of subjects respect to segmental wall thickness and percent emphysema.
Acknowledgments
COPDGene® Investigators: The members of the COPDGene® study group as of June 2010
Ann Arbor VA: Jeffrey Curtis, MD (PI), Ella Kazerooni, MD (RAD)
Baylor College of Medicine, Houston, TX: Nicola Hanania, MD, MS (PI), Philip Alapat, MD, Venkata Bandi, MD, Kalpalatha Guntupalli, MD, Elizabeth Guy, MD, Antara Mallampalli, MD, Charles Trinh, MD (RAD), Mustafa Atik, MD
Brigham and Women's Hospital, Boston, MA: Dawn DeMeo, MD, MPH (Co-PI), Craig Hersh, MD, MPH (Co-PI), George Washko, MD, Francine Jacobson, MD, MPH (RAD)
Columbia University, New York, NY: R. Graham Barr, MD, DrPH (PI), Byron Thomashow, MD, John Austin, MD (RAD)
Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr., MD (PI), Lacey Washington, MD (RAD), H Page McAdams, MD (RAD)
Fallon Clinic, Worcester, MA: Richard Rosiello, MD (PI), Timothy Bresnahan, MD (RAD)
Health Partners Research Foundation, Minneapolis, MN: Charlene McEvoy, MD, MPH (PI), Joseph Tashjian, MD (RAD)
Johns Hopkins University, Baltimore, MD: Robert Wise, MD (PI), Nadia Hansel, MD, MPH, Robert Brown, MD (RAD), Gregory Diette, MD
Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Los Angeles, CA: Richard Casaburi, MD (PI), Janos Porszasz, MD, PhD, Hans Fischer, MD, PhD (RAD), Matt Budoff, MD
Michael E. DeBakey VAMC, Houston, TX: Amir Sharafkhaneh, MD (PI), Charles Trinh, MD (RAD), Hirani Kamal, MD, Roham Darvishi, MD
Minneapolis VA: Dennis Niewoehner, MD (PI), Tadashi Allen, MD (RAD), Quentin Anderson, MD (RAD), Kathryn Rice, MD
Morehouse School of Medicine, Atlanta, GA: Marilyn Foreman, MD, MS (PI), Gloria Westney, MD, MS, Eugene Berkowitz, MD, PhD (RAD)
National Jewish Health, Denver, CO: Russell Bowler, MD, PhD (PI), Adam Friedlander, MD, David Lynch, MB (RAD), Joyce Schroeder, MD (RAD), John Newell, Jr., MD (RAD)
Temple University, Philadelphia, PA: Gerard Criner, MD (PI), Victor Kim, MD, Nathaniel Marchetti, DO, Aditi Satti, MD, A. James Mamary, MD, Robert Steiner, MD (RAD), Chandra Dass, MD (RAD)
University of Alabama, Birmingham, AL: William Bailey, MD (PI), Mark Dransfield, MD (Co-PI), Hrudaya Nath, MD (RAD)
University of California, San Diego, CA: Joe Ramsdell, MD (PI), Paul Friedman, MD (RAD)
University of Iowa, Iowa City, IA: Geoffrey McLennan, MD, PhD (PI), Edwin JR van Beek, MD, PhD (RAD), Brad Thompson, MD (RAD), Dwight Look, MD
University of Michigan, Ann Arbor, MI: Fernando Martinez, MD (PI), MeiLan Han, MD, Ella Kazerooni, MD (RAD)
University of Minnesota, Minneapolis, MN: Christine Wendt, MD (PI), Tadashi Allen, MD (RAD)
University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, MD (PI), Joel Weissfeld, MD, MPH, Carl Fuhrman, MD (RAD), Jessica Bon, MD
University of Texas Health Science Center at San Antonio, San Antonio, TX: Antonio Anzueto, MD (PI), Sandra Adams, MD, Carlos Orozco, MD, Mario Ruiz, MD (RAD)
Administrative Core: James Crapo, MD (PI), Edwin Silverman, MD, PhD (PI), Barry Make, MD, Elizabeth Regan, MD, Sarah Moyle, MS, Douglas Stinson
Genetic Analysis Core: Terri Beaty, PhD, Barbara Klanderman, PhD, Nan Laird, PhD, Christoph Lange, PhD, Michael Cho, MD, Stephanie Santorico, PhD, John Hokanson, MPH, PhD, Dawn DeMeo, MD, MPH, Nadia Hansel, MD, MPH, Craig Hersh, MD, MPH, Jacqueline Hetmanski, MS, Tanda Murray
Imaging Core: David Lynch, MB, Joyce Schroeder, MD, John Newell, Jr., MD, John Reilly, MD, Harvey Coxson, PhD, Philip Judy, PhD, Eric Hoffman, PhD, George Washko, MD, Raul San Jose Estepar, PhD, James Ross, MSc, Rebecca Leek, Jordan Zach, Alex Kluiber, Jered Sieren, Heather Baumhauer, Verity McArthur, Dzimitry Kazlouski, Andrew Allen, Tanya Mann, Anastasia Rodionova
PFT QA Core, LDS Hospital, Salt Lake City, UT: Robert Jensen, PhD
Biological Repository, Johns Hopkins University, Baltimore, MD: Homayoon Farzadegan, PhD, Stacey Meyerer, Shivam Chandan, Samantha Bragan
Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: James Murphy, PhD, Douglas Everett, PhD, Carla Wilson, MS, Ruthie Knowles, Amber Powell, Joe Piccoli, Maura Robinson, Margaret Forbes, Martina Wamboldt
Epidemiology Core, University of Colorado School of Public Health, Denver, CO: John Hokanson, MPH, PhD, Marci Sontag, PhD, Jennifer Black-Shinn, MPH, Gregory Kinney, MPH
Funding: COPDGene is supported by NHLBI Grant #'s U01HL089897 and U01Hl089856. Dr. Han is supported by funding from NHLBI Grant # K23 HL093351. Dr. Washko is supported by funding from NHLBI Grant # K23 HL089353 and an award from the Parker B. Francis Foundation. The COPDGene project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, Novartis and Sepracor.
Footnotes
The original published article is available at thorax.bmj.com.
Contributor Statement: JC, BM, NM, GW, DL, EK, FM, and MH contributed to data collection. CM, PW, LL, SM, GW, DL, EK and MKH contributed to the statistical analysis of data. CM, YC, PW, LL, SM, JC, BM, EK, DL, NM, GW, FM and MH contributed to the interpretation of data analysis and manuscript writing.
Competing Interests: There are no competing interests.
Copyright Licence Statement: The Corresponding author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licencees to permit this article (if accepted) to be published in Thorax and any other BMJPGL products to exploit all subsidiary rights, as set out in our license.
References
- 1.Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10(22):1503–19. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- 2.Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–82. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med. 1991;85(B):25–31. doi: 10.1016/s0954-6111(06)80166-6. discussion 33-7. [DOI] [PubMed] [Google Scholar]
- 4.Cote CG, Celli BR. BODE index: a new tool to stage and monitor progression of chronic obstructive pulmonary disease. Pneumonol Alergol Pol. 2009;77(3):305–13. [PubMed] [Google Scholar]
- 5.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 6.Brooks S. Surveillance for respiratory hazards. ATS News. 1982;8:12–16. [Google Scholar]
- 7.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 8.Standardization of Spirometry. 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 9.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8(3):492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 10.Tschirren J, Hoffman EA, McLennan G, Sonka M. Segmentation and quantitative analysis of intrathoracic airway trees from computed tomography images. Proc Am Thorac Soc. 2005;2(6):484–7. 503–4. doi: 10.1513/pats.200507-078DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1102–8. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 12.Patel BD, Coxson HO, Pillai SG, Agusti AG, Calverley PM, Donner CF, et al. Airway Wall Thickening and Emphysema Show Independent Familial Aggregation in COPD. Am J Respir Crit Care Med. 2008;178(5):500–5. doi: 10.1164/rccm.200801-059OC. [DOI] [PubMed] [Google Scholar]
- 13.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171(2):142–6. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 14.Kim V, Han MK, Vance GB, Make BJ, Newell JD, Hokanson JE, et al. The Chronic Bronchitic Phenotype of COPD: An Analysis of the COPDGene Study. Chest. 2011;140(3):626–33. doi: 10.1378/chest.10-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grydeland TB, Dirksen A, Coxson HO, Eagan TM, Thorsen E, Pillai SG, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181(4):353–9. doi: 10.1164/rccm.200907-1008OC. [DOI] [PubMed] [Google Scholar]
- 16.Jones PW. St. George's Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–9. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 17.Han MK, Bartholmai B, Liu LX, Murray S, Curtis JL, Sciurba FC, et al. Clinical significance of radiologic characterizations in COPD. COPD. 2009;6(6):459–67. doi: 10.3109/15412550903341513. [DOI] [PubMed] [Google Scholar]
- 18.Mair G, Maclay J, Miller JJ, McAllister D, Connell M, Murchison JT, et al. Airway dimensions in COPD: relationships with clinical variables. Respir Med. 2010;104(11):1683–90. doi: 10.1016/j.rmed.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J. 2005;26(4):630–6. doi: 10.1183/09031936.05.00045505. [DOI] [PubMed] [Google Scholar]
- 20.Sridhar MK. Why do patients with emphysema lose weight? Lancet. 1995;345(8959):1190–1. doi: 10.1016/s0140-6736(95)91984-8. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006;3(2):176–9. doi: 10.1513/pats.200508-094DO. [DOI] [PubMed] [Google Scholar]
- 22.Mineo D, Ambrogi V, Cufari ME, Gambardella S, Pignotti L, Pompeo E, et al. Variations of inflammatory mediators and alpha1-antitrypsin levels after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med. 2010;181(8):806–14. doi: 10.1164/rccm.200910-1476OC. [DOI] [PubMed] [Google Scholar]
- 23.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cohort density plot demonstrating distribution of subjects respect to pi10 and percent emphysema.
Figure S2. Cohort density plot demonstrating distribution of subjects respect to segmental wall area percent and percent emphysema.
Figure S3. Cohort density plot demonstrating distribution of subjects respect to segmental wall thickness and percent emphysema.